Abstract
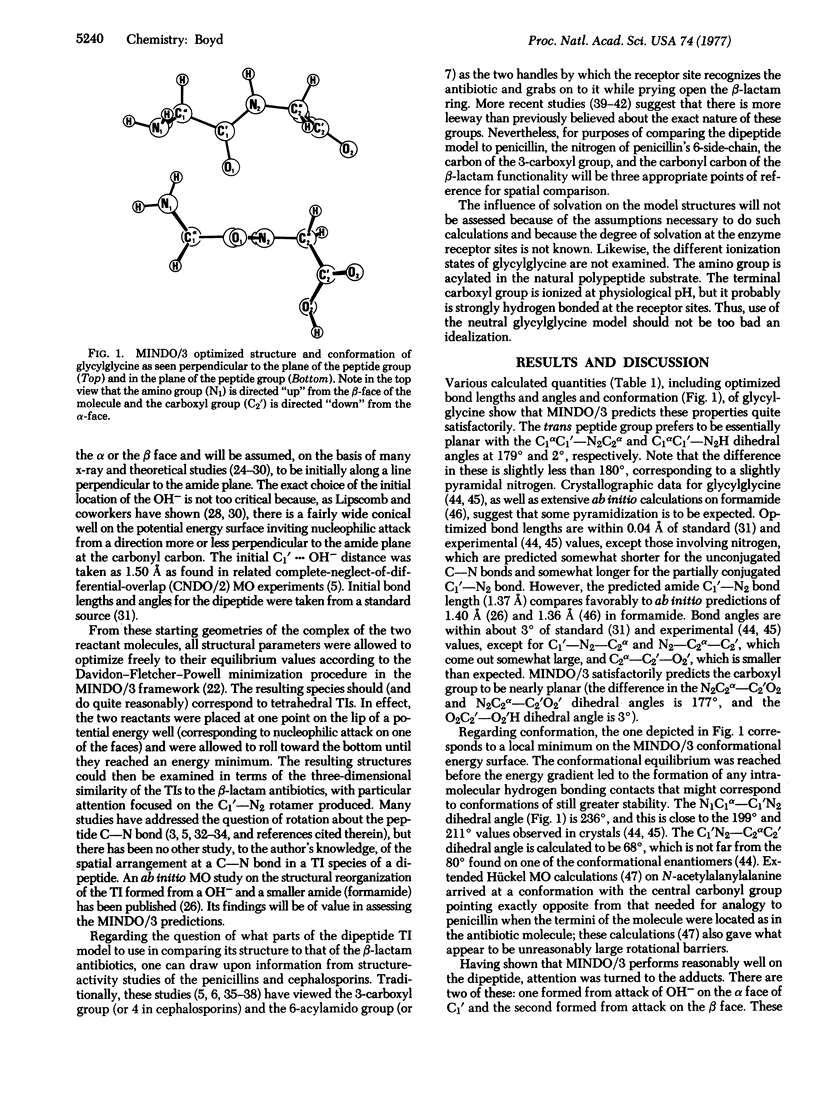
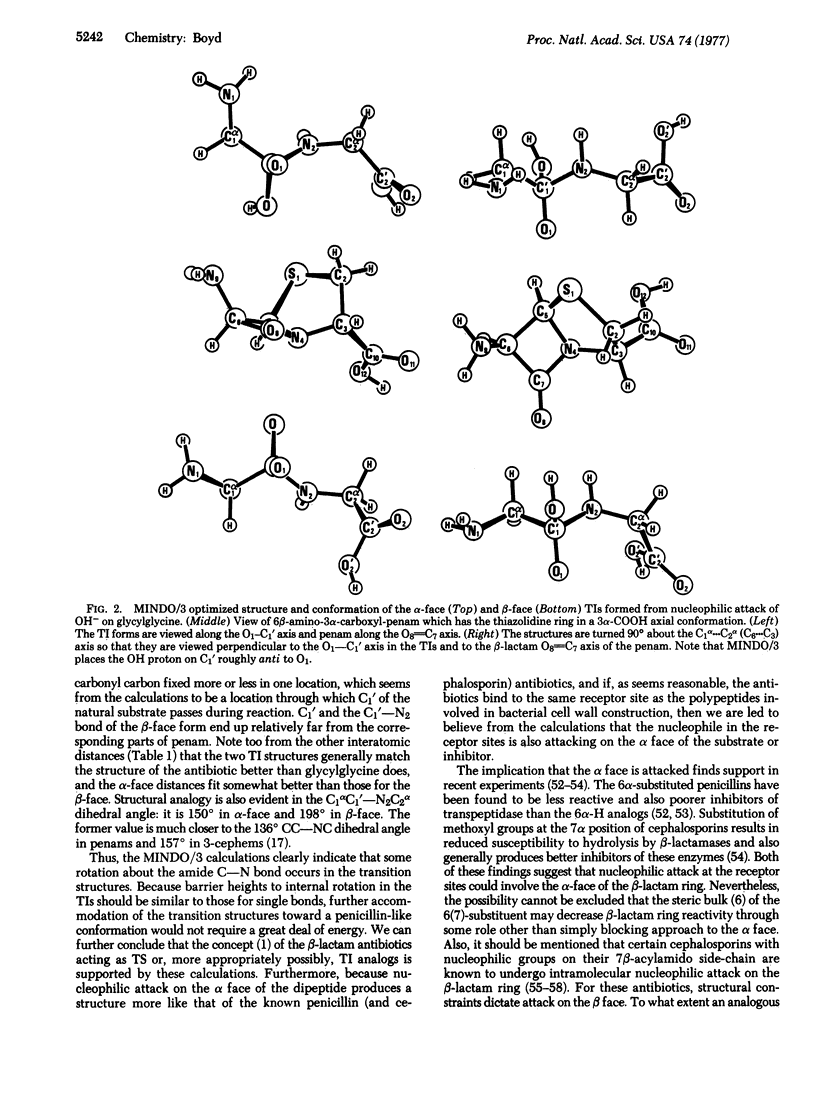
The tetrahedral adducts formed during nucleophilic attack by a hydroxyl ion on the carbonyl carbon of a model dipeptide, glycylglycine, were studied by modified-intermediate-neglect-of-differential-overlap molecular orbital calculations. This dipeptide is taken to represent the D-alanyl-D-alanine terminus of the polypeptides involved in the cross-linking transpeptidation reaction of peptidoglycan in bacterial cell walls. It was found that nucleophilic attack on one face of the carbonyl carbon leads to a transition intermediate species structurally similar to that afforded by the bicyclic nucleus of penicillins and cephalosporin antibiotics. The results support the concept that the beta-lactam antibiotics, which are known to inhibit various bacterial cell wall enzymes, may act as transition state analogs. Also, the structure formed from nucleophilic attack on the so-called alpha face of the dipeptide is more similar to the antibiotic structures than is that from attack on the opposite face. In agreement with other types of experiments, the results suggest that the alpha face may be the one approached by a nucleophile in the receptor site(s) of the appropriate cell wall enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The amino acid sequence of Staphylococcus aureus penicillinase. Biochem J. 1975 Nov;151(2):197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D. B., Hermann R. B., Presti D. E., Marsh M. M. Electronic structures of cephalosporins and penicillins. 4. Modeling acylation by the beta-lactam ring. J Med Chem. 1975 Apr;18(4):408–417. doi: 10.1021/jm00238a018. [DOI] [PubMed] [Google Scholar]
- Boyd D. B. Space-filling molecular models of four-membered rings. Three-dimensional aspects in the design of penicillin and cephalosporin antibiotics. J Chem Educ. 1976 Aug;53(8):483–488. doi: 10.1021/ed053p483. [DOI] [PubMed] [Google Scholar]
- Boyd D. B., Yeh C. Y., Richardson F. S. Optical activity of the penicillin nucleus chromophores. J Am Chem Soc. 1976 Sep 29;98(20):6100–6106. doi: 10.1021/ja00436a004. [DOI] [PubMed] [Google Scholar]
- Bundgaard H. Intramolecular nucleophilic attack of an ureido group on the beta-lactam carbonyl moiety of penicillins. Acta Pharm Suec. 1973 Sep;10(4):309–316. [PubMed] [Google Scholar]
- Carpenter C. V., Goyer S., Neuhaus F. C. Steric effects on penicillin-sensitive peptidoglycan synthesis in a membrane-wall system Gaffkya homari. Biochemistry. 1976 Jul 13;15(14):3146–3152. doi: 10.1021/bi00659a031. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Hughes J. M., Ross G. W. Inhibition of peptidoglycan cross-linking in growing cells of Escherichia coli by penicillins and cephalosporins, and its prevention by R factor-mediated beta-lactamase. Antimicrob Agents Chemother. 1976 Feb;9(2):208–213. doi: 10.1128/aac.9.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. R., Retsema J. A., Lynch J. E. Laboratory evaluation of 3-(5-tetrazolyl) penam, a new semisynthetic beta-lactam antibacterial agent with extended broad-spectrum activity. Antimicrob Agents Chemother. 1976 Jul;10(1):132–138. doi: 10.1128/aac.10.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman H. C., Paul G. L., Sabine T. M. A neutron diffraction study of perdeutero-alpha-glycylglycine. Acta Crystallogr B. 1970 Jul 15;26(7):925–932. doi: 10.1107/s0567740870003382. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N., Hammarström S., Strominger J. L. Isolation of the penicillin-binding peptide from D-alanine carboxypeptidase of Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1009–1012. doi: 10.1073/pnas.74.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Lysis by beta-lactam antibiotics: structure-activity relationships in the cephalosporins. J Appl Bacteriol. 1976 Dec;41(3):419–424. doi: 10.1111/j.1365-2672.1976.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Hamilton T. E., Lawrence P. J. The formation of functional penicillin-binding proteins. J Biol Chem. 1975 Aug 25;250(16):6578–6585. [PubMed] [Google Scholar]
- Ho P. P., Towner R. D., Indelicato J. M., Spitzer W. A., Koppel G. A. Biochemical and microbiological studies on 6-substituted penicillins. J Antibiot (Tokyo) 1972 Oct;25(10):627–628. doi: 10.7164/antibiotics.25.627. [DOI] [PubMed] [Google Scholar]
- Indelicato J. M., Norvilas T. T., Pfeiffer R. R., Wheeler W. J., Wilham W. L. Substituent effects upon the base hydrolysis of penicillins and cephalosporins. Competitive intramolecular nucleophilic amino attack in cephalosporins. J Med Chem. 1974 May;17(5):523–527. doi: 10.1021/jm00251a011. [DOI] [PubMed] [Google Scholar]
- Indelicato J. M., Wilham W. L. Effect of 6-alpha substitution in penicillins and 7-beta substitution in cephalosporins upon beta-lactam reactivity. J Med Chem. 1974 May;17(5):528–529. doi: 10.1021/jm00251a012. [DOI] [PubMed] [Google Scholar]
- Knox J. R., Kelly J. A., Moews P. C., Murthy N. S. 5-5A crystallographic structure of penicillin beta-lactamase and radius of gyration in solution. J Mol Biol. 1976 Jul 15;104(4):865–875. doi: 10.1016/0022-2836(76)90187-x. [DOI] [PubMed] [Google Scholar]
- Lee B. Conformation of penicillin as a transition-state analog of the substrate of peptidoglycan transpeptidase. J Mol Biol. 1971 Oct 28;61(2):463–469. doi: 10.1016/0022-2836(71)90393-7. [DOI] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Koppel G. A., Turner J. R. Substrate inhibition of beta-lactamases, a method for predicting enzymatic stability of cephalosporins. Antimicrob Agents Chemother. 1976 Sep;10(3):470–475. doi: 10.1128/aac.10.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Regulation of murein biosynthesis and septum formation in filamentous cells of Escherichia coli PAT 84. J Bacteriol. 1977 Mar;129(3):1593–1600. doi: 10.1128/jb.129.3.1593-1600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T. Mode of action of penicillins in vivo and in vitro in Bacillus megaterium. Antimicrob Agents Chemother. 1976 Oct;10(4):579–591. doi: 10.1128/aac.10.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renugopalakrishnan V., Rein R. Energetics of the deformation of a peptide unit. Semi-empirical molecular orbital and ab initio study of N-methyl acetamide and N-acetyl-L-alanine N-methyl amide. Biochim Biophys Acta. 1976 May 20;434(1):164–168. doi: 10.1016/0005-2795(76)90046-5. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Kleier D. A., Lipscomb W. N. Molecular orbital studies of enzyme activity: I: Charge relay system and tetrahedral intermediate in acylation of serine proteinases. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2606–2610. doi: 10.1073/pnas.72.7.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner S., Lipscomb W. N., Kleier D. A. Molecular orbital studies of enzyme activity. 2. Nucleophilic attack on carbonyl systems with comments on orbital steering. J Am Chem Soc. 1976 Aug 4;98(16):4770–4777. doi: 10.1021/ja00432a014. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Lipscomb W. N. Molecular orbital studies of enzyme activity: catalytic mechanism of serine proteinases. Proc Natl Acad Sci U S A. 1976 Feb;73(2):432–436. doi: 10.1073/pnas.73.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Ward J. B. Peptidoglycan synthesis in Bacillus licheniformis. The inhibition of cross-linking by benzylpenicillin and cephaloridine in vivo accompanied by the formation of soluble peptidoglycan. Biochem J. 1975 Jan;146(1):253–267. doi: 10.1042/bj1460253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virden R., Bristow A. F., Pain R. H. The active site of penicillinase from Staphylococcus aureus PC1. Isolation of a specific covalent complex with the substrate quinacillin. Biochem J. 1975 Aug;149(2):397–401. doi: 10.1042/bj1490397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson K. L., Roberts J. D. Conformational analysis by nuclear magnetic resonance. Nitrogen-15 and carbon-13 spectra of lactams. J Am Chem Soc. 1976 Aug 18;98(17):5082–5086. doi: 10.1021/ja00433a005. [DOI] [PubMed] [Google Scholar]
- Yamana T., Tsuji A. Comparative stability of cephalosporins in aqueous solution: kinetics and mechanisms of degradation. J Pharm Sci. 1976 Nov;65(11):1563–1574. doi: 10.1002/jps.2600651104. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. S., Scheraga H. A. Stability of cis, trans, and nonplanar peptide groups. Macromolecules. 1976 May-Jun;9(3):408–416. doi: 10.1021/ma60051a005. [DOI] [PubMed] [Google Scholar]


