Abstract
Background
Ca2+-dependent signaling through CaM Kinase II (CaMKII) and calcineurin was suggested to contribute to adverse cardiac remodeling. However, the relative importance of CaMKII versus calcineurin for adverse cardiac remodeling remained unclear.
Methods and Results
We generated double-knockout mice (DKO) lacking the 2 cardiac CaMKII genes δ and γ specifically in cardiomyocytes. We show that both CaMKII isoforms contribute redundantly to phosphorylation not only of phospholamban, ryanodine receptor 2, and histone deacetylase 4, but also calcineurin. Under baseline conditions, DKO mice are viable and display neither abnormal Ca2+ handling nor functional and structural changes. On pathological pressure overload and β-adrenergic stimulation, DKO mice are protected against cardiac dysfunction and interstitial fibrosis. But surprisingly and paradoxically, DKO mice develop cardiac hypertrophy driven by excessive activation of endogenous calcineurin, which is associated with a lack of phosphorylation at the auto-inhibitory calcineurin A site Ser411. Likewise, calcineurin inhibition prevents cardiac hypertrophy in DKO. On exercise performance, DKO mice show an exaggeration of cardiac hypertrophy with increased expression of the calcineurin target gene RCAN1-4 but no signs of adverse cardiac remodeling.
Conclusions
We established a mouse model in which CaMKII’s activity is specifically and completely abolished. By the use of this model we show that CaMKII induces maladaptive cardiac remodeling while it inhibits calcineurin-dependent hypertrophy. These data suggest inhibition of CaMKII but not calcineurin as a promising approach to attenuate the progression of heart failure.
Keywords: calcineurin, CaMKII, cardiac hypertrophy, heart failure, signal transduction
Heart failure is the leading cause of death in developed countries.1 It results from adverse cardiac remodeling on pathological stress situations such as arterial hypertension, ischemic injuries, or genetic causes. Adverse cardiac remodeling is usually described by a combined appearance of myocardial hypertrophy, activation of a fetal gene program, cell death, and interstitial fibrosis.2 Ca2+-dependent signaling pathways including CaMKII and calcineurin were both proposed to play pivotal roles in adverse cardiac remodeling.3
The protein kinase CaMKII consists of 4 different isoforms with distinct expression patterns. CaMKIIα and CaMKIIβ are enriched in the brain, but CaMKIIδ and CaMKIIγ are expressed ubiquitously. CaMKIIδ is the predominant cardiac CaMKII isoform, but CaMKIIγ is also expressed in the heart.4 In human and experimental heart failure, CaMKII expression and activity is enhanced.4,5 Phosphorylation of the ryanodine receptor RyR2 by CaMKII has been reported to cause sarcoplasmic reticulum (SR) Ca2+ leak, which in turn seems to drive heart failure development.6,7 At the epigenetic level, CaMKII inactivates the negative regulator of adverse cardiac remodeling histone deacetylase 4 (HDAC4), leading to transcriptional activation of the myocyte enhancer factor 2 (MEF2), and phosphorylates histone 3.8–14 Transgenic overexpression of the splice variants CaMKIIδB (localizes to the nucleus) and CaMKIIδC (localizes to the cytosol) promote cardiac hypertrophy and dilated cardiomyopathy, respectively.15,16 CaMKII inhibitory peptides prevent structural heart disease.17,18 Mice with a global deletion of CaMKIIδ were protected against adverse cardiac remodeling.7,8 However, in all of these models, substantial phosphorylation of the target phospholamban (PLB) was still detectable, indicating an incomplete loss-of-function.
The protein phosphatase calcineurin consists of 2 subunits: calcineurin A (CnA), which contains the catalytic site, and calcineurin B (CnB), the small regulatory Ca2+-binding subunit.19 Transgenic CnA overexpression in mice induces massive cardiac hypertrophy by dephosphorylation-dependent translocation of the transcription factor NFAT from the cytosol to the nucleus.20,21 The CnA-α and CnA-β isoforms are expressed in the myocardium, but only CnAβ was demonstrated to be stress responsive.22 Moreover, mice lacking CnAβ were protected against cardiac hypertrophy.23 Inhibition of CnA has been suggested as a treatment option for cardiac hypertrophy because studies with the calcineurin inhibitory agent cyclosporine A (CyA) have been successful in rodents.24 On the other hand, the splicing variant CnA-β1 seems to activate Akt-dependent cardioprotective pathways without inducing NFAT-dependent maladaptive hypertrophy.25
We developed a cardiomyocyte-specific double knockout model, in which the 2 cardiac isoforms, CaMKIIδ and CaMKIIγ, were both deleted. This model identifies CaMKII as the critical regulator of maladaptive cardiac remodeling and reveals that CaMKII serves as a negative regulator of calcineurin activity in vivo. Unexpectedly, we provide evidence that cardiac hypertrophy attributable to overactivation of endogenous calcineurin does not cause systolic cardiac dysfunction.
Method
Mouse Experiments
We generated conditional knockout mice containing cardiomyocyte-specific deletions of CaMKIIδ (δ-CKO), CaMKIIγ (γ-CKO), or both isoforms (double knockout mice [DKO]) by crossing previously described conditional knockout mice8,26 to transgenic mice expressing Cre recombinase under the control of the α-myosin heavy chain promoter (αMHC-Cre) or by using the mER-Cre-mER fusion protein under the control of a cardiomyocyte-specific α-MHC-promoter.27,28 As controls, littermates homozygous for the conditional CaMKIIδ and CaMKIIγ alleles without αMHC-Cre transgene (δ-FF, γ-FF, and FFFF) were used. For some experiments, C57BL/6 mice were obtained from Charles River (wild-type mice). A detailed description of the surgical methods, the exercise protocol, and in vivo imaging as well as hemodynamic measurement techniques can be found in Methods in the online-only Data Supplement. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Regierungspräsidium Karlsruhe, Germany.
Histology
The protocols for hematoxylin and eosin (H&E), Masson trichrome staining, and CD31 immunostaining can be found in Methods in the online-only Data Supplement.
Western Blotting, GST Pulldown, and Immunocytochemistry
The immunoblotting, GST-pulldown, and immunocytochemistry protocols as well as the antibodies used in this study can be found in Methods in the online-only Data Supplement.
CaMKII Kinase Activity
CaMKII kinase activity was measured by detecting the amount of endogenous CaMKII that associates to GST-HDAC4 419-670 or by radioactive kinase assay using32P-ATP.10 A detailed description can be found in Methods in the online-only Data Supplement.
Gene Expression Analysis
The RNA isolation and quantitative RT-PCR as well as the primer sequences used in this study can be found in Methods in the online-only Data Supplement.
Culture of Primary Cardiomyocytes
Adenoviruses for gene transfer into cardiomyocytes were produced as described in Methods in the online-only Data Supplement. Neonatal mouse ventricular myocytes (NMVMs) and adult mouse ventricular myocytes were isolated from wild-type and mutant mice, and neonatal rat ventricular myocytes were isolated from Wistar rats (Charles River). A detailed description is included in Methods in the online-only Data Supplement.
Epifluorescence Microscopy
Freshly isolated adult mouse ventricular myocytes were incubated with Fura-2 AM or Indo-1 AM and Ca2+ transients as well as SR Ca2+ load were measured. Adult mouse ventricular myocytes were transilluminated by red light (>650 nm) to visualize sarcomeres, and fractional shortening was measured. A detailed description is included in Methods in the online-only Data Supplement.
Statistical Analysis
Data are summarized as mean±SEM. Statistical analysis was performed with the Graph-Pad Prism Software Package Version 5.0 (GraphPad Inc). When 2 groups were compared, we used Wilcoxon–Mann–Whitney U tests. When >2 groups were compared, a Kruskal–Wallis test was applied. When we obtained a significant P value we continued with pair-wise comparisons using Wilcoxon–Mann–Whitney U tests according to the closed testing principle. A value of P<0.05 was considered statistically significant.
Results
CaMKIIδ and CaMKIIγ Contribute Redundantly to Cardiac CaMKII Activity
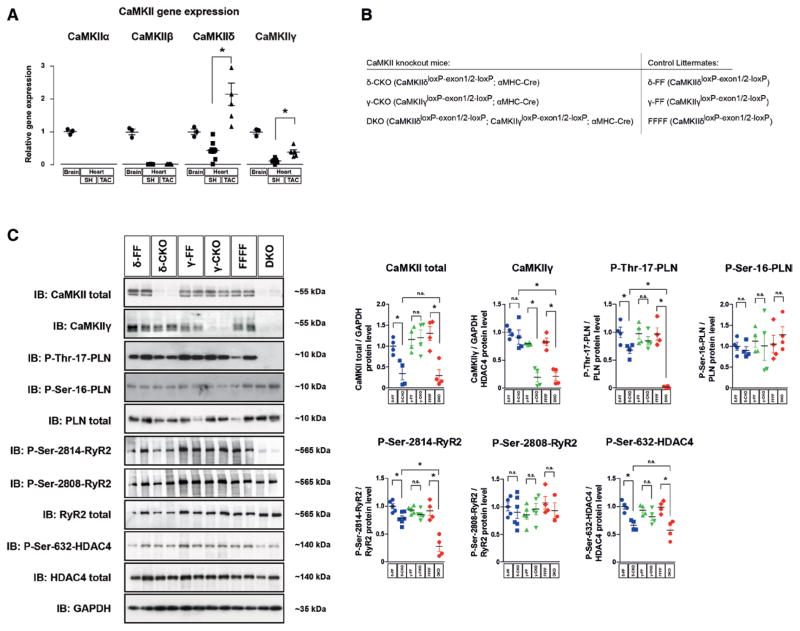
Gene-expression analysis of the 4 CaMKII genes (CaMKIIα, CaMKIIβ, CaMKIIδ, and CaMKIIγ) in RNA samples from healthy and diseased wild-type mouse hearts was performed in relation to the expression levels in the brain. In accordance with others,4 we revealed that besides CaMKIIδ, CaMKIIγ is also expressed in the heart, whereas CaMKIIα and CaMKIIβ are not (Figure 1A). Moreover, both genes were upregulated in diseased hypertrophic hearts after transverse aortic constriction (TAC)-induced pressure overload of the left ventricle. Thus, we took advantage of 2 conditional CaMKII knockout models that we developed previously,8,26 and generated cardiomyocyte- specific CaMKIIδ (δ-CKO), CaMKIIγ (γ-CKO), and CaMKIIδ/CaMKIIγ DKO (Figure 1B). As controls, αMHC-Cre negative littermates (δ-FF, γ-FF, and FFFF) were used. Western blot analysis using an antibody that was shown to recognize at least CaMKIIδ and CaMKIIγ8,26 confirmed a marked loss of CaMKII in cardiac extracts of δ-CKO and DKO (Figure 1C). With this antibody, CaMKII was almost not detectable in δ-CKO, suggesting (under the assumption that the antibody has the same affinity to CaMKIIγ as to CaMKIIδ) that CaMKIIδ is largely more abundant than CaMKIIγ in the heart (Figure 1C, see also Figure IA in the online-only Data Supplement). A CaMKIIγ-specific antibody could detect CaMKIIγ in δ-CKO but not in γ-CKO and DKO mice, suggesting that CaMKIIγ could compensate for the loss of CaMKIIδ in δ-CKO mice. Notably, phosphorylation of the CaMKII target site of PLB Thr17 was only slightly reduced in δ-CKO mice, whereas it was completely abolished in DKO mice, confirming that CaMKIIδ and CaMKIIγ exert redundant roles and largely compensate for each other. As a control, phosphorylation at the protein kinase A site PLB-Ser16 was not reduced (Figure 1C). Thus, DKO represents a so-far unique model for a complete loss of CaMKII activity in cardiomyocytes in vivo. We also analyzed the phosphorylation level of targets of interest. Consistent with PLB, phosphorylation of RyR2-Ser2814 and HDAC4-Ser632 was decreased moderately in δ-CKO but clearly in DKO animals. Again, phosphorylation at the protein kinase A site RyR2-Ser2808 was not affected by CaMKII deletion (Figure 1C).
Figure 1.
CaMKIIδ and CaMKIIγ contribute redundantly to cardiac CaMKII activity. A, Real-time polymerase chain reaction analysis of cardiac CaMKII isoforms 3 weeks after sham (SH) or transverse aortic constriction (TAC) surgery in wild-type mice. The relative expression levels were normalized to CaMKII in brain tissue. All values are reported as mean±SEM (n≥3, *P<0.05). B, To generate cardiomyocyte-specific conditional CaMKIIδ and γ single knockout (δ-CKO and γ-CKO) and CaMKIIδ/CaMKIIγ double knockout mice (DKO), CaMKIIδloxP-exon1/2-loxP (δ-FF), CaMKIIγloxP-exon1/2-loxP (γ-FF), and CaMKIIδloxP-exon1/2-loxP, CaMKIIγloxP-exon1/2-loxP (FFFF) mice were crossed to transgenic mice harboring Cre-recombinase under the control of the αMHC promoter. C, Western blot analysis using antibodies directed against CaMKII, CaMKIIγ, total PLB, phospho-PLB-Thr17, phospho-PLB-Ser16, total RyR2, phospho-RyR2-Ser2814, phospho-RyR2-Ser2808, total HDAC4, phospho-HDAC4-Ser632, and GAPDH (as loading control). Left ventricular extracts from δ-CKO, γ-CKO and DKO mice and their Cre-negative littermates (δ-FF, γ-FF, and FFFF) were analyzed (n=4 per group). Quantitative analysis of the expression and phosphorylation of the proteins is shown at right. All values are presented as mean±SEM. *P<0.05. n.s. indicates not significant.
CaMKII DKO Mice Are Viable and Do Not Display Cardiac Abnormalities
DKO mice developed normally and showed no increase in mortality or apparent morphological abnormalities. Cardiac size as indicated by heart weight/body weight ratios, ventricular chamber dimensions, or fractional shortening assessed by echocardiography were unchanged in mice at age of 12 weeks (Figure IB in the online-only Data Supplement). To study functional parameters in more detail we used a working heart preparation to standardize pre- and afterload (Figure IC in the online-only Data Supplement). We detected slight improvements in ESPVR (which was not significant in all experimental groups used in this study, see also Figure 3A) as a measure for maximal pressure that can be developed by the ventricle at any given LV volume. Based on the observation that the CaMKII sites on PLB and RyR2 were hypophosphorylated, we measured global parameters of Ca2+ handling (Figure ID and IE in the online-only Data Supplement). However, we did not detect any change in Ca2+ transients, SR Ca2+ content, diastolic Ca2+ concentration, and single-cell fractional shortening, surprisingly indicating that—at least under basal conditions—CaMKII activity is dispensable for intracellular Ca2+ handling and cardiomyocyte contractility.
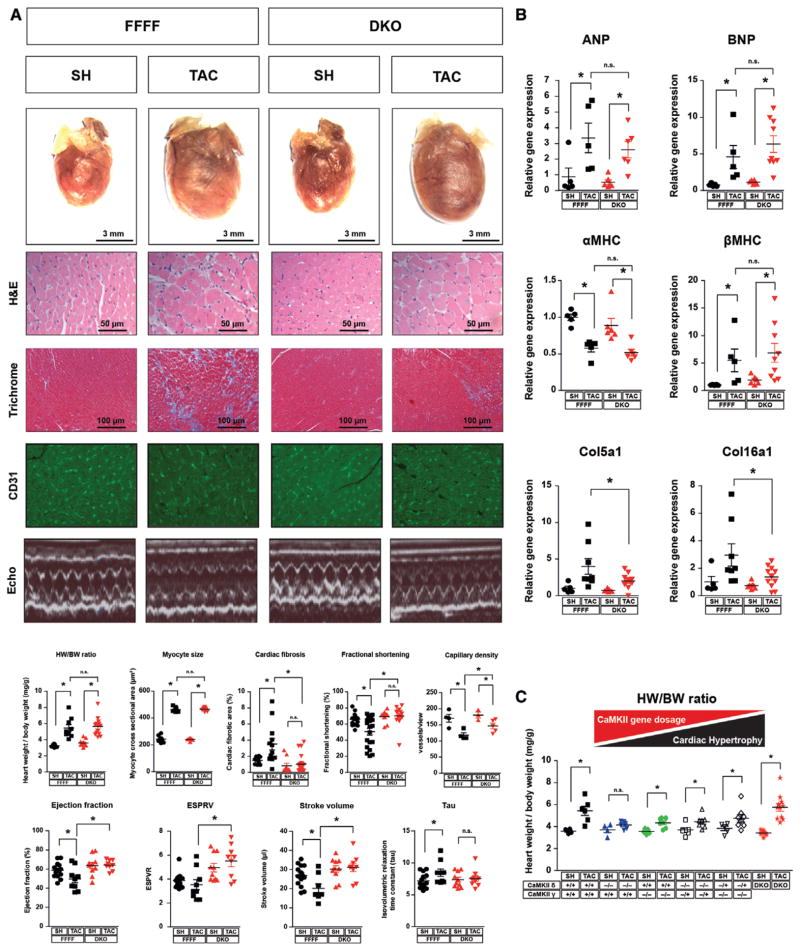
Figure 3.
Dissociation of cardiac dysfunction and interstitial fibrosis from myocardial hypertrophy. A, Double knockout (DKO) and CaMKIIδloxP-exon1/2-loxP, CaMKIIγloxP-exon1/2-loxP (FFFF) mice were randomized to either transverse aortic constriction (TAC) or sham (SH) surgery and euthanized after 3 weeks. Representative images of the total hearts, H&E, Masson trichrome, and CD31 staining, echocardiographic M-modes, and quantification of heart weight/body weight ratios, myocyte size, fibrosis area, capillary density, and fractional shortening are shown (n≥8 per group). Values of left ventricular ejection fraction, isovolumetric relaxation time constant (Tau), end systolic pressure volume relation (ESPVR), and stroke volume measured in a working heart preparation are shown (n≥7 per group). B, Fold-changes in mRNA levels of the hypertrophic markers ANP, BNP, αMHC, and βMHC and fold-changes in collagen expression (n≥5 per group). C, Heart weight/body weight ratios of SH- and TAC-operated mice with the indicated genotypes (n≥4 per group). All values are presented as mean±SEM. *P<0.05. n.s. indicates not significant.
Calcineurin Precedes CaMKII Activation on Pathological Pressure Overload
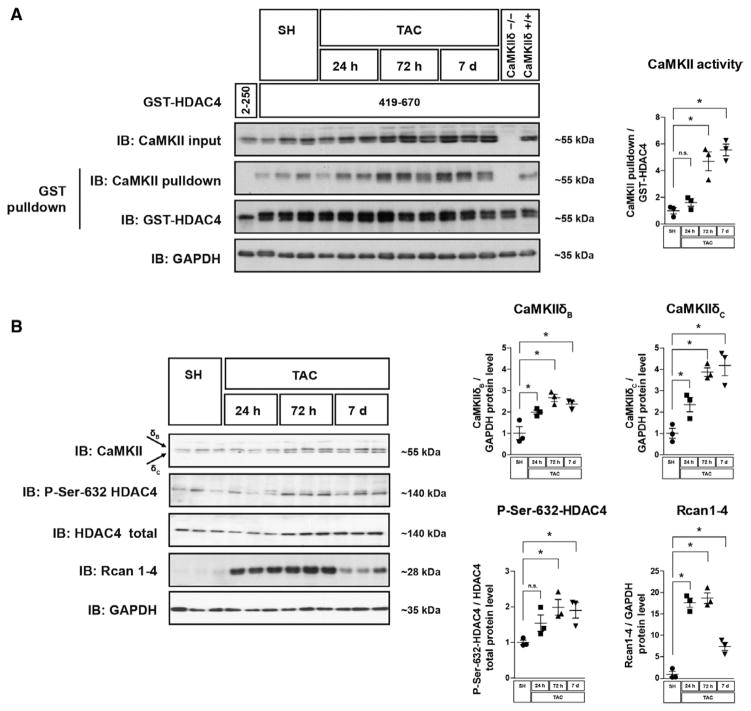
We analyzed the time course of CaMKII and calcineurin activation after TAC (Figure 2A and 2B). Starting at 24 hours after TAC, CaMKII expression (CaMKIIδC > CaMKIIδB) and CaMKII activity, as judged by its activity-dependent binding affinity to HDAC4,10 increased (≈2-fold; Figure 2A). We did not use the usual antibodies recognizing CaMKII autophosphorylation to determine CaMKII activity because in DKO 1 of these antibodies still showed a signal although CaMKII protein was gone (Figures IA and IIA in the online-only Data Supplement). As another indication for CaMKII activation, phosphorylation of HDAC4 at its CaMKII phosphorylation site Ser632 also started to increase 3 days after TAC. In contrast, expression of RCAN1-4, a specific target gene of the calcineurin-NFAT pathway, which is considered as an endogenous calcineurin reporter,29 was dramatically overexpressed (≈17-fold) 1 day after TAC (Figure 2B). These data indicate that CaMKII activation and HDAC4 phosphorylation depend on upstream signals distinct from the upstream signals of calcineurin activation.
Figure 2.
Calcineurin precedes CaMKII activation on pathological pressure overload. Western blot analyses were performed in cardiac extracts from wild-type mice. Organs were harvested at different time points after transverse aortic constriction (TAC) surgery as indicated. A, Western blot analysis after GST-HDAC4 pulldown using GST-HDAC4 419 to 670, which contains a CaMKII-activity dependent binding site. GST-HDAC4 2 to 250, which lacks a CaMKII binding domain, served as a negative control. As an additional negative control, protein lysates from global CaMKIIδ mice vs wild-type mice were used. GST-HDAC4 input was visualized by GST-immunoblotting, associated endogenous CaMKII is visualized by CaMKII-immunoblotting. The degree of CaMKII binding to GST-HDAC4 419-670 is a specific measure for CaMKII activity.10 A quantification of CaMKII binding normalized to the input of GST-HDAC4 is shown. B, Western blot analysis using antibodies directed against CaMKII, HDAC4, phospho-HDAC4-Ser632, RCAN1-4, and GAPDH. Quantitative analysis of the expression and phosphorylation of the proteins is shown. For all experiments n≥3 per group were used. All values are presented as mean±SEM. *P<0.05. n.s. indicates not significant.
Dissociation of Cardiac Dysfunction and Interstitial Fibrosis From Myocardial Hypertrophy
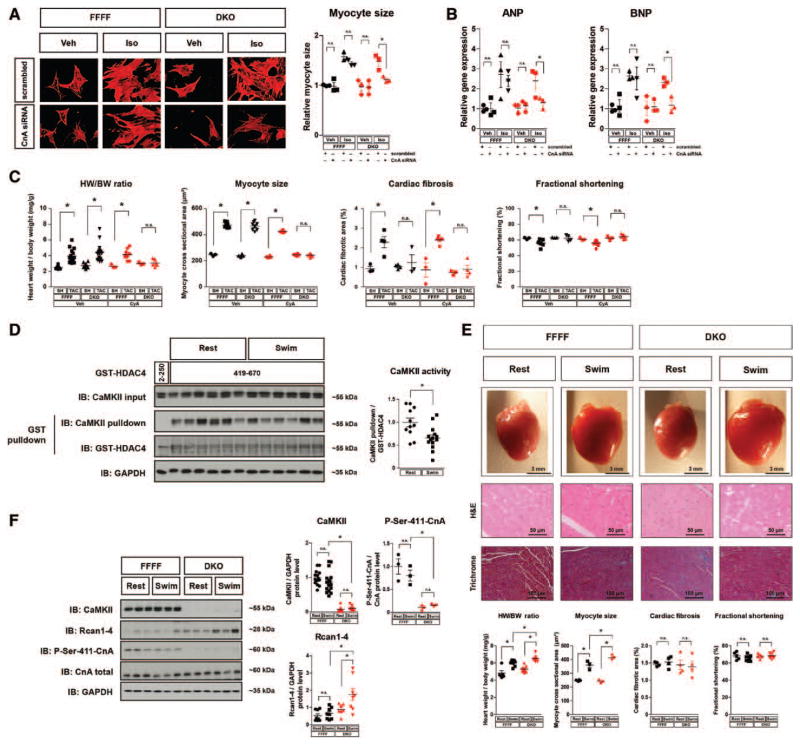
Next, we performed TAC surgeries in DKO and FFFF mice. Strikingly, DKO were protected against cardiac fibrosis, increased caspase 3/7 activity as marker for apoptosis, and systolic dysfunction and display an attenuated rarefication of capillarization (Figure 3A, see also Figure IIIA in the online-only Data Supplement). This protective effect was not associated with changes in intracellular Ca2+ handling or cellular contractility (Figure IIC and IID in the online-only Data Supplement). Surprisingly, TAC still induced cardiac and cardiomyocyte hypertrophy in DKO. Moreover, the gene expression changes of typical fetal genes (ANP, BNP, βMHC) and remodeling marker (αMHC) were similar in DKO and FFFF (Figure 3B). These data indicate a dissociation of cardiac hypertrophy and reactivation of the fetal gene program on one hand from maladaptive cardiac remodeling including interstitial fibrosis, apoptosis, reduced capillarization, and cardiac dysfunction on the other hand. To confirm these data in an independent model, we applied neurohormonal stress to DKO. We injected isoproterenol (Iso) to DKO and FFFF mice. Iso led to cardiac hypertrophy (Figure IVA in the online-only Data Supplement) and fetal gene activation in DKO as observed in FFFF mice (Figure IVB in the online-only Data Supplement). But again and consistent with the TAC data, we observed less cardiac fibrosis, apoptotic markers, and collagen expression in DKO (Figure IVA and IVB in the online-only Data Supplement, see also Figure IIIA in the online-only Data Supplement). These observations were especially surprising in view of our previous finding that global deletion of 1 isoform, CaMKIIδ, attenuated cardiac hypertrophy and fetal gene activation.8 However, in our previous model we could still detect substantial phosphorylation of the typical CaMKII target PLB-Thr17 in the myocardium because CaMKIIγ was not deleted (see also Figure 1C). Thus, we hypothesized that complete CaMKII inhibition might exert dual effects on anti-and prohypertrophic pathways. To substantiate this hypothesis we performed a gene dosage experiment (Figure 3C). Consistent with our previous findings, the homozygous deletion of either CaMKIIδ or CaMKIIγ resulted in a reduction of cardiac hypertrophy on TAC. Paradoxically, the additional heterozygous deletion of the other CaMKII isoform resulted in a less pronounced reduction of cardiac hypertrophy on TAC. Furthermore, the complete cardiac-specific deletion of all 4 CaMKIIδ and CaMKIIγ gene copies (DKO) did not prevent cardiac hypertrophy. We also analyzed the effects of the single deletion of CaMKIIδ and CaMKIIγ in isolated adult cardiomyocytes and could confirm the antihypertrophic effects of CaMKIIδ and CaMKIIγ (Figure IIIC in the online-only Data Supplement). We were also interested whether CaMKIIδ or CaMKIIγ play a specific role for apoptosis because our recent data suggested a specific role of CaMKIIγ for apoptosis at least in macrophages.30 However, TUNEL assays indicated that CaMKIIδ deletion more than CaMKIIγ deletion attenuates cardiomyocyte apoptosis and that the combined deletion is even more effective (Figure IVB in the online-only Data Supplement).
CaMKII Controls the Calcineurin-NFAT Pathway
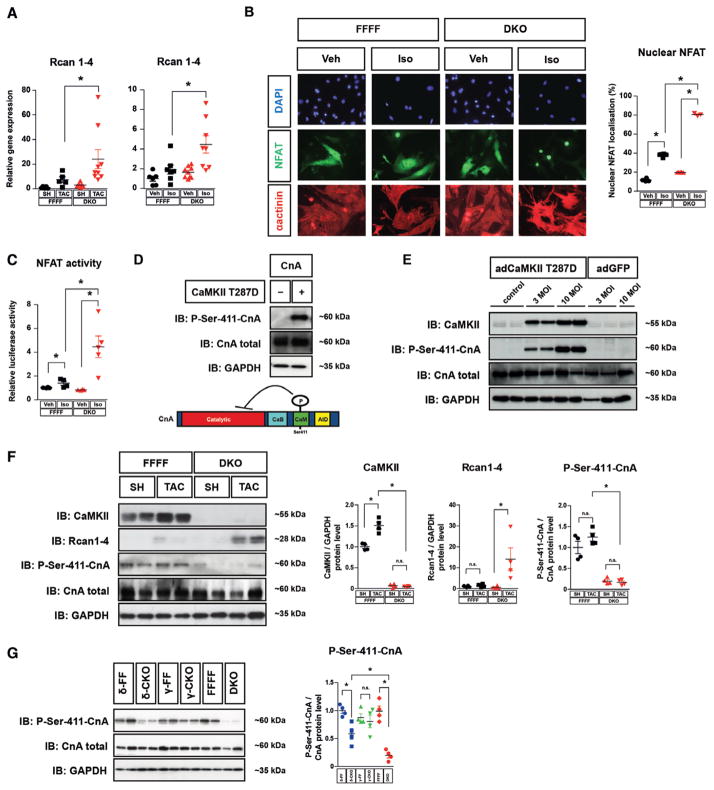
Thus, we hypothesized that complete CaMKII deletion may result in an activation of a prohypertrophic pathway. Therefore, we searched for candidate signaling molecules that are activated by TAC or Iso. Whereas protein kinase D was not activated in DKO after TAC (Figure IIA in the online-only Data Supplement), we found the calcineurin reporter gene RCAN1-4 to be strongly upregulated in DKO mice after TAC and Iso (Figure 4A, Figure IIE in the online-only Data Supplement). This could not be observed after deletion of only 1 CaMKII gene, in particular CaMKIIδ (Figure IIE in the online-only Data Supplement), indicating that complete CaMKII deletion in the heart results in calcineurin activation. Consistently, Iso induced a dramatic increase in nuclear NFAT translocation and NFAT activity in NMVMs from DKO when compared with NMVMs from FFFF (Figure 4B and 4C). In this regard, it was reported that CaMKII directly phosphorylates CnA-Ser411 in vitro but it remained unclear whether this mechanism was of biological significance in vivo.31,32 Thus, using a newly synthesized specific antibody against CnA-Ser411 (Figure 4D) we aimed to detect endogenous CnA phosphorylation. Indeed, overexpression of active CaMKIIδ (T287D) in neonatal rat ventricular myocytes resulted in hyper-phosphorylation of CnA-Ser411 (Figure 4E). In accordance, we found that CnA was strongly hypophosphorylated in cardiac lysates from sham- and TAC-operated DKO hearts, which was associated with RCAN1-4 upregulation (Figure 4F). Moreover, as compared with DKO, CnA-Ser411 hypophosphorylation was less pronounced in cardiac extracts of δ-CKO, and not found in γ-CKO, explaining why calcineurin activity was only strongly increased in DKO but not in the single knockouts (Figure 4G).
Figure 4.
CaMKII controls calcineurin A (CnA) activity. A, Real-time-PCR analysis of RCAN1-4 in hearts from animals with genotypes and treatments as indicated (≥5 per group). B and C, Neonatal mouse ventricular myocytes (NMVMs) from double knockout (DKO) and CaMKIIδloxP-exon1/2-loxP, CaMKIIγloxP-exon1/2-loxP (FFFF) mice were used for cell-based experiments. B, NFAT-GFP was adenovirally expressed in NMVMs. Cells were stained for α-actinin (shown in red) and with DAPI nuclear stain (shown in blue). The percentage of cells in which NFAT-GFP was localized to the nucleus is indicated (>100 cardiomyocytes per well; n≥3 per condition). C, NFAT-luciferase reporter assay in NMVMs (n≥3 per condition). D, Western blot analysis was performed using lysates from COS cells that were transfected with CnA in the absence and presence of constitutive active CaMKIIδ (CaMKII-T287D). A schematic diagram of CnA indicates the position of the CaMKII phosphorylation site at Ser411, which is located within the calmodulin-binding domain of CnA (AID indicates autoinhibitory domain; CaM, calmodulin-binding domain; CnB, calcineurin B-binding domain). E, Adenoviral overexpression of CaMKII-T287D (adCaMKII-T287D) in neonatal rat ventricular myocytes (NRVMs) results in hyper-phosphorylation of CnA-Ser411 compared with noninfected (control) NRVMs and NRVMs infected with GFP. F, Western blot analysis of total CaMKII, RCAN1-4, total CnA, and CnA-Ser411 in cardiac extracts from FFFF and DKO mice 3 weeks after transverse aortic constriction (TAC) or sham (SH) surgery (n≥3 per group). G, Western blot analysis of total CnA and CnA-Ser411 in δ-CKO, γ-CKO or DKO vs their Cre-negative littermates (n=4 per group). For all Western blot experiments, GAPDH was used as loading control. Quantitative analysis of the expression and phosphorylation of the proteins is shown at right (F and G). All values are presented as mean±SEM. *P<0.05. n.s. indicates not significant.
Cardiac Hypertrophy, but Not Dysfunction, Is Controlled by Calcineurin During Pathological and Physiological Hypertrophy in DKO
To test whether cardiac hypertrophy after TAC in DKO depended on calcineurin activation, we performed CnA knockdown experiments in isolated NMVMs of mutant mice. CnA knockdown, which resulted in ≈50% decrease of CnA protein (Figure VA in the online-only Data Supplement), prevented Iso-induced cellular hypertrophy and activation of fetal genes in myocytes derived from DKO but not FFFF (Figure 5A and 5B). We then repeated the TAC experiment in DKO with the calcineurin inhibitor CyA. CyA was shown to inhibit cardiac hypertrophy after TAC in dosages from 12.5 to 50 mg/kg body weight per day.33 Using a dosage of 4 mg/kg body weight per day, which did not affect TAC-induced cardiac hypertrophy in FFFF, we were able to inhibit RCAN1-4 expression as well as cardiac and cardiomyocyte hypertrophy in DKO but not FFFF (Figure 5C and Figure VB in the online-only Data Supplement), indicating that TAC-dependent hypertrophy in DKO depends on calcineurin. As CnA knockdown, CyA attenuated also the expression of fetal genes in DKO (Figure 5B and Figure VB in the online-only Data Supplement). In contrast to the antihypertrophic effects of CyA in DKO, CyA did not affect the protective effects of DKO on cardiac fibrosis or systolic function, providing evidence that—at least in this model—calcineurin-dependent cardiac hypertrophy is not maladaptive on the one hand but also not cardioprotective on the other hand. In line with the latter, we could not accumulate evidence that the potential cardioprotective calcineurin-dependent Akt pathway was activated (Figure VC in the online-only Data Supplement).
Figure 5.
Cardiac hypertrophy but not dysfunction is controlled by calcineurin during pathological and physiological hypertrophy in double knockout mice (DKO). A and B, Neonatal mouse ventricular myocytes (NMVMs) from CaMKIIδloxP-exon1/2-loxP, CaMKIIγloxP-exon1/2-loxP (FFFF) and DKO mice were transfected with two siRNAs directed against CnA or with scrambled siRNA (knockdown efficiency is shown in Figure VA in the online-only Data Supplement). NMVMs were then treated with Iso or vehicle. A, ANP and BNP gene expression in NMVMs after Iso-stimulation (n≥3 per condition). B, Cells were stained for α-actinin (shown in red) and cardiomyocyte size was determined after Iso-stimulation (>100 cardiomyocytes per well; n≥3 per condition). C, FFFF and DKO mice underwent SH or TAC surgery and were treated with cyclosporine A (CyA; 4 mg/Kg/d, 3 weeks IP; n≥7) or vehicle (n≥3) as indicated. CyA treatment was started at the day of transverse aortic constriction (TAC) or sham (SH) surgery. Three weeks later the mice were euthanized. Heart weight/body weight ratios, myocyte cross sectional areas, quantification of cardiac fibrosis from Masson trichrome–stained myocardial sections, and fractional shortening assessed by echocardiography are shown. D–F, FFFF and DKO mice were randomly assigned to a 14-day swimming or resting group, respectively. D, Cardiac CaMKII activity was determined in heart lysates by GST-HDAC4 pulldown using GST-HDAC4 419-670 (GST-HDAC4 2–250 served as specificity control). A quantification of CaMKII-activity dependent binding as measure for CaMKII activity is given (n≥11 per group). E, Representative images of whole hearts as well as H&E and Masson trichrome staining of left ventricular wall sections. Quantification of heart weight/body weight ratios, myocyte size, fibrosis area, and fractional shortening (n≥3 per group). F, Western blot analysis using antibodies directed against CaMKII, total CnA, phospho-CnA-Ser411, RCAN1-4, and GAPDH. Quantitative analysis of the expression and phosphorylation of the proteins is shown (n≥7 per group). All values are presented as mean±SEM. *P<0.05. n.s. indicates not significant.
To clarify the role of the CaMKII-calcineurin pathway in physiological hypertrophy, we performed endurance exercise training by a swimming protocol in DKO and FFFF mice (Figure 5D and 5F). Interestingly, whereas CaMKII protein levels were not altered after swimming in control mice (FFFF), CaMKII activity decreased about 30% (Figure 5D). Remarkably, we found exaggerated heart weight/body weight ratios and increased myocyte size in DKO mice after swimming, which was associated with increased expression of the calcineurin target gene RCAN1-4 (Figure 5E and 5F). Again, despite calcineurin activation and increased cardiac hypertrophy no signs for systolic cardiac dysfunction were detected, indicating that activation of endogenous calcineurin is not maladaptive.
Discussion
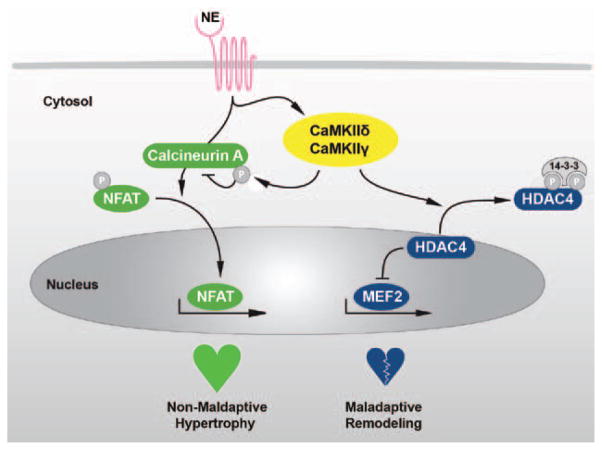
By generating mice lacking the 2 cardiac CaMKII isoforms δ and γ we introduce a new loss-of-function model with the deletion of both cardiac CaMKII isoforms in cardiomyocytes, and demonstrate that CaMKII—under unstressed conditions—is dispensable in cardiomyocytes with regard to integrity, growth, cardiomyocyte contractility, and Ca2+-handling. This is somewhat surprising in light of numerous reports about CaMKII functions. But from the drug developer’s point of view, this indicates that CaMKII inhibition does not exert major unwanted side effects on basal cardiac function. Furthermore, we show that—on pathological stress conditions—CaMKII plays a dual role in the regulation of adverse cardiac remodeling (Figure 6). On the one hand, CaMKII transduces maladaptive signaling. On the other hand, CaMKII negatively regulates calcineurin-dependent cardiac hypertrophy in vivo.
Figure 6.
Schematic model. Stimulation of β-adrenergic receptors by norepinephrine (NE) leads to an activation of the 2 Ca2+-dependent pathways calcineurin and CaMKII (mediated by the isoforms CaMKIIδ and CaMKIIγ). Activated CaMKII phosphorylates HDAC4, which leads to a translocation of HDAC4 from the nucleus to the cytosol. Thereby, the repressive effect of HDAC4 on transcription factors as myocyte enhancer factor 2 (MEF2) is interrupted and a maladaptive remodeling gene program is initiated. As a second mechanism, CaMKII phosphorylates calcineurin A (CnA), which leads to repression of the calcineurin-NFAT pathway and thereby acts antihypertrophic. Unexpectedly, the endogenous calcineurin-NFAT driven prohypertrophic pathway is not maladaptive.
Redundancy of CaMKIIδ and CaMKIIγ
Because of a lack of loss-of-function models with the deletion of both cardiac CaMKII isoforms, it was so far not possible to study all essential roles of CaMKII in myocardial disease. Moreover, most of the reported CaMKII studies used nongenetic approaches such as overexpression of peptides or pharmacological compounds such as KN-93. However, off-target effects (as, eg, described on protein kinase D or ion channels [eg, L-type Ca2+ channels])8,34,35 could have complicated the conclusions. Therefore, we focused first on the development of a specific and complete genetic mouse model, in which the 2 CaMKII genes that are expressed in cardiomyocytes are deleted. We found that CaMKIIδ and CaMKIIγ compensate for each other and act redundantly on the phosphorylation of PLB-Thr17, RyR2-Ser2814, CnA-Ser411, and HDAC4-Ser632. In contrast to previously described approaches aiming at inhibiting CaMKII,7,8,17,18,37 the new DKO model results in profound hypophosphorylation of these typical CaMKII phosphorylation targets.
CaMKII Controls Calcineurin Signaling
Previous data from our laboratory showed that homozygous CaMKIIδ knockout mice are protected against cardiac hypertrophy and interstitial fibrosis.8 Here we demonstrate that the loss of >2 cardiac CaMKII gene copies unmasks a dual role of CaMKII in regulating cardiac hypertrophy. DKO mice appear to develop a similar hypertrophic response as FFFF littermates after TAC and Iso, but histological, gene expression, and biochemical analyses revealed that cardiac hypertrophy in DKO depends on activation of endogenous calcineurin. CaMKII phosphorylates CnA-Ser411, which was shown to lead to a decrease in its phosphatase activity.31,32 Here, we show that this phosphorylation event occurs in vivo and in a CaMKII-dependent manner. Moreover, we found CnA-Ser411 hypo-phosphorylation to be associated with cardiac hypertrophy after pathological and physiological stress. Importantly and in contrast to current paradigms, the data of this study suggest that antihypertrophic effects are not required for an improved cardiac function. Moreover, a vast amount of literature suggested that calcineurin induces pathological cardiac hypertrophy, ultimately leading to heart failure.20,21,37,38 However, the latter studies used forced overexpression of a truncated CnA construct lacking the regulatory domain that is phosphorylated by CaMKII, complicating the interpretation of these findings. A CnAβ loss-of-function study seemed to prove the idea that calcineurin mediates pathological cardiac hypertrophy, but this study focused mainly on cardiac hypertrophy and not on other aspects of pathological remodeling such as cardiac dysfunction.23 In fact, histological analyses did not show a protection against cardiac fibrosis in CnAβ null mice.23 In the present study we identify DKO mice as a model with activation of endogenous calcineurin. We challenge current paradigms and conclude that—at least in the absence of CaMKII signals—activation of endogenous calcineurin is not maladaptive. Support comes from a recent study, demonstrating that calcineurin induces physiological hypertrophy in pregnant mice.39 Moreover, Heineke et al40 suggested that calcineurin exerts cardioprotective functions under certain conditions: hearts of transgenic mice with mild overexpression of CnA were protected in a murine model of dilated cardiomyopathy. Thus it is tempting to speculate that calcineurin activation in DKO contributes to the observed cardioprotective effects on TAC. Because it was suggested that the splice variant CnAβ1 exerts cardioprotection via Akt,25 we measured Akt phosphorylation. But we found no changes. We cannot completely rule out that other calcineurin-dependent pathways contribute to the cardioprotective effects but our data show that CyA-treatment after TAC in DKO did not lead to cardiac dysfunction. Thus, we conclude that—in the setting of the current study—CaM-KII-dependent but calcineurin-independent processes mediate pathological remodeling.
CaMKII Is Required for Pathological Remodeling but Not Cardiac Hypertrophy and Fetal Gene Expression
The question arises of how CaMKII regulates pathological remodeling, especially cardiac fibrosis. We have shown that HDAC4 transmits CaMKII signals to the cardiac genome via MEF2.8,10,13 Because gene deletion and overexpression studies revealed that MEF2D primarily drives cardiac fibrosis and dysfunction rather than cardiac hypertrophy,41 the CaMKII-HDAC4-MEF2 pathway is a likely pathway leading to adverse cardiac remodeling (see model in Figure 6). Likewise, HDAC4 hypophosphorylation was associated to the protective situation in DKO mice. But it was remarkable that DKO mice were not protected against reactivation of certain fetal genes including ANP, BNP, and βMHC. It is important to note that fetal gene activation is suggested to be driven not only by MEF2 but also NFAT.42 Thus, we speculate that overactivation of the calcineurin-NFAT pathway in DKO compensates for the attenuation of the CaMKII-HDAC4-MEF2 pathway with regard to ANP, BNP, and βMHC but not for attenuation of yet unidentified genes that cause cardiac fibrosis. Natriuretic peptides are actually known not to be causative but rather to be adaptive to combat the hemodynamic load and oxidative capacity of muscle cells.43,44 On the other hand, MEF2-dependent genes that cause interstitial fibrosis are not clearly described yet. Taken together, our data indicate that fetal genes associated with calcineurin-dependent hypertrophy do not cause cardiac dysfunction. Likewise, in a model of physiological hypertrophy we found no activation of CaMKII but activation of calcineurin-NFAT signaling without signs of pathological remodeling.
CaMKII Is Dispensable for Intracellular Ca2+ Handling Under Unstressed Conditions
Another puzzling observation of this study was the lack of effect on Ca2+ handling, although profound changes on phosphorylation on RyR2 and PLB were detected. We speculate that the lack of CaMKII phosphorylation on Ca2+ handling proteins might be compensated by other mechanisms such as protein kinase A–mediated phosphorylation, although we could not detect significant increases in phosphorylation at the protein kinase A sites PLB-Ser16 or RyR2-Ser2808. Knock-in mouse models with a specific mutation of these sites would clarify the specific role of CaMKII phosphorylation at PLB-Thr17 and RyR2-Ser2814. Indeed, Respress et al6 described RyR2-2814-S/A knock-in mice. Consistent with our data, they also observed an unaltered SR Ca2+ content, Ca2+ transient decay, and Ca2+ sparks at baseline, but they discovered that in the situation of heart failure, mutant mice were protected. Thus, further experiments are warranted to investigate DKO mice and Ca2+ handling in models of severe heart failure. However, it appears unlikely that an attenuation of disturbed Ca2+ handling contributes to the improved cardiac function as observed in DKO after TAC in the present study.
Summary
Taken together, we show that CaMKII is a key driver of maladaptive cardiac remodeling. This study describes maladaptive cardiac remodeling as a process of interstitial fibrosis and cardiac dysfunction but not cardiac hypertrophy and activation of commonly measured fetal genes such as ANP and BNP. Under certain conditions, CaMKII inhibits cardiac hypertrophy by a previously underestimated cross talk mechanism to calcineurin. In the absence of CaMKII signals, calcineurin does not seem to contribute to maladaptive cardiac remodeling, highlighting CaMKII and not calcineurin as a promising drug target to combat heart failure.
Supplementary Material
Clinical Perspective.
Cardiac remodeling during heart failure is usually described by a combined appearance of myocardial hypertrophy, activation of a so-called fetal gene program, cell death, and interstitial fibrosis. Ca2+-dependent signaling pathways including CaMKII and calcineurin were both proposed to play pivotal roles in adverse cardiac remodeling. By generating mice lacking the 2 cardiac CaMKII isoforms δ and γ in cardiomyocytes, we introduce a new specific and complete CaMKII loss-of-function model, and demonstrate that CaMKII—under unstressed conditions—is dispensable in cardiomyocytes with regard to integrity, growth, cardiomyocyte contractility, and Ca2+ handling. Furthermore, we show that—on pathological stress conditions— CaMKII plays a dual role in the regulation of cardiac remodeling. On the one hand, CaMKII mediates cell death, cardiac fibrosis, and left ventricular dysfunction. On the other hand, CaMKII inhibits cardiac hypertrophy and expression of certain fetal genes such as ANP and BNP. The latter is mediated through a previously underestimated cross talk to calcineurin. Importantly, in the absence of CaMKII, calcineurin does not contribute to adverse cardiac remodeling. Taken together, these data indicate that inhibition of CaMKII but not calcineurin is a promising therapeutic strategy to combat heart failure.
Acknowledgments
We thank Lonny Jürgensen, Jutta Krebs, Sylvia Katz, Claudia Heft, Michaela Oestringer, and Claudia Liebetrau for their excellent technical assistance. We thank Derk Frank for RCAN PCR primer. We thank David Stanmore for editing the manuscript. We thank Lorenz Uhlmann for statistical advice.
Sources of Funding
Drs Backs, Katus, Dobrev, and Maier were supported by the DZHK (Deutsches Zentrum für Herz-Kreislauf-Forschung - German Center for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research). Dr Backs was supported by the Deutsche Forschungsgemeinschaft (BA 2258/2-1, SFB 1118) and by the European Commission (FP7-Health-2010; MEDIA-261409). Dr Maier was supported by the Deutsche Forschungsgemeinschaft (SFB 1002, IRTG 1816) and the Fondation Leducq. Dr Lehman is recipient of a HRCMM (Heidelberg Research Center for Molecular Medicine) career development fellowship. Dr Kreusser was supported by a research grant from the Ernst-und-Berta-Grimmke foundation and by a Young Investigator grant of the University of Heidelberg. Dr Dobrev was supported by a grant from Foundation Leducq (07CVD03). Dr Maack was supported by the Deutsche Forschungsgemeinschaft (SFB 894 and Heisenberg Programme).
Footnotes
Disclosures
None.
References
- 1.Vitali E, Colombo T, Bruschi G, Garatti A, Russo C, Lanfranconi M, Frigerio M. Different clinical scenarios for circulatory mechanical support in acute and chronic heart failure. Am J Cardiol. 2005;96(12A):34L–41L. doi: 10.1016/j.amjcard.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 2.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 3.Zarain-Herzberg A, Fragoso-Medina J, Estrada-Avilés R. Calcium-regulated transcriptional pathways in the normal and pathologic heart. IUBMB Life. 2011;63:847–855. doi: 10.1002/iub.545. [DOI] [PubMed] [Google Scholar]
- 4.Colomer JM, Mao L, Rockman HA, Means AR. Pressure overload selectively up-regulates Ca2+/calmodulin-dependent protein kinase II in vivo. Mol Endocrinol. 2003;17:183–192. doi: 10.1210/me.2002-0350. [DOI] [PubMed] [Google Scholar]
- 5.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 6.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, Dealmeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH. Role of ryr2 phosphorylation at s2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Brown JH, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282:35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 10.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad S, Kunhi M, Little GH, Bai Y, An W, Bers D, Kedes L, Poizat C. Nuclear CaMKII enhances histone H3 phosphorylation and remodels chromatin during cardiac hypertrophy. Nucleic Acids Res. 2013;41:7656–7672. doi: 10.1093/nar/gkt500. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Little GH, Bai Y, Williams T, Poizat C. Nuclear calcium/calmodulin-dependent protein kinase IIdelta preferentially transmits signals to histone deacetylase 4 in cardiac cells. J Biol Chem. 2007;282:7219–7231. doi: 10.1074/jbc.M604281200. [DOI] [PubMed] [Google Scholar]
- 13.Hohl M, Wagner M, Reil JC, Müller SA, Tauchnitz M, Zimmer AM, Lehmann LH, Thiel G, Böhm M, Backs J, Maack C. HDAC4 controls histone methylation in response to elevated cardiac load. J Clin Invest. 2013;123:1359–1370. doi: 10.1172/JCI61084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreusser MM, Backs J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front Pharmacol. 2014;5:36. doi: 10.3389/fphar.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, Belke DD, Dillmann WH, Rogers TB, Schulman H, Ross J, Jr, Brown JH. The cardiac-specific nuclear delta(B) isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 17.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 19.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 20.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, Tannous P, Burchfield JS, Czubryt M, Backs J, Olson EN, Rothermel BA, Hill JA. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res. 2011;109:407–417. doi: 10.1161/CIRCRESAHA.110.228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taigen T, De Windt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci U S A. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 25.Felkin LE, Narita T, Germack R, Shintani Y, Takahashi K, Sarathchandra P, López-Olañeta MM, Gómez-Salinero JM, Suzuki K, Barton PJ, Rosenthal N, Lara-Pezzi E. Calcineurin splicing variant calcineurin Aβ1 improves cardiac function after myocardial infarction without inducing hypertrophy. Circulation. 2011;123:2838–2847. doi: 10.1161/CIRCULATIONAHA.110.012211. [DOI] [PubMed] [Google Scholar]
- 26.Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci U S A. 2010;107:81–86. doi: 10.1073/pnas.0912658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 28.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey N, Frank D, Lippl S, Kuhn C, Kögler H, Barrientos T, Rohr C, Will R, Müller OJ, Weiler H, Bassel-Duby R, Katus HA, Olson EN. Calsarcin-2 deficiency increases exercise capacity in mice through calcineurin/NFAT activation. J Clin Invest. 2008;118:3598–3608. doi: 10.1172/JCI36277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto Y, King MM, Soderling TR. Regulatory interactions of calmodulin-binding proteins: phosphorylation of calcineurin by autophosphorylated Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988;85:7001–7005. doi: 10.1073/pnas.85.18.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res. 2009;105:316–325. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 34.Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther. 2006;317:292–299. doi: 10.1124/jpet.105.097618. [DOI] [PubMed] [Google Scholar]
- 35.Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Zhu WZ, Joiner ML, Zhang R, Oddis CV, Hou Y, Yang J, Price EE, Gleaves L, Eren M, Ni G, Vaughan DE, Xiao RP, Anderson ME. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am J Physiol Heart Circ Physiol. 2006;291:H3065–H3075. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 37.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 38.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 39.Chung E, Yeung F, Leinwand LA. Calcineurin activity is required for cardiac remodelling in pregnancy. Cardiovasc Res. 2013;100:402–410. doi: 10.1093/cvr/cvt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heineke J, Wollert KC, Osinska H, Sargent MA, York AJ, Robbins J, Molkentin JD. Calcineurin protects the heart in a murine model of dilated cardiomyopathy. J Mol Cell Cardiol. 2010;48:1080–1087. doi: 10.1016/j.yjmcc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Phan D, van Rooij E, Wang DZ, McAnally J, Qi X, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest. 2008;118:124–132. doi: 10.1172/JCI33255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuwahara K, Nakao K. New molecular mechanisms for cardiovascular disease: transcriptional pathways and novel therapeutic targets in heart failure. J Pharmacol Sci. 2011;116:337–342. doi: 10.1254/jphs.10r28fm. [DOI] [PubMed] [Google Scholar]
- 43.Nishikimi T, Kuwahara K, Nakao K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol. 2011;57:131–140. doi: 10.1016/j.jjcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.