Abstract
Background
Patients with scleroderma and end-stage lung disease have a very high prevalence of gastroesophageal reflux disease (GERD). Because GERD has been associated with aspiration in those with end-stage lung disease, and because those with scleroderma are particularly prone to develop severe GERD, there is some concern that GERD may contribute to shorten survival in patients with scleroderma awaiting lung transplantation. Therefore, we hypothesized that esophageal pH-monitoring could predict survival of those with scleroderma and end-stage lung disease awaiting lung transplantation and that the severity of reflux can impact survival.
Methods
We conducted a retrospective analysis of all scleroderma patients referred for lung transplantation that underwent esophageal manometry and pH-monitoring since August, 2008. We identified 10 patients in whom we calculated and compared the area under the curve (AUC) for each receiver-operator characteristic (ROC) curve of the following variables: DeMeester score, FEV1, %predicted FEV1, FVC, %predicted FVC, DLco, and %predicted DLco.
Results
The DeMeester score nominally outperformed FEV1, FVC, and DLco. ROC curve analysis was also used to define the optimal DeMeester score (65.2) in differentiating survival status, as determined by maximizing sensitivity and specificity. Based on this value, we calculated the 1-year survival from the time of the esophageal function testing which was 100% in 7 patients with a DeMeester score of less than 65.2, and 33% in 3 patients with a score greater than 65.2 (p=0.01). The latter patients had greater total time pH <4, greater time pH <4 in the supine position, greater total episodes of reflux, and higher prevalence of absent peristalsis. The single survivor with a DeMeester score greater than 70 had also proximal reflux, underwent anti-reflux surgery, and is alive 1201 days post-transplant.
Conclusions
Our study shows that esophageal pH-monitoring can predict survival status in patients with scleroderma awaiting lung transplantation and that the severity of reflux can impact the 1-year survival rate. Therefore, esophageal pH-monitoring should be considered early in patients with scleroderma and end-stage lung disease, as this test could appropriately identify those in whom laparoscopic antireflux surgery should be performed quicker to prevent GERD and its detrimental effects in patients awaiting lung transplantation.
Keywords: Lung transplantation, Scleroderma, Connective tissue disorders, End-stage lung diseases, Gastroesophageal reflux disease, Laparoscopic antireflux surgery, Esophageal pH-monitoring
Introduction
Although small series have shown that the morbidity and mortality after lung transplantation for patients with scleroderma are equivalent to other lung transplant patients, there is still the fear that patients with scleroderma may fare worse after lung transplantation. (1,2) In fact, some lung transplant centers like ours have been reluctant in offering lung transplant to those with scleroderma and end-stage lung disease given their potential to aspiration and lung allograft compromise. This argument relies on two observations: 1) that patients with scleroderma and end-stage lung disease have a very high prevalence of gastroesophageal reflux disease (GERD) and esophageal dysmotility, and 2) that GERD has been associated with aspiration in those with end-stage lung disease. Indeed, patients with scleroderma and end-stage lung disease have a very high prevalence of GERD and esophageal dysmotility and studies such as that from University of California San Francisco on 26 patients with connective tissue disorders referred for lung transplantation have shown that the prevalence of GERD on pH-monitoring could be as high as 83% and that of impaired or absent peristalsis could be as high as 78%. (3) Similarly, studies from our center have shown that aspiration, as measured by the detection of pepsin in the bronchoalveolar fluid, leads to a quicker progression to lung transplant deterioration by promoting an augmented chemotactic and inflammatory balance of pulmonary leukocytes and immune mediators. (4, 5)
Therefore, because GERD has been associated with aspiration in those with end-stage lung disease, and because those with scleroderma are particularly prone to develop severe GERD, there is some concern that GERD may shorten survival in patients with scleroderma awaiting lung transplantation. The aim of this study was to determine if the severity of GERD could represent an effective means to predict survival in this patient population. Therefore, we hypothesized that esophageal pH-monitoring could predict survival of those with scleroderma and end-stage lung disease awaiting lung transplantation and that the severity of reflux can impact survival. The clinical implications of this study are important because our results could provide the rationale to recommend early esophageal manometry and pH-monitoring to better identify those with early stages of esophageal compromise and with milder GERD in whom laparoscopic antireflux surgery should be performed early to achieve the best chances to prevent GERD and its detrimental effects in patients awaiting lung transplantation.
Materials and Methods
Patients
We conducted a retrospective analysis of all scleroderma patients referred for lung transplantation that underwent esophageal manometry, pH-monitoring and pulmonary function tests since August 2008 and identified 10 patients for our analysis. During the study period, pulmonologists referred for esophageal testing only patients who complained of severe and daily heartburn and regurgitation. Institutional review board approval was requested and obtained prior to conducting this study (LU 205827)
Assessment of GERD and esophageal function
In our cohort, GERD was diagnosed by performing pH-monitoring using a previously described technique (6). Briefly, proton pump inhibitors were stopped for 14 days and histamine H2-receptor antagonists were stopped for 3 days before pH-monitoring. A pH catheter (Sleuth system with BioVIEW software; Sandhill Scientific Inc., Denver, CO) was placed with the distal pH sensor positioned 5 cm superior to the manometrically-determined upper border of the LES. The DeMeester score was calculated for the distal pH recordings, and a score >14.7 was considered diagnostic of GERD (7).
Esophageal function was determined by high-resolution manometry using a 32-channel solid state catheter (insight HRIM system with BioVIEW software; Sandhill Scientific Inc., Denver, CO). Specifically, the system assisted in locating the distance of the LES from the nostril and determined its pressure, length, and relaxation (normal LES pressure: 10-45 mmHg; relaxation was determined by a drop of the resting pressure to a residual pressure <8 mmHg). The esophageal body function was assessed after the patients performed 10 serial swallows in the supine position with 5 mL of normal saline. The amplitude, duration, and velocity of the peristaltic waves were simultaneously recorded. Peristaltic wave amplitude was then calculated for the distal esophagus (distal esophageal amplitude, or DEA) based on data recorded from pressure sensors located 5 and 10 cm above the LES. Esophageal motility was considered ineffective if peristaltic waves were present in <30% of the swallows with DEA <30 mmHg.
Assessment of pulmonary function
Each patient underwent resting measurements of forced vital capacity (FVC), forced expiratory volume in one seconds (FEV-1), and diffusion capacity for carbon monoxide (DLco) using standard equipment and methodology meeting American Thoracic Society Standards (8,9)
Statistical Analysis
Sensitivity was defined as the number of diseased subjects with a positive test divided by total number of diseased subjects. Specificity was defined as the number of disease-free subjects with a negative test divided by the total number of disease-free subjects. Using sensitivity plotted against 1-specificity, receiver-operator characteristic (ROC) curves were constructed for the following variables: DeMeester score, FEV1, %predicted FEV1, FVC, %predicted FVC, DLco, and %predicted DLco. Subsequently, area under the curve (AUC) was calculated for each ROC curve, and AUCs for different tests were compared using the method described by Hanley and McNeil (10). Nonparametric statistical methods were utilized. The χ2 test for association was used for differences in groups on categorical variables and the Mann-Whitney U test was used for continuous variables. Results were reported as percentages for categorical variables and as average with standard deviation for continuous variables. Statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). Significance for all tests was set at p ≤ 0.05.
Results
Since August 2008 only 10 of 32 patients with scleroderma evaluated for lung transplant were referred for esophageal function tests (31%). The study cohort therefore consisted of 10 patients with an average age of 51.3 years, an average body mass index (BMI, kg/m2) of 23.3, and was made of 10% males (Table 1). Mean survival after the esophageal function testing was 1053 ± 786 days. One patient underwent lung transplantation exactly one year after her esophageal function testing. She had a DeMeester score of 243.6, the highest score in the cohort, and she had daily symptoms of GERD and aspiration preoperatively. She died 14 days post-lung transplantation for acute on chronic upper gastrointestinal bleeding coupled with platelet dysfunction after developing chronic esophagitis and a distal esophageal erosion with an ulcer from her severe GERD.
Table 1.
Demographics and descriptive statistics of the study cohort
| Cohort (n=10) | |
|---|---|
| Age | 51.3 ± 10.7 |
| Male Gender | 10% |
| BMI | 23.3 ± 3.4 |
| DeMeester Score | 63.7 ± 72.5 |
| FEV1 | 1.4 ± 0.6 |
| FEV1, %predicted | 52.6% |
| FVC | 1.7 ± 0.9 |
| FVC, %predicted | 50.4% |
| DLCO | 5.6 ± 4.5 |
| DLCO, %predicted | 27% |
Results are reported as percentages for categorical variables and as average with standard deviation for scaled variables
The AUC with 95% confidence interval (CI) for DeMeester score, FEV1, %predicted FEV1, FVC, %predicted FVC, DLco, and %predicted DLco are shown in Table 2. The DeMeester score had the highest AUC of any metric (0.76). However, χ2 tests comparing each metric to DeMeester score did not reveal any statistically significant differences, although the ability to detect differences was limited given the sample size of 10 patients.
Table 2.
AUC with 95% confidence interval (CI) for DeMeester score, FEV1, %predicted FEV1, FVC, %predicted FVC, DLco, and %predicted DLco. DeMeester score showed the highest AUC of any metric. However, χ2 tests comparing each metric to DeMeester score did not reveal any statistically significant differences, although the ability to detect differences was limited given the sample size of 10 patients.
| AUC | 95% CI | p-value | |
|---|---|---|---|
| DeMeester Score | 0.76 | (0.38, 1.00) | - |
| FEV1 | 0.71 | (0.25, 1.00) | 0.88 |
| FEV1%predicted | 0.71 | (0.33, 1.00) | 0.86 |
| FVC | 0.71 | (0.32, 1.00) | 0.87 |
| FVC %predicted | 0.60 | (0.20, 0.99) | 0.56 |
| DLCO | 0.67 | (0.14, 1.00) | 0.77 |
| DLCO %predicted | 0.70 | (0.24, 1.00) | 0.84 |
Figure 1 shows ROC curves for DeMeester score, FEV1, %predicted FEV1, FVC, %predicted FVC, DLco, and %predicted DLco. These curves show the differences from the 45-degree line of no discrimination, indicating the accuracy of the tests at predicting survival. The DeMeester score had the highest accuracy of all tests at predicting survival (0.76), although it was not statistically superior from any other test. ROC curve analysis was also used to define the cut-off value of the DeMeester score for distinguishing survival status. We found that the optimal DeMeester score in differentiating survival status, as determined by maximizing sensitivity and specificity, was 65.2. Based on this value, we calculated the 1-year survival from the time of the esophageal function testing which was 100% in 7 patients with a DeMeester score of less than 65.2, and 33% in 3 patients with a score greater than 65.2 (p=0.01).
Figure 1.
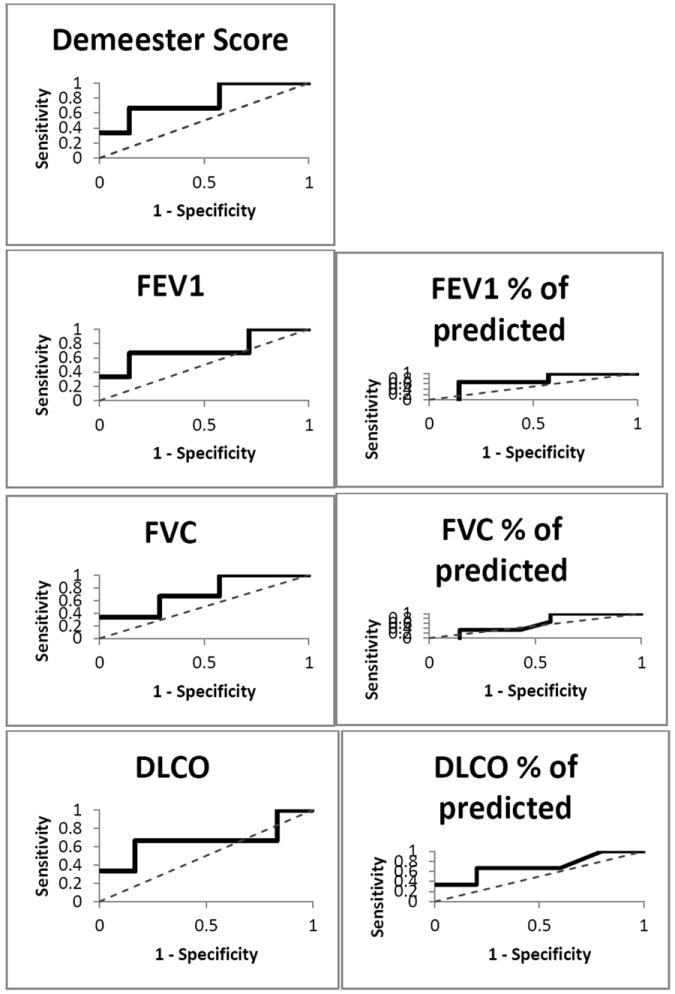
ROC curves for DeMeester score, FEV1, %predicted FEV1, FVC, %predicted FVC, DLco, and %predicted DLco. The curves show the differences from the 45-degree line of no discrimination, indicating the accuracy of tests at predicting survival.
Table 3 shows the manometric and pH-metric profile of patients with a DeMeester score of less than 65.2 (Group A), and those with a score greater than 65.2 (Group B). Those with a score greater than 65.2 (Group B) had greater total time pH <4, greater time pH <4 in the supine position, and greater total episodes of reflux over 24 hours. Moreover, esophageal peristalsis was largely ineffective in Group A and in Group B as shown by the below normal DEA in both groups. Specifically, esophageal peristalsis was absent in 14% patients in Group A and in 66% of those in Group B (p=0.09).
Table 3.
Manometric and pH-metric profile of patients with a DeMeester score of less than 65.2 (Group A), and those with a score greater than 65.2 (Group B).
| Group A (n=7) | Group B (n=3) | p-value | |
|---|---|---|---|
| Manometric profile | |||
| LES | |||
| LES pressure (mmHg) | 17.7 ± 15.9 | 21.9 ± 15.5 | 0.71 |
| LES total length (cm) | 2.4 ± 1.1 | 2.3 ± 1.2 | 0.10 |
| LES abdominal length (cm) | 1 ± 0.6 | 2 ± 1 | 0.07 |
| Esophageal body | |||
| DEA (mmHg) | 24.7 ± 12.5 | 14.7 ± 2.9 | 0.22 |
| pH-metric profile | |||
| Total time pH <4 (%) | 7.3 ± 7.3 | 46.4 ± 34.4 | 0.01 |
| Upright | 5.6 ± 9.8 | 30 ± 44.8 | 0.17 |
| Supine | 9.5 ± 9.6 | 59.2 ± 35.7 | 0.006 |
| Episodes >5min | 3 ± 3 | 14 ± 3 | 0.001 |
| Longest episode (minutes) | 45.8 ± 47.6 | 117.1 ± 80.8 | 0.11 |
| Total episodes | 25 ± 13 | 132 ± 70 | 0.002 |
| DeMeester score (normal: <14.7) | 28.2± 24.2 | 146.6 ± 84.8 | 0.006 |
| Esophageal clearance (seconds) | |||
| Total mean acid clearance time | 232 ± 187 | 402 ± 384 | 0.35 |
| Upright | 101 ± 150 | 265 ± 310 | 0.27 |
| Supine | 1180 ± 1590 | 1807 ± 2599 | 0.64 |
| Proximal pH sensor data | |||
| Total time pH <4 (normal: <1%) | 0 ± 0 | 0.7 ± 1.1 | 0.10 |
| Upright | 0 ± 0.1 | 0 ± 0 | 1 |
| Supine | 0.2 ± 0.5 | 1.2 ± 2 | 0.22 |
LES: Lower Esophageal Sphincter
DEA: Distal Esophageal Amplitude
Results are reported as percentages for categorical variables and as average with standard deviation for scaled variables
The single survivor with a DeMeester score greater than 65.2 also had proximal reflux (total time pH <4 of 1.9) and preserved peristalsis with 40% of propagated waves. Because of her clinical complaints of aspiration coupled with the finding of proximal reflux (the only patient in this study cohort) and partially preserved esophageal peristalsis, this patient underwent a laparoscopic partial fundoplication. She is still alive 1201 days post-transplant. The other two patients with a DeMeester score greater than 65.2 (one with a DeMeester score of 243.6 and the other with a score of 86.2) died of GERD related complication. Specifically, the patient with a DeMeester score of 243.6 died 14 days post-lung transplantation for acute on chronic upper gastrointestinal bleeding coupled with platelet dysfunction after she developed developing chronic esophagitis and a distal esophageal ulcer from her severe GERD. The patient with score of 86.2 had absent esophageal peristalsis with severe and daily heartburn, regurgitation and aspiration, and died on a lung transplant list as a result of respiratory failure from continuous aspiration.
Discussion
In our study of 10 patients with scleroderma awaiting lung transplantation, we found that patients with severe reflux had a lower 1-year survival rate compared to those with less severe reflux.
Calculated from ambulatory esophageal pH-monitoring data, the DeMeester score is a composite score that is used to diagnose GERD and its severity (7). As it is a technique that provides objective diagnosis, esophageal pH-monitoring is always prescribed to diagnose GERD prior to an antireflux operation (11). Specifically, in patients with end-stage lung disease (ESLD), such as those with scleroderma awaiting lung transplantation, esophageal pH-monitoring is often prescribed to diagnose GERD prior to an antireflux operation. Unfortunately, this often happens too late in the course of the disease, when the disease has progressed to esophageal aperistalsis, severe GERD, and respiratory compromise. We aimed to challenge GERD diagnosis as the sole indication to perform esophageal function testing and we argued that esophageal testing should be incorporated in the management of patients ESLD early on. We believe that it would make sense to use esophageal pH-monitoring to predict survival status in those patients in whom GERD is highly prevalent and in whom GERD may lead to lung deterioration. The results of our preliminary study confirm our hypothesis. In fact, the main findings of this study showed a nominally increased AUC of DeMeester score for predicting survival when compared to data from pulmonary function tests, including FEV1, %predicted FEV1, FVC, %predicted FVC, DLco, and %predicted DLco. Thus, the DeMeester score is at least equal to and possibly superior to other metrics gained from pulmonary function tests in predicting survival in patients with scleroderma and ESLD. This result has important clinical ramifications, as a high DeMeester score may be considered a marker for developing lung injury from aspiration, for which laparoscopic antireflux surgery can provide an effective intervention to stop aspiration and lung injury (4). Therefore the usefulness of esophageal pH-monitoring lies in understanding that in contrast to changes in pulmonary function tests, which are presumably manifestations of lung injury, changes in the DeMeester score could be predictors of lung injury and survival. It follows that esophageal pH-monitoring should be considered as important as pulmonary function tests in the management of patients with ESLD and that this test should be ordered routinely as it could appropriately identify those in whom laparoscopic antireflux surgery should be performed quicker to prevent GERD and improve survival. Although anecdotal, the evidence offered in this study by our single survivor with a DeMeester score greater than 65.2 who is still alive 1201 days post-transplant demonstrates that laparoscopic antireflux surgery could protect against GERD and aspiration-induced lung injury in these patients, and it is in line with our previous work (4,12).
The second main finding of our study, that the severity of reflux can impact the 1-year survival rate, ties up with the central message of our work reinforcing the need to prevent GERD as early as possible and to increase survival. We have shown that the 1-year survival from the time of the esophageal function testing was only 33% in those with a score greater than 65.2 in contrast to the 100% survival rate in those with a milder score and that these former patients had more supine reflux, greater total episodes of reflux over 24 hours and higher prevalence of absent esophageal peristalsis. These findings are in line with those shown by the group at University of California San Francisco, as they have shown that reflux is more severe and peristalsis is more frequently absent when ESLD is also present in those with scleroderma (3, 13). However, the group at University of California San Francisco and ours also has shown that GERD can be effectively controlled in these patients before lung transplantation and that laparoscopic fundoplication is safe in experienced hands (14). Therefore, it is reasonable to recommend early esophageal manometry and pH-monitoring to better identify those with early stages of esophageal compromise and with milder GERD in whom laparoscopic antireflux surgery should be performed early to achieve the best chances to prevent GERD and lung deterioration.
Although we acknowledge that our small sample size may be considered a main limitation, we point out two reasons why this may not be a grave concern. First, many of the studies published have enrolled a similar number of patients, as the prevalence of this specific patient population is small, and that it would take several years to enroll a powerful cohort. Second, even with this sample size limitation in mind, we believe that the data could be robust enough to spur an increased awareness to incorporate GERD testing routinely to help guiding an earlier treatment. Our referral rate for esophageal testing is 31% and we hope the results of this study will increase the number of patients who could benefit from an earlier diagnosis and treatment. As stated by Haney and Hartwig: “An ounce of prevention is worth a pound of cure” - Benjamin Franklin. If the esteemed Mr. Franklin were a lung transplant surgeon he might have rephrased his sentiment “an early Nissen is better than a late retransplant”. A laparoscopic fundoplication performed safely offers the potential to extend graft function in a low-risk, high-reward fashion.” (15)
Conclusions
Our preliminary study of 10 patients with scleroderma awaiting lung transplantation has shown that patients with severe reflux had a lower 1-year survival rate compared to those with less severe reflux. These results suggest that esophageal pH-monitoring should be considered early in patients with scleroderma and end-stage lung disease, as this test could appropriately identify those in whom laparoscopic antireflux surgery should be performed quicker to prevent GERD and its detrimental effects in patients awaiting lung transplantation. Further studies with larger sample sizes are warranted to confirm these findings.
Acknowledgments
This work was supported in part by the 2011 SAGES Research Grant Award for the study entitled: “A non-invasive test to detect markers of aspiration after lung transplantation” (Fisichella) and by the Dr. Ralph and Marian C. Falk Medical Research Trust and the NIH AA013527 (Kovacs).
Footnotes
Authors’ Contributions: PMF: Study conception and design, Drafting of manuscript. NPR: Analysis and interpretation of data. JG: Study conception, Acquisition of data. EJK: Critical revision. All authors read and approved the final manuscript.
Disclosure Information: nothing to disclose
References
- 1.Massad MG, Powell CR, Kpodonu J, Tshibaka C, Hanhan Z, Snow NJ, Geha AS. Outcomes of lung transplantation in patients with scleroderma. World J Surg. 2005;29:1510–5. doi: 10.1007/s00268-005-0017-x. [DOI] [PubMed] [Google Scholar]
- 2.Schachna L, Medsger TA, Jr, Dauber JH, Wigley FM, Braunstein NA, White B, Steen VD, Conte JV, Yang SC, McCurry KR, Borja MC, Plaskon DE, Orens JB, Gelber AC. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954–61. doi: 10.1002/art.22264. [DOI] [PubMed] [Google Scholar]
- 3.Gasper WJ, Sweet MP, Golden JA, Hoopes C, Leard LE, Kleinhenz ME, Hays SR, Patti MG. Lung transplantation in patients with connective tissue disorders and esophageal dysmotility. Dis Esophagus. 2008;21(7):650–5. doi: 10.1111/j.1442-2050.2008.00828.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisichella PM, Davis CS, Lundberg PW, Lowery E, Burnham EL, Alex CG, Ramirez L, Pelletiere K, Love RB, Kuo PC, Kovacs EJ. The protective role of laparoscopic antireflux surgery against aspiration of pepsin after lung transplantation. Surgery. 2011 Oct;150(4):598–606. doi: 10.1016/j.surg.2011.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisichella PM, Davis CS, Lowery E, Ramirez L, Gamelli RL, Kovacs EJ. Aspiration, localized pulmonary inflammation, and predictors of early-onset bronchiolitis obliterans syndrome after lung transplantation. J Am Coll Surg. 2013 Jul;217(1):90–100. doi: 10.1016/j.jamcollsurg.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis CS, Shankaran V, Kovacs EJ, Gagermeier J, Dilling D, Alex CG, Love RB, Sinacore J, Fisichella PM. Gastroesophageal reflux disease after lung transplantation: pathophysiology and implications for treatment. Surgery. 2010;148:737–744. doi: 10.1016/j.surg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson LF, DeMeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. The American Journal of Gastroenterology. 1974;62(4):325–332. [PubMed] [Google Scholar]
- 8.Brusasco V, Crapo R, Viegi G. ATS/ERS TASK FORCE: STANDARDISATION OF LUNG FUNCTION TESTING/ General considerations for lung function testing. European Respiratory Journal. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrino R, Viegi G, Brusasco V, et al. ATS/ERS TASK FORCE: STANDARDISATION OF LUNG FUNCTION TESTING/ Interpretative strategies for lung function tests. European Respiratory Journal. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 10.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 11.Jobe BA, Richter JE, Hoppo T, Peters JH, Bell R, Dengler WC, Devault K, Fass R, Gyawali CP, Kahrilas PJ, Lacy BE, Pandolfino JE, Patti MG, Swanstrom LL, Kurian AA, Vela MF, Vaezi M, Demeester TR. Preoperative Diagnostic Workup before Antireflux Surgery: An Evidence and Experience-Based Consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg. 2013 Oct;217(4):586–97. doi: 10.1016/j.jamcollsurg.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Fisichella PM, Davis CS, Lowery E, Pittman M, Gagermeier J, Love RB, Kovacs EJ. Pulmonary immune changes early after laparoscopic antireflux surgery in lung transplant patients with gastroesophageal reflux disease. J Surg Res. 2012 Oct;177(2):e65–73. doi: 10.1016/j.jss.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patti MG, Gasper WJ, Fisichella PM, Nipomnick I, Palazzo F. Gastroesophageal reflux disease and connective tissue disorders: pathophysiology and implications for treatment. J Gastrointest Surg. 2008 Nov;12(11):1900–6. doi: 10.1007/s11605-008-0674-9. [DOI] [PubMed] [Google Scholar]
- 14.Davis CS, Jellish WS, Fisichella PM. Laparoscopic fundoplication with or without pyloroplasty in patients with gastroesophageal reflux disease after lung transplantation: how I do it. J Gastrointest Surg. 2010 Sep;14(9):1434–41. doi: 10.1007/s11605-010-1233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haney JC, Hartwig MG. Nissen Fundoplication and Lung Transplantation. [October 2, 2013];ISHLT Links. 2013 Oct;5(6) http://www.ishlt.org/ContentDocuments/2013OctLinks_Haney-Hartwig.html. [Google Scholar]


