Abstract
Recombination plays an important role in the divergence of bacteria, but the frequency of interspecies and intraspecies recombination events remains poorly understood. We investigated recombination events that occurred within core genomes of 35 Vibrio strains (family Vibrionaceae, Gammaproteobacteria), from six closely related species in the so-called “Harveyi clade.” The strains were selected from a collection of strains isolated in the last 90 years, from various environments worldwide. We found a close relationship between the number of interspecies recombination events within core genomes of the 35 strains and the overall genomic identity, as inferred from calculations of the average nucleotide identity. The relationship between the overall nucleotide identity and the number of detected interspecies recombination events was comparable when analyzing strains isolated over 80 years apart, from different hemispheres, or from different ecologies, as well as in strains isolated from the same geographic location within a short time frame. We further applied the same method of detecting recombination events to analyze 11 strains of Vibrio campbellii, and identified disproportionally high number of intraspecies recombination events within the core genomes of some, but not all, strains. The high number of recombination events was detected between V. campbellii strains that have significant temporal (over 18 years) and geographical (over 10,000 km) differences in their origins of isolation. Results of this study reveal a remarkable stability of Harveyi clade species, and give clues about the origins and persistence of species in the clade.
Keywords: Vibrio, recombination, bacterial species definition, bacterial speciation
Introduction
Microbial taxonomy classifies bacteria into distinct clusters that are referred to as species. The current species definition is practical, formed based on observations made since the early days of microbiology (Drews 2000; Tindall et al. 2010). Early bacteriologists observed phenotypic similarities between some bacterial isolates, and started classifying bacteria into species based on their phenotypic characters (Drews 2000). Exhaustive analyses of phenotypic characters such as morphology, growth rates, nutrient uptake, and pathogenicity convinced microbiologists that bacteria form distinct clusters, which correspond to species observed in multicellular organisms. Microbial taxonomists later supplemented the analyses of phenotypic characters with chemotaxonomy, DNA–DNA hybridization, analysis of 16S rRNA gene sequence, and multilocus sequence analysis (MLSA). Eventually, microbial taxonomy developed polyphasic approaches which take into account all of available information to classify strains into species (Vandamme et al. 1996; Stackebrandt et al. 2002). The use of polyphasic approaches for taxonomic classification is practical, as it allows classifying strains into species based on well-defined criteria, and allows for relatively easy replication of tests used in the classification. Use of polyphasic taxonomy allowed description of numerous new bacterial species and led to changes in the taxonomy of some bacterial groups, but the core of bacterial taxonomy remained relatively unchanged in the last 50 years.
However, the current definition of bacterial species, and even the very idea that bacteria can be classified into species came into question with the advent of whole-genome sequencing and analysis of whole-genome sequence data (Doolittle and Bapteste 2007). Analysis of genome sequence data found evidence for widespread recombination among bacteria, including frequent recombination between bacteria classified as members of different species (Feil and Spratt 2001; Kunin et al. 2005). It was predicted that a high rate of recombination between species could lead to blurring of bacterial clusters, so-called “fuzzy” species (Hanage et al. 2005; Hanage 2013). Studies of a variety of bacteria groups recognized that the recombination rates vary greatly, and may depend on ecological and biological barriers (Fraser et al. 2009; Polz et al. 2013). Importantly, recombination may lead to changes in bacterial phenotypes (Hacker and Carniel 2001), which in turn could make the taxonomic classification of bacteria into species based on phenotypic characters unreliable. Furthermore, recombination was reported in sequences used for MLSA, and within sequences coding for 16S rRNA, which brought into question usefulness of sequence analyses in taxonomic classification (Smith et al. 1999; Gevers et al. 2005).
Some models of bacterial species attempted to incorporate recombination into concepts analogous to biological species concept developed for multicellular eukaryotes (Dykhuizen and Green 1991). It was proposed that recombination, which is predicted to be proportional to the overall level of sequence similarity between genomes (Zawadzki et al. 1995; Majewski et al. 2000), could allow maintaining genetic isolation between different species, and force cohesion among populations of bacteria (Fraser et al. 2007; Caro-Quintero et al. 2011). In this scenario, recombination would allow the formation of cohesive bacterial groups, which would be comparable to multicellular eukaryotic species. This concept of bacterial species was contrasted with the ecotype model, where a species is defined as an occupant of a specific ecological niche, where periodic selection allows formation and persistence of genetic clusters (Koeppel et al. 2008; Fraser et al. 2009; Shapiro and Polz 2014). However, it is unclear how either of those models corresponds to the species definition currently used by bacterial taxonomy.
The general significance of recombination in the formation of distinct clusters of bacteria, stability of those clusters, and the clusters relation to bacterial species defined using polyphasic taxonomy remains unclear. In order to interpret the species definition used by microbial taxonomists in the light of ongoing recombination between bacteria, it is important to analyze the frequency of recombination between clusters of strains described by microbial taxonomists as species. In this regard, marine bacteria in the genus Vibrio (family Vibrionaceae, Gammaproteobacteria) appear to be one of the best models. The genus is very diverse, with over 100 described species, ubiquitous in the marine environment, and bacteria in the genus have been found occupying various ecological niches (Thompson et al. 2004; Ceccarelli and Colwell 2014). Vibrio have been found as planktonic bacteria, as well as forming associations with a variety of animals (Takemura et al. 2014). Some Vibrio are pathogens of marine fishes, squids and corals, and the genus also includes important human pathogens (Thompson et al. 2004). Species in the genus have been divided into several complexes, including so-called “Harveyi clade” (Sawabe et al. 2013; Urbanczyk et al. 2013). Several species have been included in the Harveyi clade, with the core of the clade consisting of five closely related species, Vibrio harveyi, Vibrio campbellii, Vibrio owensii, Vibrio jasicida, and Vibrio rotiferianus. Importantly, the bacteria in the Harveyi clade have been studied since the early days of microbiology (Figge et al. 2011; Robertson et al. 2011), and numerous strains collected from a wide variety of locations and ecologies in the last 90 years are available for studies.
Previous studies have found a variety of recombination frequencies between Vibrio bacteria (Shapiro et al. 2012; Shapiro and Polz 2014). High frequency of recombination events was observed within the populations of Vibrio occupying the same ecological niche, with frequent exchange of niche-adaptive genes (Shapiro et al. 2012; Shapiro and Polz 2014). These recombination events were observed between bacteria classified as members of the same species, in strains isolated from a relatively limited geographic location and collected within a short time frame. These recombination events were predicted to propagate and maintain intraspecies genetic diversity, and were mainly occurring in the flexible parts of the Vibrio genome.
In an attempt to analyze the effect of recombination on the emergence of distinct bacterial clusters and the cluster relation to bacterial species delineated using polyphasic microbial taxonomy methods, we attempted to analyze the frequency of recombination events between six Vibrio species in the Harveyi clade. We aimed to identify genomic regions conserved in the six species, predict evolutionary relationship between the analyzed bacteria, compare the predicted phylogeny to classification using microbial taxonomy methods, and identify the interspecies recombination events occurring within core genomes of the Harveyi clade. The same method of detecting recombination was also applied to identify intraspecies recombination events within one of the six Vibrio species. These analyses focused on bacteria isolated worldwide, from various habitats, and over 80 years apart.
Materials and Methods
Strains
A collection of 15 Vibrio strains was obtained from various sources (see table 1). Nine strains were isolated from seawater samples collected off the coast of Miyazaki (Japan). The seawater samples were collected along 9 km of coastline, within 25-month period. Isolation of bacteria from seawater was done using the method described by Dunlap and Urbanczyk (2013). Six other strains were obtained from different collections (table 1). All 15 strains were grown at 24–28 °C on the LSW-70 broth, as described previously (Dunlap and Urbanczyk 2013). In addition to the 15 strains, genome sequence data for additional 20 Vibrio strains from the Harveyi clade were obtained from public databases. Together, the 35 strains represent a worldwide collection of Vibrio genome sequences, isolated from various environments within the last 90 years.
Table 1.
Bacterial Strains Analyzed in This Study
| Species | Strain | Year of Isolation | Ecological Information | Accession Number(s) | Reference |
|---|---|---|---|---|---|
| Vibrio campbellii | NBRC 15631T | Before 1971 | Directly from seawater, Pacific | BAOF00000000 | Baumann et al. 1971 |
| V. campbelliia | 051011E | 2011 | Directly from seawater, Miyazaki, Japan | BBKU01000001–BBKU01000219 | This study |
| V. campbelliia | 051011F | 2011 | Directly from seawater, Miyazaki, Japan | BBKV01000001–BBKV01000241 | This study |
| V. campbelliia | 051011G | 2011 | Directly from seawater, Miyazaki, Japan | BBKG01000001–BBKG01000209 | This study |
| V. campbelliia | 151112C | 2012 | Directly from seawater, Miyazaki, Japan | BBKW01000001–BBKW01000251 | This study |
| V. campbellii | 200612B | 2012 | Directly from seawater, Miyazaki, Japan | BANY00000000 | Urbanczyk et al. 2013 |
| V. campbellii | ATCC BAA-1116 | 1993 | Ocean isolate, USA | NC_022271, NC_022270, NC_022269 | Bassler et al. 1993 |
| V. campbelliia | CCS02 | 2007 | Diseased fish skin (Lates calcarifer), Australia | BBKX01000001–BBKX01000182 | Cano-Gomez et al. 2011 |
| V. campbellii | DS40M4 | Before 2010 | Directly from seawater, Africa | AGIE00000000 | Sandy et al. 2010 |
| V. campbellii | HY01 | 2004 | Dead, luminescing shrimp isolate, Thailand | AAWP00000000 | Lin et al. 2010 |
| V. campbellii | PEL22A | Before 2012 | Directly from seawater, Brazil | AHYY00000000 | Amaral et al. 2012 |
| V. harveyi | NBRC 15634T | 1935 | Dead, luminescing amphipod (Talorchestia sp.), USA | BAOD00000000 | Johnson and Shunk 1936 |
| V. harveyia | 823TEZ1 | 2005 | Gill of moribound abalone (Haliotis discus hannai), Japan | BBKY01000001–BBKY01000337 | Thompson et al. 2007 |
| V. harveyi | AOD131 | Unknown | Diseased giant grouper (Epinephelus lanceolatus), Taiwan | AOMR00000000 | |
| V. harveyi | CAIM 1792 | 2005 | Diseased shrimp (Litopenaeus vannamei), Mexico | AHHQ00000000 | Lin et al. 2010 |
| V. harveyi | VHJR7 | 2008 | Diseased Lates calcarifer, Malaysia | CAUO00000000 | Ransangan et al. 2012 |
| V. harveyi | ZJ0603 | 2008 | Diseased orange-spotted grouper (Epinephelus coioides), China | AKIH00000000 | Huang et al. 2012 |
| V. jasicida | LMG 25398T | 1999 | Healthy packhorse lobster (Jasus verreauxi), New Zealand | BAOG00000000 | Yoshizawa et al. 2012 |
| V. jasicida | 090810c | 2010 | Directly from seawater, Miyazaki, Japan | BAOC00000000 | Urbanczyk et al. 2013 |
| V. jasicidaa | 200612G | 2012 | Directly from seawater, Miyazaki, Japan | BBKZ01000001–BBKZ01000079 | This study |
| V. jasicidaa | 201212A | 2012 | Directly from seawater, Miyazaki, Japan | BBLA01000001–BBLA01000115 | This study |
| V. jasicida | MWB 21 | Before 1924 | Directly from seawater, Netherlands | BAOA00000000 | Figge et al. 2011 |
| V. owensii | LMG 25443T | Before 2010 | Diseased larvae of the ornate spiny lobster (Panulirus ornatus), Australia | BAOH00000000 | Cano-Gómez et al. 2010 |
| V. owensiia | 051011B | 2011 | Directly from seawater, Miyazaki, Japan | BBLB01000001–BBLB01000133 | This study |
| V. owensii | 1DA3 | 2007 | Isolated from coral (Phyllogorgia dilatata), Brazil | ACZC00000000 | Thompson et al. 2009 |
| V. owensiia | 47666-1 | Before 2010 | Diseased Penaeus monodon larvae, Australia | BBKN01000001–BBKN01000233 | Cano-Gómez et al. 2010 |
| V. owensii | ATCC 25919 | Before 1971 | Seawater enriched with glycerol and nitrate, USA | BANZ00000000 | Baumann et al. 1971 |
| V. owensii | LMG 25430 | 2005 | Isolated from healthy coral (Mussismilia hispida), Brazil | BAOE00000000 | Chimetto et al. 2011 |
| V. owensiia | OCN002 | Before 2012 | Diseased coral (Montipora capitata), USA | BBKO01000001–BBKO01000198 | Ushijima et al. 2012 |
| V. rotiferianus | LMG 21460T | 1999 | Isolated from rotifer (Brachionus plicatilis), Belgium | BAOI00000000 | Gomez-Gil et al. 2003 |
| V. rotiferianus | DAT722 | Before 2006 | Aquaculture tank, Australia | AFAJ00000000 | Roy Chowdhury et al. 2011 |
| V. rotiferianusa | Oz08 | 2006 | Moribound lobster larvae (Panulirus ornatus), Australia | BBLC01000001–BBLC01000964 | Cano-Gomez et al. 2011 |
| Vibrio sp.a | 090810a | 2010 | Directly from seawater, Miyazaki, Japan | BBLD01000001–BBLD01000071 | This study |
| Vibrio sp.a | 100512A | 2012 | Directly from seawater, Miyazaki, Japan | BBLE01000001–BBLE01000081 | This study |
| Vibrio sp.a | 151112A | 2012 | Directly from seawater, Miyazaki, Japan | BBLF01000001–BBLF01000214 | This study |
Note.—“Years of isolation” and “Ecological information” for some strains are only an approximation based on the information available.
aDraft genome sequences of these strains were obtained in this study.
Whole-Genome Sequencing
Genomic DNA was isolated using the DNeasy Blood and Tissue kit (Qiagen) according to the manufacturer’s instructions for Gram-negative bacteria. Draft genome sequences were obtained using the MiSeq platform (Illumina). Libraries preparation, sequencing, and assembly were done as described previously (Urbanczyk et al. 2013). The draft genome sequences were not annotated. The sequences were submitted to the DNA Data Bank of Japan (DDBJ), see table 1 for the accession numbers.
DNA Sequence Analysis
Average nucleotide identity (ANI) was calculated using whole-genome sequence data for the 35 Vibrio strains using the JSpecies program version 1.2.1 (Richter and Rosselló-Móra 2009) with default settings for calculation of ANIb.
Sequences shared by all analyzed bacterial strains were identified by a following procedure. The BLAST 2.2.28 + package was used to create a database containing whole-genome sequences of 35 Vibrio strains. The nucleotide sequences of 5,428 protein-coding genes of V. campbellii ATCC BAA-1116 were obtained from GenBank. Coding sequences of at least 500 bp long (3,776 sequences) were used as queries in searches of the genomic database of 35 Vibrio strains using the BLASTN algorithm, with 80% nucleotide sequence identity cutoff. Based on the search results, 899 nucleotide sequences that had a single match in all analyzed Vibrio strains were selected for further analysis. The 899 nucleotide sequences were manually aligned by inferred amino acid sequences using Mesquite 2.75 (http://mesquiteproject.org, last accessed July 24, 2014). During the alignment two sequences were removed from further analyses, as matching sequences recovered by BLASTN were significantly shorter for some strains. Remaining 897 alignments were concatenated, for a total of 1,032,399 characters. It should be noted that in some alignments a small number of insertions or deletions were noted. In most cases, those insertions and deletions were only present in 1 out of the 35 strains, and were likely a result of sequencing inaccuracies. As these inaccuracies were only present in one strain they were not considered as informative during phylogenetic analyses, and did not interfere with identification of recombination events.
SplitsTree version 4.12.8 (Huson and Bryant 2006) was used to construct a NeighborNet phylogenetic network using the concatenated alignment of sequences conserved in the 35 Vibrio strains, using default parameters. Phi test for recombination was performed in SplitsTree using default parameters. Phylogenetic analysis using parsimony criterion was performed using TNT version 1.1 (Goloboff et al. 2008). The analysis was carried out using 100 replicates of new technology searches, followed by 10,000 replicates of parsimony ratchet. Jackknife resampling values were calculated based on 10,000 replicates with 34% chance of character deletion. Tree predicted by the phylogenetic analysis was visualized using FigTree version 1.4.0 (http://tree.bio.ed.ac.uk/software/, last accessed October 16, 2014).
Program Structure version 2.3.4 (Falush et al. 2003) was used to examine genetic structure of the Harveyi clade. Data for the analysis were prepared based on concatenated alignment of 897 conserved sequences using the xmfa2struct program (http://www.xavierdidelot.xtreemhost.com/clonalframe.htm, last accessed July 24, 2014) using default parameters. Program Structure was run using an admixture model with 20,000 burn-in period, followed by 40,000 updates. Other parameters were set to default. For concatenated alignment of sequences of 35 strains, estimation of K (the number of populations) was set between 4 and 8. For analysis of genetic structure of V. campbellii, or genetic structure of Vibrio sp. and V. jasicida, K was set between 1 and 3.
Identification of Recombination Events
Individual alignments of 897 sequences conserved in the 35 Vibrio strains were used to identify recent recombination events using a modification of embedded quartet decomposition analysis, as implemented by Luo et al. (2011). The analysis was applied to two pairs of strains at the same time, each pair from a different clade. PAUP* version 4.0b10 (Swofford 2003) was used to make 1,000 bootstrap replicates of each alignment, with the cut-off value of 80%. The alignments that produced phylogeny incongruent with that predicted based on concatenated alignment were selected as representing a single recombination event. No assumptions were made about direction of recombination events.
Sequence data of 897 conserved protein-coding genes conserved in the Harveyi clade were also analyzed using ClonalFrame 1.1 (Didelot and Falush 2007). ClonalFrame runs consisted of 20,000 iterations, the first half was discarded as burn-in. Genealogies predicted by ClonalFrame were visualized using FigTree version 1.4.0 (http://tree.bio.ed.ac.uk/software/, last accessed October 16, 2014). Rate of recombination to mutation (r/m) was calculated based on ClonalFrame output as described by Ellegaard et al. (2013).
Results
Genome Sequencing and ANI
For the purpose of this research, we obtained draft genome sequences of 15 Vibrio strains from the Harveyi clade (table 1). Nine strains were isolated from seawater samples collected from the coast of Miyazaki prefecture in southern Japan. Remaining six strains were collected worldwide, using various isolation techniques (table 1). Whole-genome sequence data of additional 20 Vibrio strains from the Harveyi clade were obtained from public databases. The genome sequence data included the whole-genome sequences of five type strains of previously described Vibrio species, that is, V. campbellii, V. harveyi, V. jasicida, V. owensii, and V. rotiferianus. Information about origin of isolation of the 35 strains can be found in table 1.
Thirty-five Vibrio strains were initially classified into species based on the ANI between their whole-genome sequences. Calculations of ANI were conducted between all analyzed strains (supplementary table S1, Supplementary Material online). Based on the ANI calculations, the 35 strains could be divided into six groups, which contained strains with the ANI of 95% or higher. Five of the six groups contained a single type strain from previously described Vibrio species. Strains from these five groups were therefore taxonomically classified into species based on the classification of the corresponding type strains. Three strains (090810a, 100512A, and 151112A) formed a group that could not be assigned to any previously described Vibrio species, and will therefore be referred to as Vibrio sp. in this work.
Phylogenetic Analyses
Nucleotide sequences of 3,776 protein-coding genes found in V. campbellii ATCC BAA-1116 were used as queries for blast searches of the in-house database containing the whole-genome sequence data of 35 strains from the Harveyi clade (see Materials and Methods). These searches identified 897 protein-coding sequences conserved in all 35 Vibrio strains. The sequences were manually aligned and the alignments were concatenated. The resulting concatenated alignment (with 1,032,399 characters) was used for NeighborNet analysis to illustrate evolutionary relationship between the analyzed strains (fig. 1). This analysis assigned the 35 strains into six clades, which corresponded to grouping of strains based on ANI. Using the same concatenated data set for 897 protein-coding sequences, a parsimonious analysis showed same assignment of strains into clades (supplementary fig. S1, Supplementary Material online). In both NeighborNet and parsimony analyses, V. campbellii strains were divided into two subclades. Nine V. campbellii strains were assigned to the same subclades in both analyses. However, assignment of strains CCS02 and 151112C to subclades was incongruent between both analyses.
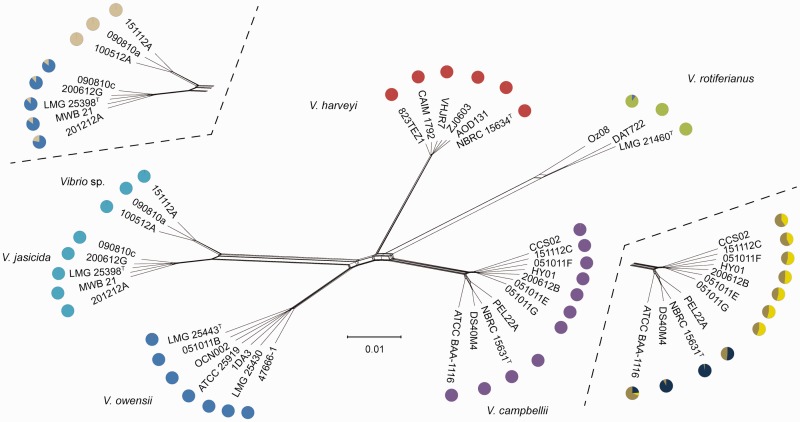
Fig. 1.—
Analysis of genetic structure in the “Harveyi clade.” Outputs from Structure analysis of 35 Vibrio strains (represented by pie charts with colors indicating proportion of estimated ancestries from five hypothetical populations; each color represents a different ancestral population) were mapped on a phylogenetic network predicted using NeighborNet analysis. The network was constructed based on analysis of concatenated alignment of 897 protein-coding sequences conserved in the six Vibrio species. The scale bar shows sequence divergence. Upper inset shows the results of an additional Structure analysis that included three Vibrio sp. and five V. jasicida strains. Lower inset shows results of a third Structure analysis, which focused on 11 V. campbellii strains.
Genetic Structure of the Harveyi Clade
Program Structure was chosen to analyze the genetic structure of Harveyi clade. The analysis revealed the pattern of admixture resulting from recombination events within the 35 strains. For the analysis, the concatenated alignment of 897 conserved sequences was used. The highest likelihood was observed for K (the number of populations) equal 5. All strains of V. rotiferianus, V. harveyi, V. owensii and V. campbellii had well-defined ancestries, whereas V. jasicida and Vibrio sp. strains shared ancestry (fig. 1). A second Structure analysis which analyzed five V. jasicida and three Vibrio sp. strains found K = 2 as the best representation of ancestry of the two species, with limited mixed ancestry between the two species (fig. 1). A Structure analysis which included 11 V. campbellii strains revealed K = 3 has the highest likelihood of the data, with mixed ancestry between the 11 strains (fig. 1).
Recombination Frequency
Phi test performed in the SplitsTree program using concatenated alignment of 897 conserved sequences found statistically significant evidence for recombination (P = 0.0). In order to identify interspecies recombination events between six Vibrio species, a method based on a phylogenetic analysis was used (see Materials and Methods). Briefly, the analysis identified recombination events between two pairs of strains, each pair of strains selected from different clades identified by the NeighborNet analysis (fig. 1). During the analysis, the assumption was that unless a recombination event occurred, results of a phylogenetic analysis of sequences of any of the 897 conserved sequences in any four strains should be congruent with phylogeny elucidated based on analysis of concatenated alignment. To test that assumption, alignments of sequences of individual genes from two pairs of strains from two different clades were bootstrapped and compared with the topology of the tree based on concatenated alignment. Results of analyses of individual genes sequences that were incongruent with those of concatenated alignment were considered to be an evidence for a recombination event between strains from the two clades. For each identified incongruence a single recombination event was assumed, with no assumption regarding direction of recombination event.
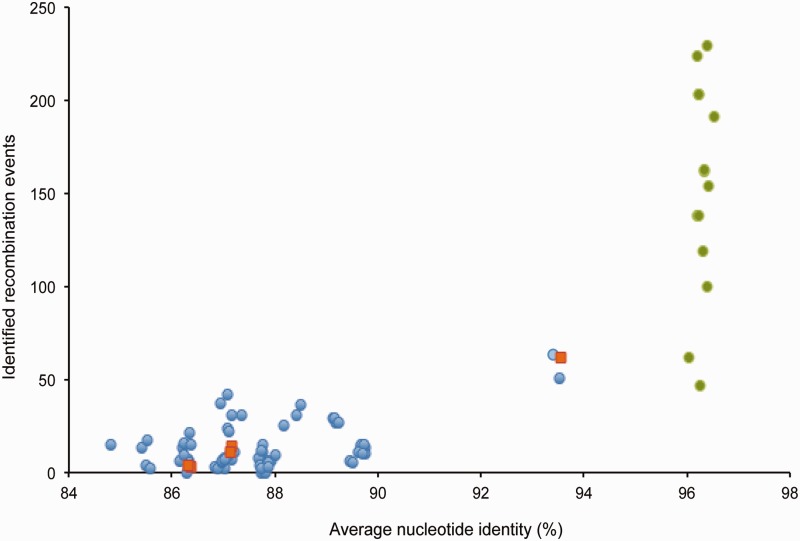
For the first analysis, pairs of strains were selected to be representative for their species. Higher priorities were given to pairs of strains isolated at distant geographical locations, from different ecological sources, using various isolation techniques, and with significant temporal differences in their origin of isolation. Information about origin of isolation of each strain is listed in table 1. The pairs of strains selected were V. campbellii 051011E and 200612B, V. campbellii 151112C and HY01, V. campbellii DS40M4 and NBRC 15631T, V. campbellii ATCC BAA-1116 and PEL22A, V. campbellii ATCC BAA-1116 and DS40M4, V. harveyi NBRC 15634T and ZJ0603, V. harveyi CAIM 1792 and VHJR7, V. jasicida LMG 25398T and 200612G, V. jasicida 090810C and MWB21, V. owensii ATCC 25919 and 051011B, V. owensii 1DA3 and LMG 25443T, V. rotiferianus LMG 21460T and Oz08, Vibrio sp. 151112A and 090810a. The number of identified recombination events was plotted versus the average ANI between strains from different clades used in the analyses (fig. 2). The results revealed close relationship between the number of interspecies recombination and the overall genome sequence identity (R2 = 0.37681, supplementary fig. S2, Supplementary Material online). Numbers of detected recombination events between each pair of strains are also listed in the supplementary table S2, Supplementary Material online.
Fig. 2.—
Relationship between the number of identified recombination events and the ANI. Each marker represents the number of recombination events identified between two pairs of strains from different clades plotted against average ANI between analyzed strains from different clades. Blue circles represent results of analysis of interspecies recombination events; orange squares represent results of analysis of strains isolated in the Miyazaki prefecture within a short time frame; green circles indicate results of analysis of intraspecies recombination events.
A second analysis of recombination events included pairs of strains isolated recently (within 25 months) from the same geographical location (shallow coastal water in the Miyazaki prefecture), using the same collection technique (see Materials and Methods). The pairs of strains were V. campbellii 151112C and 051011E, V. campbellii 051011E and 200612B, V. jasicida 090810c and 200612G, and Vibrio sp. 151112A and 090810a. Strains Vibrio sp. 151112A and V. campbellii 151112C were isolated from the same 50 ml seawater sample. Similar to results of analysis of interspecies recombination in strains collected worldwide from various ecologies, analysis of strains collected in Miyazaki prefecture (fig. 2, supplementary fig. S2 and table S2, Supplementary Material online) found close relationship between the number of recombination events and overall genome sequence identity.
A third analysis focused on identification of recombination events within a single species, V. campbellii. The same method used to analyze interspecies recombination events between six Vibrio species was used to identify the number of intraspecies recombination events, in particular recombination events between V. campbellii strains from two different subclades. For the analysis, V. campbellii strains were divided into two groups, corresponding to two subclades identified using NeighborNet analysis, and the number of recombination events between strains from two different subclades was elucidated. Results of the analysis found disproportionally high numbers of intraspecies recombination events (fig. 2 and supplementary table S2, Supplementary Material online).
The disproportionally high numbers of intraspecies recombination events within V. campbellii were identified in the analyses that include two strains, namely ATCC BAA-1116 and PEL22A (supplementary table S2, Supplementary Material online). The same two strains have shown atypical admixture patter in the analysis of V. campbellii genetic structure (fig. 1, lower inset). In order to determine whether these results are due to a major recombination event involving many genes, or due to multiple small events, conserved sequences that shown evidence of recombination were mapped on strain ATCC BAA-1116 chromosomes (supplementary fig. S3, Supplementary Material online). No conserved protein-coding genes were found on the strain plasmid. Results of the mapping have found sequences showing evidence of recombination to be spread along both chromosomes of the strain ATCC BAA-1116 (supplementary fig. S3, Supplementary Material online), which suggests that the strain was involved in numerous intraspecies recombination events, and pattern of admixture in V. campbellii revealed by Structure analysis is not a result of a single recombination event involving large number of sequences.
ClonalFrame Analysis
Analysis of interspecies and intraspecies recombination events in the Harveyi clade was also conducted using ClonalFrame software (Didelot and Falush 2007). ClonalFrame uses multilocus sequence data to predict clonal relationship between bacterial strains and estimates probability that a given site is altered through recombination. ClonalFrame was applied to three data sets, each containing aligned 897 protein-coding sequences conserved in the Harveyi clade. The first data set contained 11 V. campbellii strains, second data set included five V. jasicida and three Vibrio sp. strains, and the third data set contained three Vibrio sp. strains and V. jasicida 090810c. For each data set, genealogies predicted by the ClonalFrame software were similar to those predicted by phylogenetic and NeighborNet analyses (supplementary fig. S4, Supplementary Material online). Analyses of the first two data sets, which included multiple strains from a single species, identified high number of recombining sites in each strain, between 5,883 and 10,788 (supplementary fig. S4, Supplementary Material online). In the third analysis, which included three Vibrio sp. and a single V. jasicida strains, between 7,995 and 10,055 recombining sites were identified among Vibrio sp. strains, but only six recombining sites were identified in V. jasicida 090810c. These results can be interpreted as evidence that intraspecies recombination events have greater effect on genealogies of Harveyi clade bacteria than interspecies recombination events.
Discussion
Reported here are the results of a series of analyses of recombination events that occurred between 35 strains representing six closely related Vibrio species in the Harveyi clade. The analyses focused on recombination events that occurred within core regions of the bacteria genomes. The number of identified interspecies recombination events was in all cases correlated with the overall genomic identity between the analyzed strains, with increased number of recombination events occurring between genomes with higher average nucleotide sequence identity (fig. 2). In contrast, the number of identified intraspecies recombination events between some (but not all) strains of V. campbellii from different subclades was disproportionally high.
In this study, the number of identified interspecies recombination events was relatively low, and related to the overall genome sequence identity (supplementary fig. S2, Supplementary Material online). ClonalFrame analysis also predicted low effect of interspecies recombination on genealogies of the Harveyi clade bacteria (supplementary fig. S4, Supplementary Material online). Furthermore, the number of identified interspecies recombination events was independent of the origin of isolation. We found a good correlation between the number of interspecies recombination events and the overall genome identity when analyzing strains isolated over 80 years apart, as well as in strains isolated only few months apart. We also found no correlation between the number of interspecies recombination events and the strains habitats, as we found comparable number of interspecies recombination events between planktonic strains and in strains associated with marine animals. We also found comparable numbers of interspecies recombination events between strains isolated from different hemispheres, or between strains isolated from a limited geographical location (i.e., 9-km coastline in the Miyazaki prefecture). A low number of interspecies recombination events was found even when analyzing strains from different species isolated from the same 50 ml sample of seawater. Despite similar ecologies and presumably ample opportunities for exchange of genetic material, the number of interspecies recombination events within core genome of the strains isolated in the Miyazaki prefecture was in all cases correlated with the overall genomes sequence identity. Many factors likely influence recombination between Harveyi clade species, but the results reported here suggest that shared ecology of bacteria had little influence over the frequency of interspecies recombination in the core genomic regions.
In this regard, it should be stressed the method used in here allows calculating frequency of recombination between sequences conserved in the six Vibrio species, and are a part of the bacteria core genomes. The analyzed conserved sequences had 1.03 million nucleotides, which constitutes less than 25% of most genomes used in this study. The frequency of recombination between flexible parts of the Vibrio genomes is likely different from that reported here. Previous studies found frequent exchange of niche-adaptive genes located in flexible parts of Vibrio genomes, with higher rate of recombination between strains isolated from the same ecological niche (Shapiro et al. 2012; Polz et al. 2013). Here, we found no obvious correlation between the number of recombination events in the core genomes and the bacteria ecology.
In contrast to the results of analysis of interspecies recombination events, we found a high number of intraspecies recombination events that occurred within the core genomes of some V. campbellii strains from two different subclades. The number of identified intraspecies recombination events was higher than would be expected based on the overall sequence identity between the strains. ClonalFrame analysis also identified high number of recombining sites in the analyses that included multiple strains from a single species. High frequency of intraspecies recombination was found between strains isolated over 18 years apart, and from distinct geographical locations. It should be noted that the disproportionally high frequency of recombination events was only observed between some, but not all V. campbellii strains, namely ATCC BAA-1116 and PEL22A. The two strains also show a distinct admixture pattern in the analysis of V. campbellii genetic structure (fig. 1, lower inset). At this moment, it is not clear why frequency of intraspecies recombination events differs between some V. campbellii strains. Based on the results of mapping of recombining sequences on strain ATCC BAA-1116 chromosomes, it is likely that the pattern of interspecies recombination observed in this V. campbellii is a result of numerous independent events, and not due to a single event involving multiple genes. Furthermore, our analysis only identified recombination events occurring between strains from different V. campbellii subclades, and did not estimate frequency of recombination events between strains from the same subclade. The method used in this study to identify recombination events relies on understanding of evolutionary relationship between the analyzed strains, but we were unable to confidently determine the relationship between strains from the same V. campbellii subclade. It is likely that the number of intraspecies recombination events between strains from the same subclade is higher than that identified between strains from different subclades.
The number of inter- and intraspecies recombination events reported here encouraged us to analyze the genetic structure of Harveyi clade using program Structure. Initially, the analysis found five ancestral populations with very limited admixture. The five ancestral populations corresponded to five out of six species identified based on results of ANI, reticulation in the NeighborNet network, and results of a parsimonious analysis. Initial Structure analysis could not distinguish two closely related species, V. jasicida and Vibrio sp. However, a closer inspection of results of Structure analysis suggested limited admixture of V. jasicida and Vibrio sp. An analysis of genetic structure that focused on V. jasicida and Vibrio sp. strains identified two ancestral populations, with only limited admixture. In contrast to the situation found in V. jasicida and Vibrio sp., analysis of a genetic structure within V. campbellii strains identified three ancestral populations, with high degree of admixture. Overall, we interpret results of these Structure analyses as an evidence of relatively low levels of admixture between the six Vibrio species, and consider the initial prediction of shared ancestry between V. jasicida and Vibrio sp. as a result of too little data available to find a true genetic structure of the 35 strains. We predict that separation of Vibrio sp. and V. jasicida was a relatively recent event, whereas separation of other species occurred earlier. Results of these Structure analyses therefore support classification of the 35 strains into six species, and are in agreement with our conclusion about the frequency of interspecies and intraspecies recombination events.
The results of this study revealed clear-cut boundaries between the analyzed Vibrio species. Based on the ANI (supplementary table S1, Supplementary Material online) we were able to assign all analyzed strains into six groups, which correspond to five Vibrio species with validly published names and one previously undescribed species. This classification was also supported by reticulation revealed by the NeighborNet (fig. 1), and the evolutionary relationship evident from the results of parsimonious analysis (supplementary fig. S2, Supplementary Material online), as well as genetic structure of the 35 strains. Relatively, low levels of identified interspecies recombination events and high frequency of intraspecies recombination events also supported classification of the 35 strains into six species. Importantly, the use of large number of sequence data for MLSA allowed good resolution of relationship between the six species, despite evidence of recombination within some of the sequences used for the analysis. Remarkably, strains analyzed in this study could be confidently classified into species despite significant geographical or temporal distance between their isolation. The strains analyzed here were sampled over 80 years apart, from distant geographic locations and from varied environments, yet the 35 strains formed well-defined, remarkably cohesive and genetically similar groups.
Results of this study revealed Harveyi clade bacteria as very stable, cohesive groups, and can be used as a basis for future studies of evolutionary processes responsible for the emergence of species in the clade. Based on results of this study, we suggest that studies of Harveyi clade diversification processes should analyze a large number of strains, isolated from a wide variety of habitats, and from diverse geographical locations. With respect to the bacterial species definition, the remarkable cohesiveness of species in the Harveyi clade was observed in groups of strains classified as members of the same species using definition currently used in bacterial taxonomy. We conclude that the current bacterial species definition adequately describes Harveyi clade diversity, and is a good starting point for discussion of speciation in other Vibrio clades. It remains to be determined whether other Vibrio species show similar stability and cohesiveness as Harveyi clade bacteria.
Supplementary Material
Supplementary figures S1–S4 and tables S1 and S2 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
The authors thank Leigh Owens and Lone Høj (AIMS) for providing strains CCS02, Oz08 and 47666-1, Tomoo Sawabe (Hokkaido University) for providing strain 823TEZ1, and Sean Callahan and Blake Ushijima (University of Hawaii) for providing strain OCN002. This work was supported by the Program to Disseminate Tenure Tracking System from the Japanese Ministry of Education, Culture, Sports, Science and Technology; by a grant for Scientific Research on Priority Areas from the University of Miyazaki; as well as Kurita Water and Environment Foundation Grant.
Literature Cited
- Amaral GR, et al. Genome sequence of the bacterioplanktonic, mixotrophic Vibrio campbellii strain PEL22A, isolated in the Abrolhos Bank. J Bacteriol. 2012;194:2759–2760. doi: 10.1128/JB.00377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Baumann P, Baumann L, Mandel M. Taxonomy of marine bacteria: the genus Beneckea. J Bacteriol. 1971;107:268–294. doi: 10.1128/jb.107.1.268-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Gómez A, Goulden EF, Owens L, Høj L. Vibrio owensii sp. nov., isolated from cultured crustaceans in Australia. FEMS Microbiol Lett. 2010;302:175–181. doi: 10.1111/j.1574-6968.2009.01850.x. [DOI] [PubMed] [Google Scholar]
- Cano-Gomez A, Høj L, Owens L, Andreakis N. Multilocus sequence analysis provides basis for fast and reliable identification of Vibrio harveyi-related species and reveals previous misidentification of important marine pathogens. Syst Appl Microbiol. 2011;34:561–565. doi: 10.1016/j.syapm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Caro-Quintero A, et al. Unprecedented levels of horizontal gene transfer among spatially co-occurring Shewanella bacteria from the Baltic Sea. ISME J. 2011;5:131–140. doi: 10.1038/ismej.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli D, Colwell RR. Vibrio ecology, pathogenesis, and evolution. Front Microbiol. 2014;5:256. doi: 10.3389/fmicb.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimetto LA, et al. Vibrio communis sp. nov., isolated from the marine animals Mussismilia hispida, Phyllogorgia dilatata, Palythoa caribaeorum, Palythoa variabilis and Litopenaeus vannamei. Int J Syst Evol Microbiol. 2011;61:362–368. doi: 10.1099/ijs.0.019729-0. [DOI] [PubMed] [Google Scholar]
- Didelot X, Falush D. Inference of bacterial microevolution using multilocus sequence data. Genetics. 2007;175:1251–1266. doi: 10.1534/genetics.106.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle WF, Bapteste E. Pattern pluralism and the Tree of Life hypothesis. Proc Natl Acad Sci U S A. 2007;104:2043–2049. doi: 10.1073/pnas.0610699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G. The roots of microbiology and the influence of Ferdinand Cohn on microbiology of the 19th century. FEMS Microbiol Rev. 2000;24:225–249. doi: 10.1111/j.1574-6976.2000.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Dunlap PV, Urbanczyk H. Luminous bacteria. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The prokaryotes. New York: Springer; 2013. pp. 492–528. [Google Scholar]
- Dykhuizen DE, Green L. Recombination in Escherichia coli and the definition of biological species. J Bacteriol. 1991;173:7257–7268. doi: 10.1128/jb.173.22.7257-7268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegaard KM, Klasson L, Näslund K, Bourtzis K, Andersson SG. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet. 2013;9:e1003381. doi: 10.1371/journal.pgen.1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil EJ, Spratt BG. Recombination and the population structures of bacterial pathogens. Annu Rev Microbiol. 2001;55:561–590. doi: 10.1146/annurev.micro.55.1.561. [DOI] [PubMed] [Google Scholar]
- Figge MJ, Robertson LA, Ast JC, Dunlap PV. Historical microbiology: revival and phylogenetic analysis of the luminous bacterial cultures of M. W. Beijerinck. FEMS Microbiol Ecol. 2011;78:463–472. doi: 10.1111/j.1574-6941.2011.01177.x. [DOI] [PubMed] [Google Scholar]
- Fraser C, Alm EJ, Polz MF, Spratt BG, Hanage WP. The bacterial species challenge: making sense of genetic and ecological diversity. Science. 2009;323:741–746. doi: 10.1126/science.1159388. [DOI] [PubMed] [Google Scholar]
- Fraser C, Hanage WP, Spratt BG. Recombination and the nature of bacterial speciation. Science. 2007;315:476–480. doi: 10.1126/science.1127573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D, et al. Opinion: re-evaluating prokaryotic species. Nat Rev Microbiol. 2005;3:733–739. doi: 10.1038/nrmicro1236. [DOI] [PubMed] [Google Scholar]
- Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. [Google Scholar]
- Gomez-Gil B, Thompson FL, Thompson CC, Swings J. Vibrio rotiferianus sp. nov., isolated from cultures of the rotifer Brachionus plicatilis. Int J Syst Evol Microbiol. 2003;53:239–243. doi: 10.1099/ijs.0.02430-0. [DOI] [PubMed] [Google Scholar]
- Hacker J, Carniel E. Ecological fitness, genomic islands and bacterial pathogenicity. A Darwinian view of the evolution of microbes. EMBO Rep. 2001;2:376–381. doi: 10.1093/embo-reports/kve097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanage WP. Fuzzy species revisited. BMC Biol. 2013;11:41. doi: 10.1186/1741-7007-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanage WP, Fraser C, Spratt BG. Fuzzy species among recombinogenic bacteria. BMC Biol. 2005;3:6. doi: 10.1186/1741-7007-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, et al. Draft genome sequence of the fish pathogen Vibrio harveyi strain ZJ0603. J Bacteriol. 2012;194:6644–6645. doi: 10.1128/JB.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Johnson FH, Shunk IV. An interesting new species of luminous bacteria. J Bacteriol. 1936;31:585–593. doi: 10.1128/jb.31.6.585-593.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppel A, et al. Identifying the fundamental units of bacterial diversity: a paradigm shift to incorporate ecology into bacterial systematics. Proc Natl Acad Sci U S A. 2008;105:2504–2509. doi: 10.1073/pnas.0712205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin V, Goldovsky L, Darzentas N, Ouzounis CA. The net of life: reconstructing the microbial phylogenetic network. Genome Res. 2005;15:954–959. doi: 10.1101/gr.3666505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, et al. Comparative genomic analyses identify the Vibrio harveyi genome sequenced strains BAA-1116 and HY01 as Vibrio campbellii. Environ Microbiol Rep. 2010;2:81–89. doi: 10.1111/j.1758-2229.2009.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, et al. Genome sequencing of environmental Escherichia coli expands understanding of the ecology and speciation of the model bacterial species. Proc Natl Acad Sci U S A. 2011;108:7200–7205. doi: 10.1073/pnas.1015622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski J, Zawadzki P, Pickerill P, Cohan FM, Dowson CG. Barriers to genetic exchange between bacterial species: Streptococcus pneumoniae transformation. J Bacteriol. 2000;182:1016–1023. doi: 10.1128/jb.182.4.1016-1023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polz MF, Alm EJ, Hanage WP. Horizontal gene transfer and the evolution of bacterial and archaeal population structure. Trends Genet. 2013;29:170–175. doi: 10.1016/j.tig.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransangan J, Lal TM, Al-Harbi AL. Characterization and experimental infection of Vibrio harveyi isolated from diseased Asian seabass (Lates calcarifer) Malays J Microbiol. 2012;8:104–115. [Google Scholar]
- Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LA, Figge MJ, Dunlap PV. Beijerinck and the bioluminescent bacteria: microbiological experiments in the late 19th and early 20th centuries. FEMS Microbiol Ecol. 2011;75:185–194. doi: 10.1111/j.1574-6941.2010.01004.x. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury P, et al. Genome sequence of Vibrio rotiferianus strain DAT722. J Bacteriol. 2011;193:3381–3382. doi: 10.1128/JB.05089-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy M, et al. Vanchrobactin and anguibactin siderophores produced by Vibrio sp. DS40M4. J Nat Prod. 2010;73:1038–1043. doi: 10.1021/np900750g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabe T, et al. Updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front Microbiol. 2013;4:414. doi: 10.3389/fmicb.2013.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro BJ, et al. Population genomics of early events in the ecological differentiation of bacteria. Science. 2012;336:48–51. doi: 10.1126/science.1218198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro BJ, Polz MF. Ordering microbial diversity into ecologically and genetically cohesive units. Trends Microbiol. 2014;22:235–247. doi: 10.1016/j.tim.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NH, Holmes EC, Donovan GM, Carpenter GA, Spratt BG. Networks and groups within the genus Neisseria: analysis of argF, recA, rho, and 16S rRNA sequences from human Neisseria species. Mol Biol Evol. 1999;16:773–783. doi: 10.1093/oxfordjournals.molbev.a026162. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E, et al. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol. 2002;52:1043–1047. doi: 10.1099/00207713-52-3-1043. [DOI] [PubMed] [Google Scholar]
- Swofford D. 2003 PAUP*: phylogenetic analysis using parsimony (* and other methods). Version 4. Sunderland (MA): Sinauer Associates. [Google Scholar]
- Takemura AF, Chien DM, Polz MF. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front Microbiol. 2014;5:38. doi: 10.3389/fmicb.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CC, et al. Genomic taxonomy of Vibrios. BMC Evol Biol. 2009;9:258. doi: 10.1186/1471-2148-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FL, Gomez-Gil B, Vasconcelos AT, Sawabe T. Multilocus sequence analysis reveals that Vibrio harveyi and V. campbellii are distinct species. Appl Environ Microbiol. 2007;73:4279–4285. doi: 10.1128/AEM.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FL, Iida T, Swings J. Biodiversity of Vibrios. Microbiol Mol Biol Rev. 2004;68:403–431. doi: 10.1128/MMBR.68.3.403-431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall BJ, Rosselló-Móra R, Busse HJ, Ludwig W, Kämpfer P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol. 2010;60:249–266. doi: 10.1099/ijs.0.016949-0. [DOI] [PubMed] [Google Scholar]
- Urbanczyk H, Ogura Y, Hayashi T. Taxonomic revision of Harveyi clade bacteria (family Vibrionaceae) based on analysis of whole genome sequences. Int J Syst Evol Microbiol. 2013;63:2742–2751. doi: 10.1099/ijs.0.051110-0. [DOI] [PubMed] [Google Scholar]
- Ushijima B, Smith A, Aeby GS, Callahan SM. Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS One. 2012;7:e46717. doi: 10.1371/journal.pone.0046717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme P, et al. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa S, et al. Vibrio jasicida sp. nov., a member of the Harveyi clade, isolated from marine animals (packhorse lobster, abalone and Atlantic salmon) Int J Syst Evol Microbiol. 2012;62:1864–1870. doi: 10.1099/ijs.0.025916-0. [DOI] [PubMed] [Google Scholar]
- Zawadzki P, Roberts MS, Cohan FM. The log-linear relationship between sexual isolation and sequence divergence in Bacillus transformation is robust. Genetics. 1995;140:917–932. doi: 10.1093/genetics/140.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.