Abstract
Intrinsically photoreceptive retinal ganglion cells (ipRGC) contain the photopigment melanopsin and convey retinal light inputs to the circadian system via the retinohypothalamic tract (RHT) projection to the suprachiasmatic nucleus (SCN). The principal neurotransmitter of this projection is glutamate, and ipRGCs use the vesicular glutamate transporter 2 (VGLUT2) to package glutamate into synaptic vesicles. However, these neurons contain other potential neurotransmitters, such as PACAP. To test the role of glutamate in mediating ipRGC light inputs into the SCN, we crossed mice in which Cre-recombinase expression is driven by the melanopsin promotor (Opn4Cre/+) with mice in which the second exon of VGLUT2 is flanked by loxP sites (VGLUT2fl/fl), producing ipRGCs that are unable to package glutamate into synaptic vesicles. Such mice had free-running circadian rhythms that did not entrain to a 12:12 light-dark (12:12 LD) cycle, nor did they show a phase delay after a 45 minute light pulse administered at circadian time (CT) 14. A small subset of the mice did appear to entrain to the 12:12 LD cycle with a positive phase angle to lights-off; a similar entrainment pattern could be achieved in free-running mice if they were exposed to a 12:12 LD cycle with light of a greater intensity. Glutamate transmission from the ipRGCs is necessary for normal light entrainment of the SCN at moderate (0.35 W/m2) light levels, but residual transmission (possibly by PACAP in ipRGCs or by other RGCs) can weakly entrain animals, particularly at very high (6.53 W/m2) light levels, although it may be less effective at suppressing locomotor activity (light masking).
Keywords: Retina, suprachiasmatic nucleus (SCN), melanopsin, vesicular glutamate transporter 2 (VGLUT2), light entrainment, masking
INTRODUCTION
Retinal light inputs synchronize or entrain the suprachiasmatic nucleus (SCN), the brain’s master circadian timekeeper, to the environmental light-dark cycle (Moore et al., 1995; Abrahamson & Moore, 2001; Berson et al., 2002; Pickard et al., 2002). In the retina, a sub-class of ganglion cells that are intrinsically photoreceptive (ipRGC) due to the expression of the photopigment melanopsin provide light input to the SCN, via the retinohypothalamic tract (RHT) (Gooley et al., 2001; Hannibal et al., 2002; Hattar et al., 2002). Numerous anatomical studies suggest glutamate as the primary RHT neurotransmitter (Moffett et al., 1990; van den Pol, 1991; Castel et al., 1993; de Vries et al., 1993), but these same cells also express pituitary adenylate cyclase activating polypeptide (PACAP) and might contain smaller amounts of other co-transmitters (Morin & Allen, 2006). SCN neurons express both AMPA and NMDA glutamate receptors as well as PAC1 PACAP receptors, each of which exerts an excitatory influence on SCN neuronal activity. Glutamate agonists mimic the effects of light on free-running circadian rhythms in vitro, and antagonists block light-induced circadian phase-shifts in vivo; however, mice in which the PACAP PAC1 receptor has been deleted have impaired entrainment to light (Cahill & Menaker, 1989; Kim & Dudek, 1991; Ohi et al., 1991; Colwell & Menaker, 1992; Shibata et al., 1994). In addition, the SCN receives glutamatergic inputs from other brain areas besides ipRGCs, and PAC1 receptors are sensitive to vasoactive intestinal peptide (VIP, expressed in neurons in the core of the SCN) as well as PACAP. Thus experiments that block these receptors entirely do not test their role in relaying retinal input to SCN neurons. Hence, we used a genetically specified means to prevent glutamate neurotransmission from ipRGCs.
The ipRGCs use the vesicular glutamate transporter 2 (VGLUT2) to package glutamate into synaptic vesicles (Fujiyama et al., 2003; Fyk-Kolodziej et al., 2004; Wassle et al., 2006; Johnson et al., 2007). To target glutamatergic neurotransmission specifically in ipRGCs, we bred mice expressing Cre-recombinase under the control of the Melanopsin promotor (OPN4Cre/+) (Ecker et al., 2010) with mice that carried loxP sites flanking the second exon of the VGLUT2 gene (VGLUT2flox/flox) (Tong et al., 2007). These mice show deficits in neonatal photoaversion (Delwig et al., 2013), and here we tested them for deficits in circadian photoentrainment. Our findings suggest a primary role of glutamate in transmitting light information from ipRGCs to the circadian system.
MATERIALS AND METHODS
Animals
All protocols used in this study were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. All animals were bred in our facilities and kept in a 12:12 LD environment from birth until use in the experiments at around 8–11 weeks of age. Mice had ad lib access to water and standard chow. Mice were derived from two previously described lines: OPN4Cre/Cre (Ecker et al., 2010) and VGLUT2fl/fl (Tong et al., 2007). We used mice that were OPN4Cre/+;VGLUT2flox/flox to test the role of glutamate in photoentrainment and littermate OPN4+/+;Vglut2flox/flox as controls.
Circadian rhythms measurement
Mice were kept in light tight, temperature controlled recording chambers. Each recording chamber contained one light fixture attached to the wall at the level of the cage top, and two cages were placed in such a way that the entire cage was illuminated with approximately the same amount of light. For all experiments except those using high light intensity (e.g., 12 hr LD, LL, 6 hr phase shifts, 45 min light pulses and 2 hr LD cycles), a broad-spectrum spot light LED (PAR20 36 LEDs, superbrightLEDs.com) emitting 0.35 W/m2 was used. For bright light experiments, a broad-spectrum CFL (GE helical 42W) emitting 6.52 W/m2 was used. Wheel running activity was measured in cages outfitted with a stainless steel running wheel (4.5 inch, or 11.4 cm diameter, Mini Mitter, Bend, OR). Each wheel revolution closed a switch monitored by computer using Dataquest A.R.T. software, v 4.3 (DSI) and data were collected in 5 min. bins and plotted in standard actogram format using ACT-500 ClockLab analysis software (ActiMetrics, Wilmette, IL).
Cell counting
Counts of c-Fos immunoreactive neurons from each animal were made bilaterally on 3 sections containing the SCN, 120 μm apart. Counting boxes included both the ventrolateral and dorsomedial SCN. Counters were blinded to the genotype / light treatments in each case. Only cells with a labeled nucleus were counted, an Abercrombie correction was applied (Guillery, 2002), and the mean number of cells on each side of the brain per section is reported.
Data analysis
Circadian measures were calculated in ClockLab (Actimetrics, Wilmette, IL). χ2 periodogram data was generated to calculate period length, while the regression line fit of activity onsets was used to calculate phase shifts. Means, standard errors and p values were derived in Excel.
RESULTS
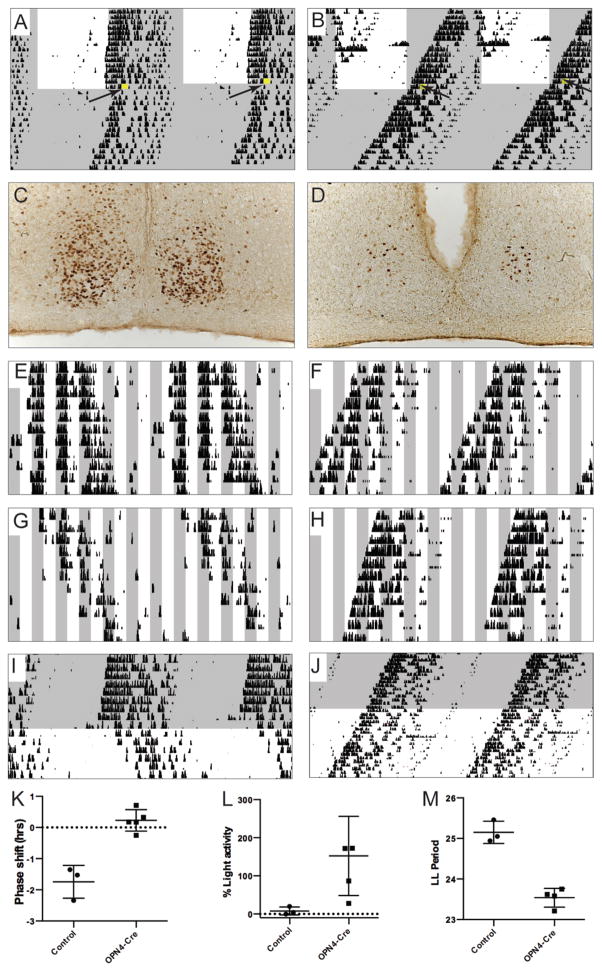
In 12:12 LD conditions (0.35 W/m2), most Opn4Cre/+;VGLUT2flox/flox mice (14/18) failed to entrain to 12:12 LD and exhibited free-running periods in LD, with a circadian period significantly shorter than control animals (23.55 ± 0.09 hrs, mean ± S.D., Opn4Cre/+;VGLUT2flox/flox, n = 18 vs 24.00 ± 0.00 hrs control, n = 6; p = 0.007, Fig. 1A, B). Free-running periods in DD did not differ between groups (23.47 ± 0.08 hrs Opn4Cre/+;VGLUT2flox/flox n = 12; vs 23.49 ± 0.22 hrs control n = 3, p = 0.88, Fig. 1A, B). A 45-minute light pulse administered at circadian time (CT) 14 (0.35 W/m2) elicited a phase delay in all control animals (−1.74 ± 0.30 hrs, n = 3, Fig. 1A, K), whereas it had no effect when administered to Opn4Cre/+;VGLUT2flox/flox animals (0.23 ± 0.15 hrs, n = 5, p = 0.0006, Fig. 1B, K). Congruent with these observations, a 45-minute long light-pulse administered at CT 14 reliably elicited expression of the immediate early gene c-Fos in the SCN of control mice (805.47 ± 99.07, n = 6, Fig. 1C), whereas c-Fos expression was substantially lower in Opn4Cre/+;VGLUT2flox/flox mice under these conditions (224.13 ± 27.35, n = 6, p = 0.0018, Fig. 1D), and not significantly different from dark adapted mice of either genotype (229.33 ± 25.57, n = 3, p = 0.91 control and 139.40 ± 41.35, n = 4, p = 0.11 Opn4Cre/+;VGLUT2flox/flox). To test negative light masking (light-induced locomotor suppression), mice were kept on a 2 hr light-dark cycle for 10 days (0.35 W/m2). While locomotor activity was strongly inhibited in control animals during the light exposure during their active cycle (7.9 ± 6.05 % of activity measured in darkness, n = 3, Fig. 1E, G, L), Opn4Cre/+;VGLUT2flox/flox mice showed no decrease in locomotor activity during the 2 hr lights-on period compared to the 2 hr dark period immediately following (123.8 ± 32.9 % of dark activity, n = 5, Fig. 1F, H, L). Finally, while control animals exhibited lengthened circadian periods in continuous light (LL) (0.35 W/m2) compared to continuous darkness (25.15 ± 0.16 hrs in LL vs 23.49 ± 0.22 hrs in DD, n = 3, p = 0.013, Fig 1 I, M), this was not observed in Opn4Cre/+;VGLUT2flox/flox mice (23.54 ± 0.095 hrs in LL vs 23.68 ± 0.02 hrs in DD, n = 4, p = 0.33, Fig. 1J, M).
Figure 1. Impaired light entrainment in Opn4Cre/+;VGLUT2flox/flox mice.
Light grey shading indicates lights-off in the recording chamber, while a white background indicates lights-on. A, B: A 45-minute light pulse at the beginning of the subjective dark phase (CT14), illustrated in yellow, leads to a phase delay in control animals (A), but has no effect on Opn4Cre/+;VGLUT2flox/flox mice. C, D: Similarly, the 45-minute light pulse elicits robust c-Fos expression in the SCN of control mice (C), but not in Opn4Cre/+;VGLUT2flox/flox mice (D). E – H: Control (E, G) and Opn4Cre/+;VGLUT2flox/flox (F, H) mice both express free-running rhythms in a 2:2 LD cycle. However, only control mice inhibit their activity during lights-on (negative masking, E, G), whereas Opn4Cre/+;VGLUT2flox/flox do not (F, H). I, J: Control mice (I) exhibit lengthened periods during LL, while Opn4Cre/+;VGLUT2flox/flox (J) mice do not experience lengthened periods during LL vs DD. K–M: Summary plots. K: plot of the magnitude of the phase shift in response to the light pulse in control and Opn4Cre/+;VGLUT2flox/flox (“OPN4-Cre”) mice. L: % of total activity during lights-on compared to lights-off in control and Opn4Cre/+;VGLUT2flox/flox (“OPN4-Cre”) mice. M: Control vs Opn4Cre/+;VGLUT2flox/flox (“OPN4-Cre”) LL period lengths.
Similar to previous results in mice with ablated ipRGCs (Opn4aDTA/DTA) (Guler et al., 2008), Opn4Cre/+;VGLUT2flox/flox segregated into two groups: while the majority (n = 14, “phenotype A” in Guler et al., 2008) were free running under any pattern of light exposure (Fig. 1B, F, H, J), a minority of animals (n=4; “phenotype B”) were weakly light responsive (control: Fig. 2A, phenotype B: Fig. 2B, C). Note that phenotype B mice do not simply have free-running periods close to 24-hours, but the phase of activity onset slowly advances after the LD cycle is abruptly advanced and tends to slowly delay after the cycle is abruptly delayed, suggesting photoentrainment. However, since locomotor activity starts up to 6 hrs before lights off, these animals have an extremely positive phase angle to lights-off.
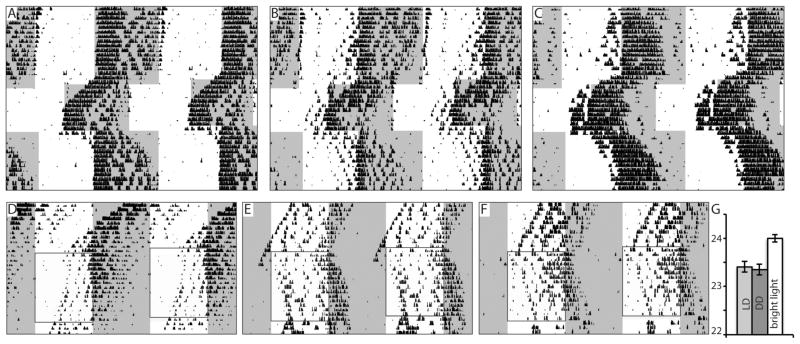
Figure 2. Circadian photoresponsiveness in a subset of Opn4Cre/+;VGLUT2flox/flox mice.
A – C: Wheel running activity of a control mouse (A), and phenotype B Opn4Cre/+;VGLUT2flox/flox mice (B, C). Note that circadian locomotor activity appears to be photosensitive to 6 hour phase advances and delays but the phase angle of activity onset to lights-off is advanced. D–F: In the presence of very bright light (6.53 W/m2, boxed area), free-running phenotype A Opn4Cre/+;VGLUT2flox/flox mice in 0.35 W/m2 LD (white and grey shading indicates light-dark cycle) respond to a bright 12:12 LD cycle with apparent entrainment (yellow and grey shading indicates light-dark cycle). G: Bar graph depicting period lengthening of phenotype A mice in very bright light.
Because the ipRGCs in our mice were still intact and contained other putative neurotransmitters, we hypothesized that exposure to a more intense light stimulus might increase the release of those transmitters and possibly overcome to some extent the effects of the Opn4Cre/+;VGLUT2flox/flox genotype. We therefore exposed 8 free-running “phenotype A” mice (LD period at 0.35 W/m2 for two weeks: 23.35 ± 0.12, vs DD period: 23.41 ± 0.11, p = 0.72) to a 12:12 LD cycle in bright light for two weeks (6.53 W/m2, Fig. 2D – F). In these mice, period length increased to 24.0 ± 0.09 hrs (vs 23.35 ± 0.12 LD at 0.35 W/m2, n = 8, p = 0.001, Fig. 2G) suggesting photoresponsiveness of the circadian system to light at higher light intensities. However, activity onset began 5.38 ± 0.92 hours before ZT12. The circadian phase of the animal prior to 6.53 W/m2 light did not affect this early activity onset: while the Zeitgeber time of 6.53 W/m2 light onset for each animal was the same as the 0.35 W/m2 light (7:00AM – 7:00PM), the circadian phase of each mouse at the onset of 6.53 W/m2 light was different, with 5 animals in the active phase and 3 in the rest phase. Combined, these results suggest that bright light was able to entrain Opn4Cre/+;VGLUT2flox/flox mice, although with an advanced phase angle to the dark onset.
DISCUSSION
Here we demonstrate that mice lacking the ability to package glutamate into synaptic vesicles of melanopsin-expressing ipRGCs have substantial deficits in photoentrainment. About 75% of mice express phenotype A, in which under 0.35 W/m2 light the mice have free-running locomotor activity rhythms with a slightly shorter period in LD, and do not respond to phase shifts or light pulses or express negative light masking (light-induced locomotor activity suppression). Under bright (6.53 W/m2) lights, these same mice demonstrate photoentrainment, but with an advanced circadian phase (by 5–6 hours). The 25% of mice showing phenotype B demonstrate a similar pattern (24 hour entrainment but early onset of activity) under 0.35 W/m2 light. These observations indicate that, under the relatively dim lighting conditions that nocturnal wild mice typically encounter, they are dependent upon glutamatergic phototransmission from the ipRGCs to maintain normal circadian entrainment of locomotor activity. However, the remaining retinal transmission, e.g., via PACAP and PAC1 receptors from ipRGCs, or from other RGCs that project to the SCN, is capable of maintaining a 24 hour, phase advanced rhythm in a minority of mice under light conditions (0.35 W/m2) that are likely to be at the upper end of what mice encounter in the wild, and that exposure to very bright lights can cause the remainder of mice lacking glutamatergic transmission to show a similar pattern.
There are several caveats in this study. First, we could not directly measure the elimination of glutamatergic transmission by ipRGCs in mice of the Opn4Cre/+;VGLUT2flox/flox genotype. However, Tong et al. demonstrated that glutamatergic transmission is eliminated in neurons from the VGLUT2flox/flox genotype when they express Cre-recombinase, and Hattar and colleagues showed that nearly all melanopsin-expressing neurons in the retina express Cre-recombinase in the Opn4Cre/+ line, so this is likely (Tong et al., 2007; Guler et al., 2008). Second, the Opn4Cre/+ mice express Cre-recombinase in some central neurons at some point during development, so that VGLUT2 may have been eliminated from those neurons as well. However, none of these other sites provide input to the SCN (cf. Hattar paper vs. Krout and Loewy, 2002). Hence it is likely that this reduced sensitivity of photoentrainment to light is due to deletion of VGLUT2, and glutamatergic transmission, from the ipRGCs.
The remaining photoresponsiveness of our phenotype B OPN4Cre/+;VGLUT2fl/fl mice is reminiscent of the small subset of mice lacking ipRGCs reported to exhibit weak photoresponsiveness (Guler et al., 2008). Thus, even in the absence of ipRGC inputs, there are other pathways that can cause some degree of photoresponsiveness. In fact, we were previously unable to find melanopsin mRNA in as many as 20% of the RGCs that innervate the SCN (Gooley et al., 2001). While some of these cells may express melanopsin at levels too low for immunohistochemical detection, some may represent RGCs that project to the SCN but do not produce melanopsin, at least in adulthood.
However, the entrainment pattern in phenotype B mice, with onset of activity 5–6 hours before the dark period, was similar to that in the remaining OPN4Cre/+;VGLUT2fl/fl mice when exposed to bright light (6.53 W/m2). This pattern suggests that the remaining transmission from ipRGCs, e.g., via PACAP and PAC1 receptors, may be sufficient to maintain the 24 hr rhythm when the ipRGCs are driven by light inputs at levels beyond those that mice in the wild would ordinarily see (because they are nocturnal and avoid bright environments). On the other hand, the 5–6 hour phase advance is puzzling. This raises the possibility that the circadian phototransmission is completely blocked by glutamate deletion, but that altered processing of photic information in an SCN-projecting region such as the intergeniculate leaflet can inhibit running wheel activity during the first half of the bright light exposure.
Note: While this work was in the final stages of revision, another report (Purrier et al., 2014) was published using an independently derived mouse line with identical genotype, in which the animals were studied only with high intensity (900 lux) lighting. In comparison, the lighting used in this study was 130 lux (0.35 W/m2) and 2000 lux (6.53 W/m2). They report a circadian pattern similar to the mice we studied under more intense (6.53 W/m2) lighting, but did not identify the loss of circadian synchrony we found at lower light levels.
Acknowledgments
Funding
This work was funded by grants from the U.S. Department of Health and Human Services - National Institutes of Health. Grant support for HSG: NS067476; CBS: NS072337, AG09975; JL: NS061841, NFS051609
References
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain research. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Menaker M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain research. 1989;479:76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. The European journal of neuroscience. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. NMDA as well as non-NMDA receptor antagonists can prevent the phase-shifting effects of light on the circadian system of the golden hamster. Journal of biological rhythms. 1992;7:125–136. doi: 10.1177/074873049200700204. [DOI] [PubMed] [Google Scholar]
- de Vries MJ, Nunes Cardozo B, van der Want J, de Wolf A, Meijer JH. Glutamate immunoreactivity in terminals of the retinohypothalamic tract of the brown Norwegian rat. Brain research. 1993;612:231–237. doi: 10.1016/0006-8993(93)91665-f. [DOI] [PubMed] [Google Scholar]
- Delwig A, Majumdar S, Ahern K, LaVail MM, Edwards R, Hnasko TS, Copenhagen DR. Glutamatergic neurotransmission from melanopsin retinal ganglion cells is required for neonatal photoaversion but not adult pupillary light reflex. PLoS One. 2013;8:e83974. doi: 10.1371/journal.pone.0083974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Hioki H, Tomioka R, Taki K, Tamamaki N, Nomura S, Okamoto K, Kaneko T. Changes of immunocytochemical localization of vesicular glutamate transporters in the rat visual system after the retinofugal denervation. The Journal of comparative neurology. 2003;465:234–249. doi: 10.1002/cne.10848. [DOI] [PubMed] [Google Scholar]
- Fyk-Kolodziej B, Dzhagaryan A, Qin P, Pourcho RG. Immunocytochemical localization of three vesicular glutamate transporters in the cat retina. The Journal of comparative neurology. 2004;475:518–530. doi: 10.1002/cne.20199. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. The Journal of comparative neurology. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Fremeau RT, Jr, Duncan JL, Renteria RC, Yang H, Hua Z, Liu X, LaVail MM, Edwards RH, Copenhagen DR. Vesicular glutamate transporter 1 is required for photoreceptor synaptic signaling but not for intrinsic visual functions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7245–7255. doi: 10.1523/JNEUROSCI.0815-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. The Journal of physiology. 1991;444:269–287. doi: 10.1113/jphysiol.1991.sp018877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Williamson L, Palkovits M, Namboodiri MA. N-acetylaspartylglutamate: a transmitter candidate for the retinohypothalamic tract. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8065–8069. doi: 10.1073/pnas.87.20.8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. The Journal of comparative neurology. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Ohi K, Takashima M, Nishikawa T, Takahashi K. N-methyl-D-aspartate receptor participates in neuronal transmission of photic information through the retinohypothalamic tract. Neuroendocrinology. 1991;53:344–348. doi: 10.1159/000125740. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Smeraski CA, Tomlinson CC, Banfield BW, Kaufman J, Wilcox CL, Enquist LW, Sollars PJ. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2701–2710. doi: 10.1523/JNEUROSCI.22-07-02701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purrier N, Engeland WC, Kofuji P. Mice deficient of glutamatergic signaling from intrinsically photosensitive retinal ganglion cells exhibit abnormal circadian photoentrainment. PLoS One. 2014;9:e111449. doi: 10.1371/journal.pone.0111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Watanabe A, Hamada T, Ono M, Watanabe S. N-methyl-D-aspartate induces phase shifts in circadian rhythm of neuronal activity of rat SCN in vitro. Am J Physiol. 1994;267:R360–364. doi: 10.1152/ajpregu.1994.267.2.R360. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991;11:2087–2101. doi: 10.1523/JNEUROSCI.11-07-02087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Regus-Leidig H, Haverkamp S. Expression of the vesicular glutamate transporter vGluT2 in a subset of cones of the mouse retina. The Journal of comparative neurology. 2006;496:544–555. doi: 10.1002/cne.20942. [DOI] [PubMed] [Google Scholar]