Summary
Background
Stress is a critical risk factor affecting both the development of and the relapse to drug addictions. Drug addictions are caused by genetic, environmental and drug-induced factors. The objective of this hypothesis-driven association study was to determine if genetic variants in stress-related genes are associated with heroin addiction.
Methods
112 selected genetic variants in 26 stress-related genes were genotyped in 852 case subjects and 238 controls of predominantly European ancestry. The case subjects are former heroin addicts with a history of at least one year of daily multiple uses of heroin, treated at a methadone maintenance treatment program (MMTP). The two most promising SNPs were subsequently tested in an African-American sample comprising of 314 cases and 208 control individuals.
Results
Nineteen single nucleotide polymorphisms (SNPs) in 9 genes (AVP, CRHR1, CRHR2, FKBP5, NR3C2, AVPR1A, GAL, GLRA1, and NPY1R) showed nominally significant association with heroin addiction. The associations of two FKBP5 SNPs that are part of one haplotype block, rs1360780 (intron 2) and rs3800373 (the 3' untranslated region), remained significant after correction for multiple testing (Pcorrected =0.03; OR = 2.35, Pcorrected = 0.0018; OR = 2.85, respectively). The two SNPs also showed nominally significant association (P <0.05) with heroin addiction in an independent African-American cohort. FKBP5 is a co-chaperone that regulates glucocorticoid sensitivity. These FKBP5 SNPs were previously associated with diverse affective disorders and showed functional differences in gene expression and stress response. This study also supports our and others’ previous reports of association of the GAL SNP rs694066 and the AVPR1A SNPs rs11174811, rs1587097 and rs10784339 with heroin and general drug addiction, respectively.
Conclusions
This study suggests that variations in the FKBP5 gene contribute to the development of opiate addiction by modulating the stress response. These findings may enhance the understanding of the interaction between stress and heroin addiction.
Keywords: Genetic variants, association study, heroin addiction, stress, HPA axis, glucocorticoid sensitivity, FKBP5, AVPR1A, GAL
1. Background
Addiction to opiates and the illicit abuse of prescription opioids is a growing epidemic. Addiction to drugs is a chronic relapsing brain disease caused by a combination of genetic, epigenetic, environmental and drug-induced factors. Stress is a critical risk factor affecting both the development of addictive disorders, by promoting drug seeking and excessive drug intake, and the relapse to addictive behaviors, since drug withdrawal can increase stress response, and stress increases reward-seeking behavior, such as reinstatement of drug-taking behavior (Koob and Kreek, 2007; Sinha, 2008; Ulrich-Lai and Herman, 2009; Kreek et al., 2012). Studies showed a high rate of various types of childhood trauma exposure and affective disorders comorbidity among individuals with opioid dependence (Mills et al., 2005; Nelson et al., 2006; Sansone et al., 2009). The response to stress is influenced by genetic and environmental factors and has high inter-individual variability. A plastic neural circuitry that includes the hippocampus, amygdala, hypothalamus, brainstem and prefrontal cortex coordinates the response systems (McEwen and Gianaros, 2011).
Adrenal secretion of glucocorticoids is one of the mechanisms of response to stress. Stress exposure, as well as endogenous opioids and drugs of abuse, activate the hypothalamic-pituitary-adrenal (HPA) axis. Consequently, corticotropin-releasing hormone (CRH, CRF) and arginine vasopressin (AVP) are released from the hypothalamic paraventricular nucleus (PVN) and are transported to the anterior pituitary and stimulate adrenocorticotropic hormone (ACTH) secretion, which in turn stimulates glucocorticoid synthesis and release from the adrenal cortex. CRF is also produced in other brain regions and activates the sympathetic nervous system to release epinephrine and norepinephrine from the adrenal glands. It also stimulates the mesocorticolimbic dopamine system that mediates the rewarding effects associated with drug use. High levels of glucocorticoids can “sensitize” CRF systems in the extra hypothalamic brain stress systems (extended amygdala)(Koob, 2010).
Glucocorticoids regulate the activity of the HPA axis through negative feedback via the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR). These receptors regulate the expression of genes necessary for coping with stress. The functions of the GR and the MR are partly moderated by chaperone proteins including the heat shock protein 90 (Hsp90) co-chaperone FKBP5 (FKBP51, FK506-binding protein 51). Glucocorticoids have many other effects when bound to glucocorticoid receptors (e.g., modulation of cardiovascular function, immunologic status, arousal, learning and memory). They can also alter the methylation patterns of genes (Stephens and Wand, 2012).
Numerous molecular genetic studies have evaluated the association between polymorphisms in stress-related genes and affective disorders (Domschke and Reif, 2012), but only a few studies have reported an association of variants in these genes with specific drug addictions. We have previously performed association studies of heroin addiction that include several genes related to stress response (Levran et al., 2008; Proudnikov et al., 2008; Levran et al., 2009). These studies identified, in these genes, association of SNPs in the galanin gene (GAL) in European Americans, the AVP receptor gene (AVPR1A) in African Americans, and the ACTH receptor gene (MC2R) in Hispanics. A different AVPR1A SNP was shown to be associated with general drug use disorders by another group (Maher et al., 2011), and NPY2R SNPs were associated with alcohol and cocaine dependence (Wetherill et al., 2008).
Here, we report the results of a case-control hypothesis-driven association study of 112 SNPs from 26 genes related to stress response, with heroin addiction, in a sample of 1090 subjects of predominantly European ancestry. The study is a major expansion of our previous study (Levran et al., 2008) to which 517 samples, 12 additional stress-related genes, and several new SNPs in genes included in the previous study were added. The study employed more stringent inclusion criteria for ancestry, based on biographic ancestry scores obtained by STRUCTURE analysis of 155 Ancestry Informative Markers (AIMs). This study included a validation sample of different ethnicity (African-American) for the most significant results.
2. Methods
2.1. Subjects
Discovery sample
The 1090 subjects of this study are part of a larger cohort recruited by the Kreek laboratory for the study of the genetics of specific drug addictions. There were 852 cases (33% female; mean age 40±12) and 238 controls (49% female; mean age 42±16). The subjects were selected based on phenotype (history of severe heroin addiction, normal controls) and self-identified European ancestry (including a Middle-Eastern contribution). Other ethnicities were excluded (e.g., Africans, Hispanics, Asians, Native Americans or mixed ancestry). Ancestry was verified by a family history questionnaire and STRUCTURE analysis (see below), and specific inclusion criteria were employed to obtain relative homogeneity and to limit population stratification. To be included in the discovery sample, an individual had to show at least a 50% European, Middle-Eastern or both ancestry contributions and less than 25% contribution from another specific ancestry. This study is a major expansion of our previous study (Levran et al., 2008). The current study includes a majority of the samples from the original study.
The case subjects were former heroin addicts with a history of at least one year of daily multiple uses of heroin, treated at a MMTP at the time of recruitment. The case subjects were recruited at the Rockefeller University Hospital (n = 238), the Manhattan Campus of the VA NY Harbor Health Care System (n = 55) and the Dr. Miriam and Sheldon G. Adelson Clinics for Drug Abuse Treatment and Research, in Las Vegas (n = 264) and Israel (n = 295). The control sample was mainly from the NYC community (n = 208) and was recruited at the Rockefeller University Hospital by newspaper and online advertisement. Additional controls were recruited at the Adelson clinic in Israel (n = 30). Ascertainment of cases and controls was made by personal interview performed in a similar manner at the recruiting places, using several instruments: the Addiction Severity Index (McLellan et al., 1992), Kreek-McHugh-Schluger-Kellogg Scale (KMSK) (Kellogg et al., 2003) and Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). The following exclusion criteria from the healthy control category were used: (1) at least one instance of drinking to intoxication or any illicit drug use in the previous 30 days; (2) a history of alcohol drinking to intoxication or illicit drug use, more than twice a week, for more than six consecutive months, and (3) cannabis use for more than 12 days in the previous 30 days or past cannabis use for more than twice a week for more than four years. Subjects with active DSM-IV axis I disorder were excluded from the study. All subjects completed a family history questionnaire and relatives were excluded from the study. The Institutional Review Boards of the Rockefeller University Hospital, the VA New York Harbor Healthcare System and the Tel Aviv Sourasky Medical Center (Helsinki Committee) approved the study. All subjects signed informed consent for genetic studies.
Validation sample
The two most significant FKBP5 SNPs were subsequently tested in an independent African-American (>50% African ancestry, non-Hispanic) sample comprising of 314 (37% female) cases and 208 (52% female) control individuals. The average African contribution was ~80% in both cases and controls, with similar pattern of distribution of the other ancestry clusters (5% each of Europe, Middle East and Central Asia). The case sample was recruited at the Rockefeller University Hospital (n = 230), the Manhattan Campus of the VA NY Harbor Health Care System (n = 63) and the Dr. Miriam and Sheldon G. Adelson Clinic for Drug Abuse Treatment and Research in Las Vegas (n = 21).The control sample was recruited at the Rockefeller University Hospital. Ethnicity verification by STRUCTURE analysis, recruitment and ascertainment were as described for the discovery sample.
2.2. Genes/SNPs selection and array design
Twenty-seven genes were selected based on their known function in response to stress (Table 1, Table S1). In addition to the genes included on the original hypothesis-driven “addiction” array (GS0007064-OPA; Illumina, San Diego, CA, USA) (Hodgkinson et al., 2008) that was used in our original association studies (Levran et al., 2008; Levran et al., 2009), we have added 12 stress-related genes to the current array (GS0013101-OPA). Out of the 68 new SNPs from stress-related genes that were selected for the new array based on previous reports of potential functionality and/or association with stress response, 13 SNPs were not technically suitable for this platform based on the Illumina Assay Design Tool, and 55 SNPs were added to the new array. A total of 143 SNPs from these stress-related genes were included in the array, out of which 88 SNPs were from the original “addiction” array (Table S1).
Table 1.
Stress-related genes
| Symbol | Gene name |
|---|---|
| AVP | arginine vasopressin |
| AVPR1A | arginine vasopressin receptor 1A |
| AVPR1B | arginine vasopressin receptor 1B |
| CARTPT | CART prepropeptide |
| CCK | cholecystokinin |
| CRH | corticotropin releasing hormone |
| CRHBP | corticotropin releasing hormone binding protein |
| CRHR1 | corticotropin releasing hormone receptor 1 |
| CRHR2 | corticotropin releasing hormone receptor 2 |
| FKBP5 | FK506-binding protein 51 |
| GAL | galanin |
| GALR1 | galanin receptor 1 |
| GLRA1 | glycine receptor, alpha 1 |
| HCRTR1 | hypocretin receptor 1 |
| HCRTR2 | hypocretin receptor 2 |
| MC2R | melanocortin 2 receptor |
| NPY | neuropeptide Y |
| NPY1R | neuropeptide Y receptor Y1 |
| NPY2R | neuropeptide Y receptor Y2 |
| NPY5R | neuropeptide Y receptor Y5 |
| NR3C1 | nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor) |
| NR3C2 | nuclear receptor subfamily 3, group C, member 2 (mineralocorticoid receptor) |
| OXT | oxytocin |
| OXTR | oxytocin receptor |
| PITX1 | paired-like homeodomain transcription factor 1 |
| SERPINA6 | corticosteroid binding globulin |
Genes are sorted by alphabetical order
2.3. Genotyping
Blood samples were taken and DNA was extracted and quantified using standard methods. DNA (700 ng) was precipitated as described (Levran et al., 2008). Genotyping was performed on a 1536-plex GoldenGate Custom Panel at the Rockefeller University Genomics Resource Center according to the manufacturer’s protocol. Random samples were genotyped in duplicate. Analysis was performed with BeadStudio software v2.3.43 (Illumina). The genotype data for all SNPs were visually inspected to verify and correct automatic calling. Genotype data were filtered based on SNP call rates (> 99%), minor allele frequency (MAF) in controls > 0.05, and deviation from Hardy-Weinberg equilibrium (HWE) in controls (P < 0.001) (Table S1). Of the 143 SNPs genotyped, 14 SNPs were excluded from analysis based on low quality (n = 6), more than 3 clusters (n = 4), or failure on some of the plates that did not reflect ambiguous clustering (n = 4), and 17 SNPs were excluded from analysis based on MAF < 0.05 in this sample. Since the HCRT gene was represented by only one SNP with MAF < 0.05 in this sample, this gene is not included in the final gene list. The total analysis was performed with 112 SNPs from 26 genes (Table S1).
2.4. Assessment of ancestry contribution using AIMs
Of the original 186 AIMs from the GS0007064-OPA panel (Hodgkinson et al., 2008), 171 SNPs with adequate quality were included in the new panel, and 155 AIMs with high quality scores were used for analysis. Biographic Ancestry Scores (e.g., fractions of genetic affiliation of the individual in each cluster) were estimated by STRUCTURE 2.2 with seven clusters (K). Each subject was anchored against genotypes of 1051 samples from 51 worldwide populations represented in the Human Genome Diversity Cell Line Panel (http://www.cephb.fr/HGDP-CEPH-Panel), as described (Ducci et al., 2009). To be included in the discovery sample, an individual had to show at least a 50% European, Middle-Eastern, or both ancestry contribution and less than 25% contribution from another specific ancestry. The decision to include both European and Middle-Eastern clusters was based on their low population differentiation (Tian et al., 2009). Studies showed especially close relationship between Middle Eastern and Southern European populations (Atzmon et al., 2010). To be included in the validation sample, an individual had to show at least a 50% African ancestry contribution.
In the discovery sample, the mean (SD) European ancestry contribution score was 0.73 (0.32) with median of 0.89. The mean Middle Eastern score was 0.21 (0.30) with median of 0.03. The means and medians of the other five ancestries were <0.05 (<0.05). In the validation sample, the mean African score was 0.8 (0.11) with median of 0.82. The European, Middle Eastern and Central Asia mean and median scores were 0.05±0.01 (0.04). The means of the other three ancestries’ scores were 0.01±0.01.
From the original cohort of self-identified European and/or Middle-Eastern subjects, 57 subjects were excluded because they did not meet the inclusion criteria. Seven subjects were excluded based on a major conflict between their self-identified ethnicity and STRUCTURE results. Twenty-two subjects with ambiguous self-identified ancestry and six subjects who self-identified as African Americans were included based on STRUCTURE results.
2.5. Statistical analysis
Quality control for the SNP genotypes was carried out as follows. Exact tests for deviation from HWE were performed with the PLINK program, with SNPs to be rejected based on the recommended threshold of P < 0.001 in control individuals. Pairwise linkage disequilibrium (LD) and haplotype blocks were estimated using Haploview 4.2. Analysis of block of LD was performed using the Confidence Intervals algorithm for block definition (Gabriel et al., 2002), with stringent criteria of LD (D' lower bound of 0.9). Association analysis was performed for each SNP separately by logistic regression in the PLINK program, where in different analyses the genotype was coded as a linear allelic effect (genotypes AA, AB, and BB were given numerical values 0, 1, and 2, respectively), and as two groups reflecting a dominant or recessive inheritance model. Sex was included as a covariate. Ancestry contribution scores (European and Middle Eastern for the discovery sample and African for the validation sample) were initially included as predictor covariates but had no significant effects on the dependent variable so they were not included in the final analysis. The multiple testing issue was addressed by assuming 67 independent SNPs following the method suggested by Duggal et al. (Duggal et al., 2008). Based on analysis of block of LD in controls, there are 45 “non-independent” SNPs, including seven redundant SNPs in complete LD (r2 = 1) so that, with Bonferroni correction, P values were corrected by division of 0.05 by 67. P values were further adjusted for testing three models of inheritance by division by 1.7 (= √3) based on Mantel (Mantel, 1980). Thus, P = 4.4E-04 (0.05/113.9) was chosen as the threshold for significance.
3. Results
A total of 1090 subjects (852 cases and 238 controls) met the inclusion criteria and were included in the association analyses. The ethnicity of all subjects was verified as predominantly European using STRUCTURE analysis of 155 AIMs (Fig. 1). Genotypes of 112 SNPs (MAF > 0.05) from 26 stress-related genes were analyzed for association with heroin addiction (Table 1, Table S1). The European and Middle Eastern ancestry contribution scores did not show independent effects on the dependent variable indicating that the associations are not due to population stratification. No SNP showed deviation from HWE (P < 0.001) in controls. One SNP (rs10213647) showed deviation from HWE in cases only (P = 0.0004). Based on LD analysis in controls, there are 45 correlated SNPs, including seven redundant SNPs in complete LD (r2 = 1), suggesting an “effective” number of 67 independent SNPs.
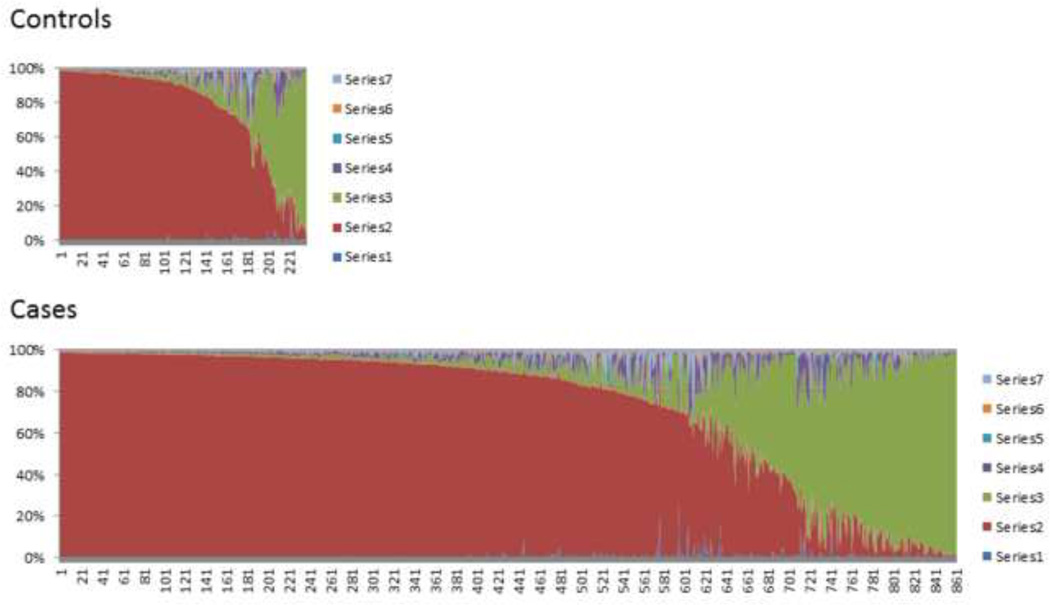
Figure 1. Schematic representation of the individual admixture estimates.
The estimates are based on STRUCTURE analysis using K=7. Each vertical line represents one individual, and subjects are displayed according to their predominant cluster contribution (see Methods). The clusters correspond to the geographical regions based on the HGDP sample. Color code: Africa (1) (blue), Europe (2) (red), Middle East (3) (green), Central Asia (4) (purple), Far East Asia (5) (cyan), Oceania (6) (amber), and America (7) (light blue).
Nineteen SNPs in nine genes showed nominally significant association of genotype with heroin addiction (Table 2). The top signals are from the following genes: AVP, CRHR1, CRHR2, FKBP5, NR3C2, AVPR1A, GAL, GLRA1 and NPY1R. Two tightly-linked FKBP5 SNPs, rs1360780 and rs3800373, from intron 2 and the 3' UTR, respectively, remained significant after correction for multiple testing (Pcorrected = 0.03; OR = 2.35; 95% CI,1.5–3.7 & Pcorrected = 0.0018; OR = 2.85; 95% CI,1.8–4.6, respectively). The two SNPs also showed nominally significant association in the same direction (P = 0.04, OR = 1.49; 95% CI, 1.01–2.18 & P = 0.03, OR = 1.54; 95% CI 1.04–2.30, respectively) with heroin addiction in an independent African-American sample comprising of 314 cases and 208 control individuals that was used for validation and generalization. The African ancestry contribution score did not show independent effects on the dependent variable indicating that the associations are not due to population stratification.
Table 2.
Details of SNPs with nominally significant associations (P < 0.05)
| Gene | SNP | Location | Allelesa | MAF Case |
MAF Control |
P | Modeb | OR (95% CI) | Risk allele |
|---|---|---|---|---|---|---|---|---|---|
| AVP | rs2282018 | intron | T/C | 0.37 | 0.42 | 0.027 | D | 1.41 (1.04–1.92) | T |
| AVPR1A | rs3021529 | 5' UTR | C/T | 0.14 | 0.17 | 0.011 | D | 1.50 (1.10–2.06) | C |
| rs3803107 | 3' UTR | C/T | 0.15 | 0.20 | 2.7E-03 | D | 1.61 (1.18–2.18) | C | |
| rs11174811 | 3' UTR | C/A | 0.14 | 0.18 | 4.2E-03 | D | 1.57 (1.15–2.15) | C | |
| rs10784339 | 3' UTR | G/C | 0.17 | 0.21 | 0.022 | D | 1.42 (1.05–1.92) | G | |
| rs1587097 | 3' intergenic | C/T | 0.11 | 0.14 | 0.026 | D | 1.57 (1.15–2.15) | C | |
| CRHR1 | rs242939 | intron | A/G | 0.09 | 0.06 | 0.038 | A | 1.54 (1.02–2.33) | G |
| CRHR2 | rs255102 | intron | A/T | 0.35 | 0.32 | 0.048 | D | 1.34 (1.00–1.80) | T |
| FKBP5 | rs9470080 | intron | C/T | 0.29 | 0.34 | 3.3E-03 | R | 1.97 (1.25–3.09) | C |
| rs1360780 | intron | C/T | 0.27 | 0.33 | 3.0E-04 | R | 2.35 (1.48–3.72) | C | |
| rs7757037 | intron | A/G | 0.51 | 0.44 | 1.1E-03 | D | 1.70 (1.24–2.34) | A | |
| rs3800373 | 3' UTR | A/C | 0.26 | 0.33 | 1.6E-05 | R | 2.85 (1.77–4.58) | A | |
| GAL | rs694066 | intron | C/T | 0.12 | 0.07 | 5.2E-03 | A | 1.72 (1.17–2.51) | T |
| rs3136541 | intron | T/C | 0.40 | 0.32 | 3.2E-03 | A | 1.39 (1.12–1.73) | C | |
| GLRA1 | rs2915885 | intron | T/C | 0.41 | 0.35 | 0.027 | A | 1.27 (1.03–1.57) | C |
| rs9324714 | intron | T/A | 0.41 | 0.35 | 0.038 | A | 1.25 (1.01–1.55) | A | |
| rs1428159 | intron | A/G | 0.41 | 0.36 | 0.049 | A | 1.24 (1.00–1.53) | G | |
| NPY1R | rs4518200 | 5' intergenic | A/C | 0.10 | 0.12 | 0.030 | D | 1.47 (1.03–2.08) | C |
| NR3C2 | rs1040288 | intron | C/G | 0.45 | 0.52 | 0.011 | A | 1.31 (1.06–1.61) | Gc |
SNPs are listed in alphabetical order. SNPs in each gene are sorted by relative position. Bolded SNPs are significant after correction for multiple testing.
Alleles are listed as major/minor in Europeans;
Inheritance modes: R=recessive; D=dominant; A=additive;
The risk allele is the major allele in the case sample but the minor allele in the control sample.
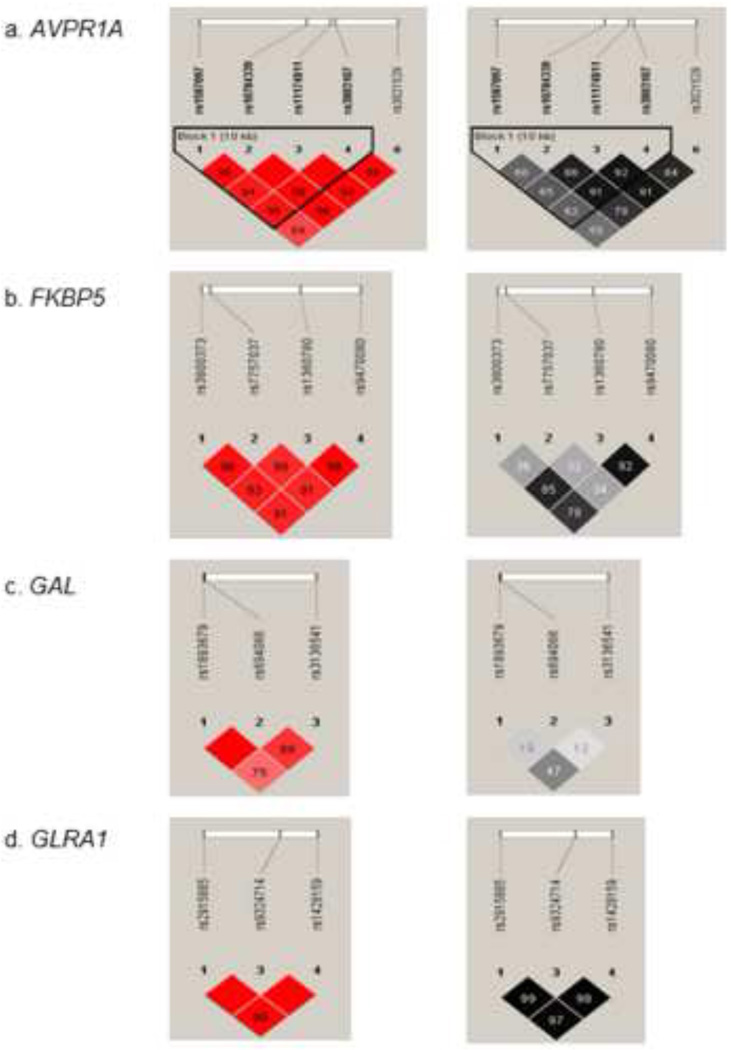
LD analysis revealed that some of the top results are likely to be related, as some SNPs are in strong LD. As is shown in Fig. 2, the four FKBP5 SNPs are linked (D' >.89), of which 3 SNPs (rs3800373, rs1360780, and rs9470080) are tightly linked (r2 >.77). The three GLRA1 and the five AVPR1A SNPs are tightly linked (r2 > .96, r2 > .62, respectively). In contrast, the two GAL SNPs showed lower levels of LD.
Figure 2. Pairwise Linkage Disequilibrium (LD).
LD between SNPs in four genes was derived from genotypes of controls. The pairwise correlation between SNPs was measured as D' (in red) and r2 (in black). The values are shown (x100) in each box. The color scheme indicates the magnitude of the value. When the value is equal to 1.0 no number is given. a. AVPR1A. b. FKBP5. c. GAL. d. GLRA1.
4. Discussion
The strongest results of this study are of four linked FKBP5 SNPs, two of which (rs1360780 and rs3800373, from intron 2 and the 3' UTR, respectively) remained significantly associated with heroin addiction after correction for multiple testing. The odd ratios (OR) were relatively high (> 2) and the more abundant alleles (C and A, respectively) were the “risk alleles”, while the less abundant alleles can be considered “protective alleles”. The two SNPs also showed nominally significant association in an independent African-American sample that was used for validation and generalization. These two FKBP5 SNPs are in strong LD across different ethnic groups. The FKBP5 gene was not included in our previous association study (Levran et al., 2008).
FKBP5 is considered a negative transcriptional regulator of steroid receptors. FKBP5 is a component of steroid hormones receptor hetero-complexes along with the Hsp90 and the p23 protein. It binds the immunosuppressive drugs FK506 (tacrolimus) and rapamycin. FKBP5-Hsp90 hetero-complex regulates GR sensitivity via an ultra-short negative feedback loop (Galigniana et al., 2012). When cortisol binds the GR in the cytoplasm, the complex enters the nucleus and GR regulates transcription of glucocorticoid-responsive genes. FKBP5 regulates GR sensitivity by preventing translocation of the GR complex to the nucleus.
Several rodent studies shed light on the relationship between stress, drug addiction, and FKBP5. Chronic morphine administration and precipitated withdrawal were shown to change Fkbp5 expression in the locus ceruleus and the VTA, and blockade of FKBP5 with FK506 attenuated dependence development (Homayoun et al., 2003; McClung et al., 2005). Recent studies found that Fkbp5 expression in the mouse striatum was strongly activated by acute and chronic administration of opioids (Piechota et al., 2012). Fkbp5 whole brain expression was shown to be regulated by oxycodone (Hassan et al., 2010). Fkbp5 KO mice display a reduced response to chronic social defeat stress. Under basal conditions, deletion of Fkbp5 did not change anxiety or depression-like behavior. However, exposure to stressors led to a more active coping behavior and decreased HPA axis reactivity and GR expression (Touma et al., 2011; Hartmann et al., 2012). Glucocorticoid administration was shown to decrease DNA methylation in Fkbp5 in brain and blood, and this decrease was associated with behavioral deficits (Lee et al., 2011; Yang et al., 2012).
FKBP5 SNPs were shown to be associated with stress response and affective disorders (e.g., (Binder, 2009; Willour et al., 2009; Velders et al., 2011)). Impaired GR sensitivity was detected in major depression patients that were carriers of the FKBP5 rs1360780 T allele (Menke et al., 2013). FKBP5 SNPs were associated with baseline and peak cortisol response to social stress test in healthy individuals (Mahon et al., 2013). An fMRI study of African-American subjects showed that carriers of the rs1360780 T allele had an attention bias toward threat, increased hippocampal activation to stress, and difference in hippocampal shape during performance of an attention-bias task (Fani et al., 2013).
Several studies support the hypothesis that genetic or epigenetic modifications in FKBP5 modulate the effect of the environment on the HPA axis and the risk for stress-related disorders (gene×environment interaction). These studies suggest that dysregulation of the HPA axis function in childhood may have long-lasting effects. FKBP5 variants were shown to interact with childhood trauma to predict depression, suicidality, aggression, and PTSD (e.g.,(Binder et al., 2008; Xie et al., 2010; Appel et al., 2011; Bevilacqua et al., 2012; Boscarino et al., 2012; Roy et al., 2012; Klengel et al., 2013)). Binder’s group (Binder et al., 2004; Binder et al., 2008; Klengel et al., 2013) identified the less abundant alleles of four SNPs (H2 haplotype, including the rs1360780 T allele) to be the “risk alleles” that moderate child abuse-related risk for adult PTSD, in African Americans. They also showed that this allele increased FKBP5 expression and caused relative GR resistance. Similar results were obtained by others (Xie et al., 2010; Appel et al., 2011). In contrast, Roy et al. (Roy et al., 2010; Roy et al., 2012) identified the more abundant alleles of these SNPs (H1 haplotype, including rs1360780 C allele) as the “risk alleles” for suicide attempt in substance-dependent African Americans. They found no association of FKBP5 SNPs and substance dependence (alcohol, cocaine, or opiate). A study of aggression in Italian males from the same group (Bevilacqua et al., 2012) reported a dosage effect of rs1360780 alleles with larger effect of the less abundant allele. The Bevilacqua et al. study suggested an association of the more abundant FKBP5 haplotype with substance dependence in a subgroup of 127 subjects with mixed-drug dependencies and high comorbidity with Axis I psychiatric disorders. Notably, the current study does not include subjects with active DSM-IV axis I disorder; therefore the addiction is not secondary to psychiatric disorders in this cohort. Since this study has limited information about trauma exposure, we cannot address the suggestion of gene × environment interaction at this point.
A possible mechanism of action of FKBP5 SNP rs1360780 was suggested by a recent study by The Binder’s group (Klengel et al., 2013). They found that differential DNA CpG methylation in FKBP5, in carriers of the FKBP5 rs1360780 T allele that were exposed to childhood trauma, increased the risk of developing stress-related psychiatric disorders in adulthood. A bioinformatics analysis suggested that SNP rs3800373 may cause the loss of exon splicing enhancer motive that may cause exon skipping (Liu et al., 2000).
Several other SNPs were identified by this study with only nominal significant association that may not reflect true association; nonetheless, additional support for these findings is provided by several previous association studies of related phenotypes and evidence of functionality. The AVPR1A 3' UTR SNPs rs11174811, rs1587097 and rs10784339 were previously associated with a non-specific drug use disorder in European-Americans (Maher et al., 2011). SNP rs11174811 is located in potential seed recognition sites for microRNAs (miRs) miR-526b and miR-578 and was shown to disrupt miR/mRNA interactions, in vitro (Nossent et al., 2011). The other two AVPR1A SNPs are in strong LD with SNP rs11174811. SNP rs3803107 is located very close (358 bp) to SNP rs11174811, so their association signals are most probably related. We have previously reported an association between another AVPR1A SNP (rs3759292) in the 5' flanking region and heroin addiction in African Americans (Levran et al., 2009). SNP rs3759292 is rare in European populations and was excluded from analysis in this study based on very low allele frequency (< 0.05).
The finding of this study also supports our previous report of association between GAL SNP rs694066 and heroin addiction (Levran et al., 2008). This variant was also shown to be associated with depression in a gender-dependent manner in Han Chinese (Wang et al., 2013). A GAL haplotype that includes SNPs rs694066 and rs3136541 was associated with alcoholism in Finnish and Plains American Indian men (Belfer et al., 2006).
In addition, CRHR1 SNP rs242939 was associated with depressive disorders in Han Chinese (Liu et al., 2006; Xiao et al., 2011; Liu et al., 2013) and British females (Engineer et al., 2013). NPY1R SNP rs4518200 is in strong LD with the 3' UTR SNP rs4552421 that is part of a haplotype that was associated with nicotine dependence in Han Chinese (Wei et al., 2012).
This study supports the hypothesis that atypical GR sensitivity underlies the pathophysiology of drug addiction, and contributes to addiction continuation and relapse (Kreek et al., 2005). Drug exposure generates chronic stress, activates the stress response and creates reward sensitization and a negative emotional state. Active heroin addicts have hypo-responsive (blunted) HPA system that may be caused by a preexisting trait or consequence of long term heroin abuse and/or exposure to trauma. The modulation of the stress response by FKBP5 variants, possibly in combination with other stress-related genes variants, may contribute to the development of heroin dependence by increasing sensitivity to both stress and heroin effect.
The identification of specific stress-related genetic variants involved in heroin addiction has potential clinical implications. It identifies treatment targets, supports treatment options suggested by animal studies, and has the potential to optimize therapeutic interventions by identifying patients who are at specific risk for stress-related relapse and/or patients that would benefit from specific interventions based on their genotype, especially early in the addiction cycle.
In summary, this study provides evidence for the involvement of specific genetic variants in several stress-related genes with heroin addiction. It is plausible that combinations of alleles and gene-gene interactions underlie the genetic basis of heroin addiction. Future studies with greater statistical power should examine the contributions of simultaneous variations in several genes. The study suggests that functional genetic variations in the FKBP5 gene, which is involved in glucocorticoid sensitivity, affect the risk for heroin addiction. It is important to verify whether the associations of these gene variants are specific to heroin addiction or are shared with other drug addictions. Additional association and functional studies utilizing different drug addictions, populations with different ancestries, and broader SNP coverage are required to further corroborate these findings and define the role of FKBP5 and the other genes variations indicated, as contributing factors to heroin addiction.
Supplementary Material
Acknowledgements
We thank all the clinical staff who enrolled and assessed subjects for this study, including S. Linzy, RN, A. Sason, P. Casadonte, MD, E. Ducat, NP and B, Ray, NP. We are grateful to P-H. Shen, PhD, and D. Goldman, PhD, from the NIH/NIAAA, for STRUCTURE analysis, S. Russo for proofreading, and C. Zhao, PhD, and B. Zhang, from the Rockefeller Genomic Resource Center, for their excellent assistance in genotyping.
This work was supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and the Shanxi Scholarship Council of China (LY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors have any conflicts of interest to declare with respect to this manuscript.
Contributors: Study design: OL and MJK; Acquisition of data: EP, JR, MR, and MA; Data analysis: OL, JO, and YI; Data interpretation: OL and MJK; Manuscript writing: OL; Critical revision: MJK, JO. All authors reviewed the manuscript.
Supporting Information
Table S1. SNPs list
References
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, Biffar R, Nauck M, Volzke H, Freyberger HJ, Grabe HJ. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Hao L, Pe'er I, Velez C, Pearlman A, Palamara PF, Morrow B, Friedman E, Oddoux C, Burns E, Ostrer H. Abraham's children in the genome era: major Jewish diaspora populations comprise distinct genetic clusters with shared Middle Eastern Ancestry. American Journal of Human Genetics. 2010;86:850–859. doi: 10.1016/j.ajhg.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Hipp H, McKnight C, Evans C, Buzas B, Bollettino A, Albaugh B, Virkkunen M, Yuan Q, Max MB, Goldman D, Enoch MA. Association of galanin haplotypes with alcoholism and anxiety in two ethnically distinct populations. Molecular Psychiatry. 2006;11:301–311. doi: 10.1038/sj.mp.4001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, Enoch MA. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Archives of General Psychiatry. 2012;69:62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature Genetics. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Erlich PM, Hoffman SN, Zhang X. Higher FKBP5, COMT, CHRNA5, and CRHR1 allele burdens are associated with PTSD and interact with trauma exposure: implications for neuropsychiatric research and treatment. Neuropsychiatric Disease and Treatment. 2012;8:131–139. doi: 10.2147/NDT.S29508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Reif A. Behavioral genetics of affective and anxiety disorders. Current Topics in Behavioral Neurosciences. 2012;12:463–502. doi: 10.1007/7854_2011_185. [DOI] [PubMed] [Google Scholar]
- Ducci F, Roy A, Shen PH, Yuan Q, Yuan NP, Hodgkinson CA, Goldman LR, Goldman D. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. American Journal of Psychiatry. 2009;166:1031–1040. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal P, Gillanders EM, Holmes TN, Bailey-Wilson JE. Establishing an adjusted p-value threshold to control the family-wide type 1 error in genome wide association studies. BMC Genomics. 2008;9:516. doi: 10.1186/1471-2164-9-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer N, Darwin L, Nishigandh D, Ngianga-Bakwin K, Smith SC, Grammatopoulos DK. Association of glucocorticoid and type 1 corticotropin-releasing hormone receptors gene variants and risk for depression during pregnancy and post-partum. Journal of Psychiatric Research. 2013;47:1166–1173. doi: 10.1016/j.jpsychires.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Fani N, Gutman D, Tone EB, Almli L, Mercer KB, Davis J, Glover E, Jovanovic T, Bradley B, Dinov ID, Zamanyan A, Toga AW, Binder EB, Ressler KJ. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA Psychiatry. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Galigniana NM, Ballmer LT, Toneatto J, Erlejman AG, Lagadari M, Galigniana MD. Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51. Journal of Neurochemistry. 2012;122:4–18. doi: 10.1111/j.1471-4159.2012.07775.x. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, Cheung-Flynn J, Cox MB, Smith DF, Holsboer F, Muller MB, Schmidt MV. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Hassan HE, Myers AL, Lee IJ, Chen H, Coop A, Eddington ND. Regulation of gene expression in brain tissues of rats repeatedly treated by the highly abused opioid agonist, oxycodone: microarray profiling and gene mapping analysis. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2010;38:157–167. doi: 10.1124/dmd.109.029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol and Alcoholism. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Khavandgar S, Mehr SE, Namiranian K, Dehpour AR. The effects of FK506 on the development and expression of morphine tolerance and dependence in mice. Behavioural Pharmacology. 2003;14:121–127. doi: 10.1097/00008877-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. American Journal of Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. Journal of Clinical Investigation. 2012;122:3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, Purcell RH, Huo Y, Rongione M, Potash JB, Wand GS. A measure of glucocorticoid load provided by DNA methylation of Fkbp5 in mice. Psychopharmacology. 2011;218:303–312. doi: 10.1007/s00213-011-2307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behavior. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, Randesi M, Rotrosen J, Casadonte P, Linzy S, Ott J, Adelson M, Kreek MJ. Heroin addiction in African Americans: a hypothesisdriven association study. Genes Brain Behavior. 2009;8:531–540. doi: 10.1111/j.1601-183X.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Chew SL, Cartegni L, Zhang MQ, Krainer AR. Exonic splicing enhancer motif recognized by human SC35 under splicing conditions. Molecular and Cellular Biology. 2000;20:1063–1071. doi: 10.1128/mcb.20.3.1063-1071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu W, Yao L, Yang C, Xiao L, Wan Q, Gao K, Wang H, Zhu F, Wang G, Xiao Z. Negative life events and corticotropin-releasing-hormone receptor1 gene in recurrent major depressive disorder. Scientific Reports. 2013;3:1548. doi: 10.1038/srep01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, Li W. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neuroscience Letters. 2006;404:358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Maher BS, Vladimirov VI, Latendresse SJ, Thiselton DL, McNamee R, Kang M, Bigdeli TB, Chen X, Riley BP, Hettema JM, Chilcoat H, Heidbreder C, Muglia P, Murrelle EL, Dick DM, Aliev F, Agrawal A, Edenberg HJ, Kramer J, Nurnberger J, Tischfield JA, Devlin B, Ferrell RE, Kirillova GP, Tarter RE, Kendler KS, Vanyukov MM. The AVPR1A Gene and Substance Use Disorders: Association, Replication, and Functional Evidence. Biological Psychiatry. 2011;70:519–527. doi: 10.1016/j.biopsych.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. Assessing laboratory evidence for neoplastic activity. Biometrics. 1980;36:381–399. [PubMed] [Google Scholar]
- McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. Journal of Neuroscience. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Menke A, Klengel T, Rubel J, Bruckl T, Pfister H, Lucae S, Uhr M, Holsboer F, Binder EB. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes Brain Behavior. 2013;12:289–296. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- Mills KL, Lynskey M, Teesson M, Ross J, Darke S. Post-traumatic stress disorder among people with heroin dependence in the Australian treatment outcome study (ATOS): prevalence and correlates. Drug and Alcohol Dependence. 2005;77:243–249. doi: 10.1016/j.drugalcdep.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Lynskey MT, Bucholz KK, Madden PA, Statham DJ, Martin NG. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: a twin study. Psychological Medicine. 2006;36:1473–1483. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- Nossent AY, Hansen JL, Doggen C, Quax PH, Sheikh SP, Rosendaal FR. SNPs in microRNA binding sites in 3'-UTRs of RAAS genes influence arterial blood pressure and risk of myocardial infarction. American Journal of Hypertension. 2011;24:999–1006. doi: 10.1038/ajh.2011.92. [DOI] [PubMed] [Google Scholar]
- Piechota M, Korostynski M, Sikora M, Golda S, Dzbek J, Przewlocki R. Common transcriptional effects in the mouse striatum following chronic treatment with heroin and methamphetamine. Genes Brain Behavior. 2012;11:404–414. doi: 10.1111/j.1601-183X.2012.00777.x. [DOI] [PubMed] [Google Scholar]
- Proudnikov D, Hamon S, Ott J, Kreek MJ. Association of polymorphisms in the melanocortin receptor type 2 (MC2R, ACTH receptor) gene with heroin addiction. Neuroscience Letters. 2008;435:234–239. doi: 10.1016/j.neulet.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy a, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. Journal of Psychiatric Research. 2012;46:72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone RA, Whitecar P, Wiederman MW. The prevalence of childhood trauma among those seeking buprenorphine treatment. Journal of Addictive Diseases. 2009;28:64–67. doi: 10.1080/10550880802545101. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Research. 2012;34:468–483. [PMC free article] [PubMed] [Google Scholar]
- Tian C, Kosoy R, Nassir R, Lee A, Villoslada P, Klareskog L, Hammarstrom L, Garchon HJ, Pulver AE, Ransom M, Gregersen PK, Seldin MF. European population genetic substructure: further definition of ancestry informative markers for distinguishing among diverse European ethnic groups. Molecular Medicine. 2009;15:371–383. doi: 10.2119/molmed.2009.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Bull DR, Ionescu IA, Heinzmann JM, Knapman A, Siebertz A, Depping AM, Hartmann J, Hausch F, Schmidt MV, Holsboer F, Ising M, Cox MB, Schmidt U, Rein T. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biological Psychiatry. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews: Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, Hek K, Hofman A, Verhulst FC, Kivimaki M, Van Duijn CM, Walker BR, Tiemeier H. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology. 2011;36:1053–1061. doi: 10.1016/j.psyneuen.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Li H, Yang YT, Tie CL, Li F, Xu ZQ, Wang CY. Association of galanin and major depressive disorder in the chinese han population. PloS One. 2013;8:e64617. doi: 10.1371/journal.pone.0064617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Chu C, Wang Y, Yang Y, Wang Q, Li T, Zhang L, Ma X. Association study of 45 candidate genes in nicotine dependence in Han Chinese. Addictive Behaviors. 2012;37:622–626. doi: 10.1016/j.addbeh.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Wetherill L, Schuckit MA, Hesselbrock V, Xuei X, Liang T, Dick DM, Kramer J, Nurnberger JI, Jr, Tischfield JA, Porjesz B, Edenberg HJ, Foroud T. Neuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence. Alcoholism, Clinical and Experimental Research. 2008;32:2031–2040. doi: 10.1111/j.1530-0277.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, Zandi PP, Lee RS, MacKinnon DF, Mondimore FM, Schweizer B, Bipolar Disorder Phenome G, Consortium NGIBD, DePaulo JR, Jr, Gershon ES, McMahon FJ, Potash JB. Family-based association of FKBP5 in bipolar disorder. Molecular Psychiatry. 2009;14:261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Liu W, Gao K, Wan Q, Yang C, Wang H, Wang X, Wang G, Liu Z. Interaction between CRHR1 and BDNF genes increases the risk of recurrent major depressive disorder in Chinese population. PloS One. 2011;6:e28733. doi: 10.1371/journal.pone.0028733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Ewald ER, Huo Y, Tamashiro KL, Salvatori R, Sawa A, Wand GS, Lee RS. Glucocorticoid-induced loss of DNA methylation in non-neuronal cells and potential involvement of DNMT1 in epigenetic regulation of Fkbp5. Biochemical and Biophysical Research Communications. 2012;420:570–575. doi: 10.1016/j.bbrc.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.