Summary
Background
The visual system is now known to be composed of image-forming and non-image-forming pathways. Photoreception for the image-forming pathway begins at the rods and cones, whereas that for the non-image-forming pathway also involves intrinsically photosensitive retinal ganglion cells (ipRGCs), which express the photopigment melanopsin. In the mouse retina, the rod and cone photoreceptors become light responsive from postnatal day 10 (P10); however, the development of photosensitivity of the ipRGCs remains largely unexplored.
Results
Here, we provide direct physiological evidence that the ipRGCs are light responsive from birth (P0) and that this photosensitivity requires melanopsin expression. Interestingly, the number of ipRGCs at P0 is over five times that in the adult retina, reflecting an initial overproduction of melanopsin-expressing cells during development. Even at P0, the ipRGCs form functional connections with the suprachiasmatic nucleus, as assessed by light-induced Fos expression.
Conclusions
The findings suggest that the non-image-forming pathway is functional long before the mainstream image-forming pathway during development.
Introduction
In mice, the rod and cone photoreceptor cells are born during the embryonic period of development (gestation 21 days) but mature anatomically and functionally in the postnatal retina. Cone cells differentiate at embryonic day 10 (E10) [1, 2], and gene expression of the ultraviolet-sensitive cone opsin has been detected from E15 [3] and of the long-wavelength-sensitive cone opsin from postnatal day 7 (P7) [3, 4]. Rod cells are born at E12 [1, 2], and rod opsin-gene expression begins at P5 [4]. Photoreceptor outer-segment membranes first appear at P4–6 [3, 5] and mature by P20 [3]; this process is mirrored by the accumulation at P6 to P20 of 11-cis retinyl ester, a precursor of 11-cis retinaldehyde, which is required for photopigment function [6]. These developmental events correlate with the earliest recording of retinal photosensitivity at P10, when ON/OFF light responses of the retinal ganglion cells (RGCs) are detectable [7]. Functionality of the rod and cone cells is preceded by spontaneous Ca2+ waves in the ganglion-cell layer [8], a phenomenon thought to be important for activity-dependent retinal and cortical development [9–11].
A novel class of photoreceptor, recruited specifically for non-image-forming irradiance detection, has been recently identified in the inner retina [12, 13]. In mice, these cells comprise a subset of the RGCs (approximatley 1%) that project mainly to the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract [13, 14]. These intrinsically photosensitive RGCs (ipRGCs) depolarize in response to light [12] and form an electrically coupled network in the ganglion-cell layer [15]. Melanopsin, the photopigment for inner-retinal photosensitivity, is expressed in ipRGCs [13, 16]. Heterologously expressed melanopsin forms a fully functional photopigment [17–19], whereas melanopsin knockout mice (Opn4–/–) exhibit attenuated phase shifting [20, 21] and diminished pupillary responses to bright light [22]. All major non-image-forming visual capabilities are lost in mice lacking melanopsin and functional rod and cone cells [23, 24].
The melanopsin gene is expressed from E10.5 [25] during mouse retinal development, coinciding with the emergence of the RGCs (E9) [2, 26]. The retinohypothalamic tract is present at P0 in murine species [27], and, on the basis of Fos induction as a marker of neuronal activity in the SCN, the first signs of functional retinal output have been detected at P4 [28]. These studies would suggest that light responsiveness of the ipRGCs in mice might develop early. Indeed, in rats it has been shown that light stimulates Fos induction in melanopsin-expressing retinal ganglion cells from the day of birth [29] and that photic input to the SCN is achieved from P0 [29–31].
Here, we physiologically characterize ipRGC light responses in the neonatal mouse retina. We find melanopsin-dependent intrinsic light responses in ganglion-cell-layer neurons from the day of birth, albeit with reduced sensitivity. Surprisingly, over 13% of the ganglion-cell-layer neurons were light responsive at P0, and this high level of neonatal photosensitivity was mainly attributed to an overproduction of melanopsin-expressing ganglion cells. We show that the ipRGCs already form functional connections with the SCN at the day of birth, whereas in melanopsin knockout mice, functional innervation of the SCN is delayed until P14, coinciding with the maturation of rod- and cone-cell photosensitivity [7].
Results
Development of Intrinsic Photosensitivity in the Ganglion-Cell Layer
To assess retinal photosensitivity, we employed a fluorescent imaging technique [15]. Retinae (with ganglion-cell side up) loaded with the Ca2+-sensitive indicator FURA-2AM were exposed to 470 nm (2.9 × 1015 photons/s/cm2) stimulation interleaved between image acquisitions for a 1 min period.
Retinal Ca2+ Waves
A technical consideration in the measurement of neuronal responses during early retinal development is the presence of spontaneous correlated waves of cellular Ca2+ influx [8]. We determined the spatiotemporal properties of Ca2+-wave activity in wild-type (Figures 1A–1C; Movies S1 and S2 in the Supplemental Data available with this article online) and melanopsin knockout (Opn4–/–; Figure 1C) mouse strains. At birth (P0–1), the interwave interval in wild-type retinae was 203 ± 17 s (4 retinae), and in Opn4–/– mice it was 169 ± 19 s (6 retinae; p > 0.05 cf. wild-type). An age-dependent increase in the interwave interval for both strains was observed (wild-type P4–5: 307 ± 27 s, 4 retinae; p < 0.05 cf. P0–1. Opn4–/– P2–4: 253 ± 17 s, 3 retinae; p < 0.05 cf. P0–1). To examine inner-retinal light responsiveness independently of the spontaneous activity, we applied 1 min light stimuli during the interwave interval.
Figure 1. Retinal Ca2+ Waves.
(A) Change in FURA-2 fluorescence (images acquired every 2 s) in the ganglion-cell layer of a flat-mounted P4 wild-type retina (first panel) during a Ca2+ wave. The scale bar represents 100 μm.
(B) Traces of the change in fluorescence in the ganglion-cell layer (averaged for 10 cells in a selected region) in a P0 and a P4 wild-type retina over a 15 min period. Spontaneous Ca2+ waves were observed spreading across the retina (see also Movies S1 and S2).
(C) Developmental changes in the interwave interval (mean ± SEM) in wild-type and Opn4–/– retinae from P0 to P5. Significant age-dependent differences in the interwave interval were observed for each strain (p < 0.05).
Wild-Type Mice
We have previously shown that in the adult rodless-coneless (rdrd/cl) mouse retina, 2.7 ± 0.3% of the neurons in the ganglion-cell layer show an increase in intracellular Ca2+ on exposure to 470 nm light [15]. We undertook an investigation of the developmental emergence of this response. We first studied light responsiveness of the retina at P4–5 because the SCN has been reported to receive light input at P4 [28]. Robust light-evoked increases in fluorescence were observed in a significant proportion of the neurons in the ganglion-cell layer. The majority of the light responses were of a “sustained” phenotype, where the fluorescence change remained elevated at the end of the light-stimulation period. “Transient” response phenotypes were also observed, characterized by a single fluorescence transient and rapid decay before the end of the stimulus. Overall, light responses were detected in 5.4 ± 1.4% of the total cells sampled in the ganglion-cell layer (8 retinae; 86 of 1513 cells; 77% sustained, 23% transient; data not shown).
To preclude any input to the ganglion-cell layer from early-functioning rod or cone cells [32, 33], we investigated light responsiveness in the presence of glutamate-receptor analogs. Perfusion of P4–5 isolated retinae with Ringer solution containing DL-AP4 or PDA (general antagonists of ionotropic glutamate receptors) in combination with L-AP4 (agonist of metabotropic glutamate receptors) had no effect on the number of cells responding to light in the ganglion-cell layer (6 retinae; 71 of 1097 cells; data not shown), suggesting that light responses were intrinsic to the cells themselves. Even though the glutamate-receptor analogs did not inhibit light responsiveness, they did moderately suppress Ca2+-wave activity at P4–5 [34].
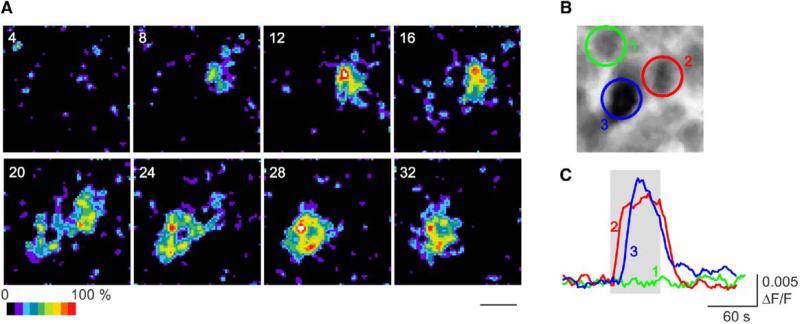
Surprisingly, at earlier stages of development, light responses were also apparent in the inner retina. Even on the day of birth (P0), cells in the ganglion-cell layer showed light-induced increases in fluorescence with a predominantly sustained response phenotype (Figure 2 and Movie S3). Moreover, the percentage of cells that responded to light was even higher at P0–1, being 13.7 ± 2.4% (6 retinae; 192 of 1414 cells; 94% sustained, 6% transient). This is more than twice the percentage observed at P4–5. Again, application of glutamate-receptor analogs had no effect on the number of cells responding to light at P0–1. The data suggest not only that ipRGCs are present at birth, but also that there is a significantly higher percentage of light-responsive cells relative to P4–5 (p < 0.05) and to adult [15].
Figure 2. Light Responsiveness at Birth.
(A) and (B) Series of sequential images (acquired every 4 s) depicting the change in fluorescence in a group of cells (B) during 470 nm stimulation (applied at time point 0; 2.9 × 1015 photons/s/cm2; see also Movie S3). The scale bar represents 10 μm.
(C) Traces of the change in fluorescence in three cells in this preparation. Cell 1 showed no fluorescence change during light stimulation, whereas cells 2 and 3 demonstrated significant light-evoked increases in fluorescence.
Melanopsin Knockout Mice
Intrinsically photosensitive RGCs in adult retina express melanopsin [13], and ablation of the melanopsin gene in Opn4–/– mice results in the complete loss of intrinsic light responses from the ganglion-cell layer [22]. Likewise, in neonatal Opn4–/– mice, we detected no light-evoked increases in FURA-2 fluorescence in the ganglion-cell layer at P0–4 (11 retinae; 0 of 2155 cells; light stimulus of 2.9 × 1015 photons/s/cm2 at 470 nm). This result confirms that the light responses observed in neonatal wild-type retinae originated from melanopsin-expressing ipRGCs.
Developmental Reduction in the Number of ipRGCs Cell-to-Cell Coupling
It is possible that the larger percentage of light-responsive cells at birth is due to greater electrical coupling between ipRGCs and other ganglion/amacrine cells. We therefore recorded the percentage of cells in the ganglion-cell layer that were light responsive before, during, and after the application of 10 μM carbenoxolone, a gap-junction blocker (Figure 3A). Before drug application, 90 of 485 cells in P0–1 retinae responded to light stimulation (2 retinae; 2.9 × 1015 photons/s/cm2 at 470 nm). In the presence of carbenoxolone, the number of light-responsive cells was reduced by 33% (60 of 485 cells). At P4–5, 15 of 320 cells (2 retinae) in the ganglion-cell layer responded to light stimulation, and this was reduced by 44% (8 of 320 cells) during carbenoxolone treatment (P0–1 cf. P4–5: p > 0.05). Thus, whereas electrical coupling does exist in the neonatal retina, it cannot account for the higher percentage of light-responsive cells at P0–1 relative to P4–5. Interestingly, in adult retinae, 56% of the light responses were due to electrical coupling [15].
Figure 3. Developmental Loss of Light-Responsive Cells.
(A) Proportion of cells (mean ± SEM) that were light responsive before (control), during (carb.), and after (recovery) application of the gap-junction blocker carbenoxolone (10 μM) in P0–1 and P4–5 retinae. There was a 33% loss in light-responsive cells at P0–1 and a 44% loss at P4–5 during drug treatment.
(B) Typical examples of melanopsin expression in P0, P4, P14, and adult retinae. The scale bar represents 50 μm.
(C) The number of melanopsin-positive cells per mm2 retina (mean ± SEM) has been plotted against developmental age. An overproduction of melanopsin-positive cells in the early neonate was observed. There is a significant difference in the number of melanopsin-positive cells at P0 or P4 relative to P14 levels (one-factor ANOVA, F3,6 = 10.874, p < 0.01, and post hoc Fishers LSD test: P14 versus P0, p < 0.05; P14 versus P4, p < 0.01) but no significant difference in melanopsin expression between P14 and adult retinae (p > 0.05).
Melanopsin Expression
During vertebrate retinal development, there is a general overproduction of the ganglion cells [2, 35]. Ganglion-cell genesis is completed prenatally [26] and followed by a period of cell death between P2 and P6 [36, 37]. We determined the density of melanopsin-expressing cells in the neonatal retina. Using immunohistochemistry, we confirmed that melanopsin was present in ganglion-cell-layer neurons from the day of birth (Figures 3B and 3C) [25, 38]. The number of melanopsin cells per square millimeter of retina was 181.7 ± 35.1 cells/mm2 at P0 (3 retinae) and 225.6 ± 14.1 cells/mm2 at P4 (3 retinae). We found similar numbers with 3,3′-diaminobenzidine (DAB) immunohistochemistry (data not shown). In comparison, the melanopsin cell density is significantly lower at P14 (61.9 ± 18.1 cells/mm2; 2 retinae; one-factor analysis of variance [ANOVA], F3,6 = 10.874, p < 0.01, and post hoc Fishers LSD test: P0 versus P14, p < 0.05; P4 versus P14, p < 0.01), where it is similar to that in adult (63.3 ± 6.4 cells/mm2; 2 retinae; P14 versus adult, p > 0.05).
The overproduction of melanopsin-expressing ganglion cells in the neonatal retina may contribute to the high proportion of light-responsive cells that we detected with Ca2+ imaging at the same developmental age. It is interesting that melanopsin-expression levels peaked at P4–5 although light responsiveness peaked at P0–1. This may reflect a loss of functionality in some melanopsin cells at P4–5. Because ganglion-cell death during retinal development also peaks at P4–6 [36, 37], it is likely that a proportion of the melanopsin-expressing cells in P4–5 retinae are moving toward apoptotic cell death [39].
Developmental Changes in Light Sensitivity
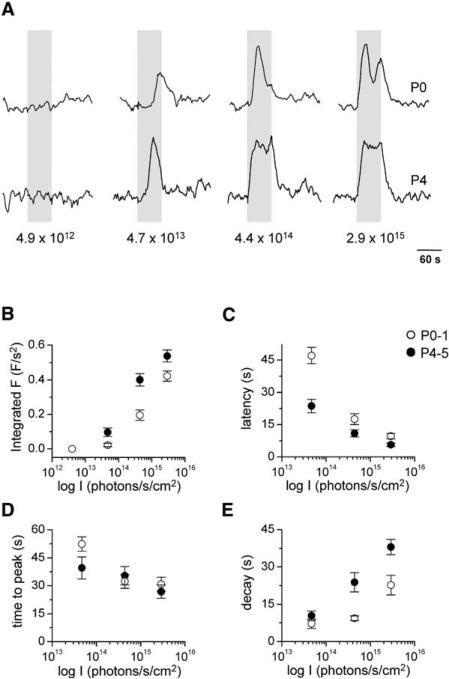
The sustained response phenotype of ipRGCs from P0–1 (4 retinae, 19 cells) and P4–5 (5 retinae, 17 cells) wild-type retinae was characterized after stimulation with 4.9 × 1012–2.9 × 1015 photons/s/cm2 light at 470 nm (Figure 4A).
Figure 4. Developmental Changes in Light Sensitivity.
(A) Typical examples of the change in fluorescence in a cell from a P0 retina and in a cell from a P4 retina after application of increasing intensities of 470 nm stimulation. Responses have been normalized to the maximum response amplitude for each cell.
(B) Intensity dependence of the total change in fluorescence during light stimulation in cells from P0–1 (open circle) and P4–5 (closed circle) retinae. Significant differences between the responses from P0–1 and P4–5 cells were observed (p < 0.05 at 4.7 × 1013–2.9 × 1015 photons/s/cm2).
The intensity dependence of the response latency (C), time to peak (D), and response decay (E) are plotted for P0–1 and P4–5 light responses. Significant differences between the P0–1 and P4–5 data were found for the latency (p < 0.05 at 4.7 × 1013–2.9 × 1015 photons/s/cm2) and decay (p < 0.05 at 4.4 × 1014–2.9 × 1015 photons/s/cm2) parameters. There was no age-dependent difference in the time-to-peak data. All data are presented as mean ± SEM.
Intensity-Response Relationship
We have plotted the total change in fluorescence (integrated fluorescence) during light stimulation as a function of the log light intensity in Figure 4B. For both P0–1 and P4–5 cells, the integrated fluorescence increased with light intensity. However, the P0–1 intensity-response relation was shifted to higher intensities relative to the P4–5 relation, revealing a lower light sensitivity at birth (P0–1 cf. P4–5: p < 0.05 at 4.7 × 1013–2.9 × 1015 photons/s/cm2).
The peak response amplitude also increased with light intensity, saturating at ~4.4 × 1014 photons/s/cm2 (data not shown). We found no significant differences between the amplitude data from P0–1 and P4–5 cells. Because the light-sensitive cells were capable of generating larger increases in fluorescence, as observed during Ca2+-wave activity, the peak amplitude attained was not limited by saturation of the fluorescent indicator.
Kinetics of the Light-Response Onset
The time taken to reach 50% of the peak amplitude from the start of the light stimulus was measured (“response latency”). For both P0–1 and P4–5 cells, the response latency decreased with increasing levels of illumination (Figure 4C). However, at each light intensity tested, the average response latency was shorter at P4–5 than at P0–1 (p < 0.05). The time-to-peak amplitude from the start of the light stimulus was also measured (Figure 4D). The time-to-peak parameter was shorter at higher levels of illumination. There was no significant difference between the time-to-peak data from P0–1 and P4–5 cells.
Kinetics of the Light-Response Offset
The “response decay” was measured as the time between the peak amplitude and the initial drop to 50% of the peak amplitude. The light response took longer to decay at higher levels of illumination for both P0–1 and P4–5 cells (Figure 4E). However, light responses from P0–1 cells tended to have a more complex decay component than those from P4–5; after stimulation at 2.9 × 1015 photon/s/cm2, P0–1 responses often had a second peak in fluorescence (e.g., Figure 4A), a feature not observed at P4–5. The decay time at P0–1 was significantly shorter than at P4–5 over the intensity range of 4.4 × 1014–2.9 × 1015 photons/s/cm2 (p < 0.05).
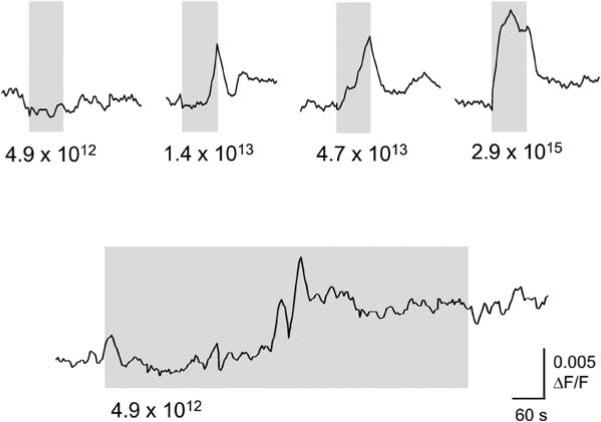
Temporal Integration
The threshold for light responsiveness was at approximately 5 × 1013 photons/s/cm2 (Figures 4A and 4B). However, longer-duration pulses elicited light responses at lower levels of illumination. In Figure 5, the change in fluorescence is shown for a single cell from a P4 retina after successive applications of increasing intensities of 1 min stimuli. The threshold intensity for this cell was at 1 × 1013 photons/s/cm2. A prolonged light stimulus at a lower intensity was able to elicit a response, albeit with a much longer response latency. This response is unlikely to represent spontaneous activity because the recording was made in the presence of synaptic blockers, the kinetics of the initial response are slower than observed during Ca2+-wave activity, and the Ca2+ level remained elevated during stimulation. Thus, ipRGCs can integrate light information over relatively long time periods.
Figure 5. Effect of Increasing Stimulus Duration.
An intensity-dependent increase in fluorescence levels can be seen in a cell from a P4 retina (top panel). The duration of the light pulses was 1 min. At 4.9 × 1012 photons/s/cm2, no light response was observed. Application of this same light stimulus for a longer duration induced a light response after 4 min.
Fos Induction in the SCN of Wild-Type and Opn4–/– Mice during Development
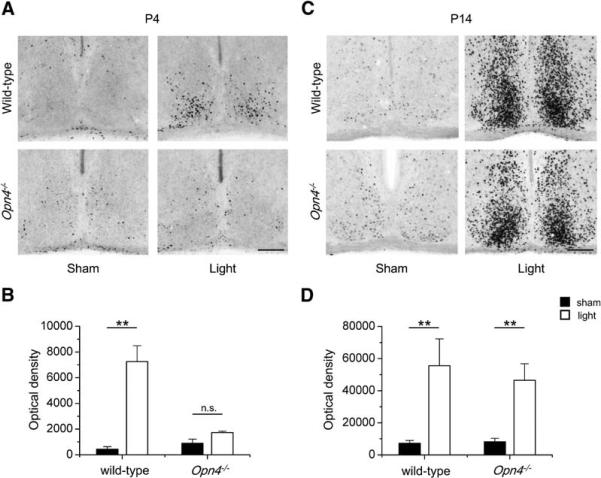
The main target of the ipRGC axonal projections is the SCN. The immediate early gene c-Fos has been used as a marker of light-induced neuronal activity in SCN neurons [40], and in P4 mice, a 60 min light pulse induces Fos expression in this brain region [28]. Using immunohistochemistry, we confirmed that Fos (integrated optical density) is induced in the SCN of P4 wild-type mice (n = 4; sham versus light p < 0.01), but not in the SCN of Opn4–/– mice (n = 4; sham versus light p > 0.05), by a 30 min light pulse administered at 4 hr after lights off (Figures 6A and 6B). For Opn4–/– mice at P4, we tried a light-pulse duration of up to 90 min but still did not detect Fos induction (data not shown), confirming that melanopsin expression is required for functional retinal connections to the SCN in early neonates. In Opn4–/– mice at P14, light did induce Fos expression in the SCN (n = 3; sham versus light, p < 0.001), as was also observed in age-matched wild-type animals (n = 3; sham versus light, p < 0.01; Figures 6C and 6D). This result is consistent with the emergence of functional rod/cone outputs to the SCN at the end of the second postnatal week [7].
Figure 6. Fos Induction in the SCN at P4–5 and P14.
The photomicrographs show Fos immunoreactivity in coronal brain sections containing the SCN of wild-type and Opn4–/– mice at P4 (A) and P14 (C). Animals were maintained on a 12:12 light-dark cycle and a light pulse (2 mW/cm2) or a sham pulse was applied 4 hr after lights off. The scale bar represents 100 μm. Histograms of the integral optical density of Fos (mean ± SEM) in the SCN are shown for wild-type and Opn4–/– mice at P4–5 (B) and P14 (D). Significant differences between light and sham treatments are indicated (** = p < 0.01; n.s. = non significant).
Retinal innervation of the SCN was assessed in mice at P0. At this early age, the melanopsin-expressing ganglion cells (Figure 7A) send projections mainly to the ventral SCN (Figure 7B), as is revealed by blue X-gal labeling in Opn4–/– animals in which the melanopsin gene is replaced by a tau-LacZ coding sequence. In adult mice, the retinal projection also extends into the dorsal region of the SCN (Figures 7C and 7D). We show that retinal innervation of the SCN is functional in P0 wild-type mice; a 90 min light pulse proved to be consistently effective in inducing Fos in the SCN (n = 4; sham versus light, p < 0.001; Figure 7E), whereas a 30 min light pulse was insufficient (data not shown). Thus, the SCN receives functional retinal connections from melanopsin-expressing ipRGCs on the day of birth.
Figure 7. Functional Retinal Innervation of the SCN at P0.
(A) In P0 Opn4–/– mice (tau-LacZ+/+), retinal ganglion cells that would express melanopsin are labeled with X-gal (blue stain; the scale bar represents 20 μm).
(B) The axons of these ganglion cells form the retinohypothalamic tract and innervate the SCN (coronal brain sections; the scale bar represents 100 μm).
(C) and (D) In adult mice, fewer melanopsin-expressing ganglion cells are present in the retina relative to P0 (the scale bar represents 20 μm), and these neurons send extensive projections to the SCN. D indicates dorsal, V indicates ventral, and the scale bar represents 100 μm.
(E) Photomicrographs showing Fos immunoreactivity in the SCN of P0 wild-type mice after a 90 min light pulse or sham treatment. The scale bar represents 100 μm. The integral optical density of Fos (mean ± SEM) in the SCN of P0–1 wild-type mice has been quantified, and there is a significant difference between the sham and light treatment conditions (p < 0.001).
Discussion
A Functional Photosensitive Non-Image-Forming System Is Present at Birth
The rod and cone photoreceptors that mediate image-forming vision are among the last cells to mature during mouse retinal development [3–5]. Photosensitivity of the image-forming pathway has been detected from P10 [7], just before eye opening (P12–14). In contrast, the melanopsin-expressing ipRGCs that primarily subserve non-image-forming functions are present from E10 [25]. We have shown that these ipRGCs are light responsive from the day of birth (Figure 2), in a period during which it has been widely assumed that there is no functional light detection in the mouse retina. It is interesting that the non-image-forming photoreceptors are functional nearly 2 weeks before eye opening; this may reflect the function of the ipRGCs as irradiance detectors rather than as image-forming photoreceptors.
The ipRGC light responses persisted in the presence of a glutamate-receptor blockade, suggesting that photosensitivity was intrinsic to the ganglion-cell-layer neurons and not mediated via glutamatergic inputs from any early-functioning rod or cone cells [32, 33]. Intrinsic light responses of ganglion-cell-layer neurons were absent in neonatal melanopsin knockout mice, confirming that melanopsin expression is a prerequisite for inner-retinal photosensitivity and also consistent with recent studies that confirm that melanopsin expression alone is sufficient to engender photoresponsiveness [17–19].
It has been previously shown that retinal projections to the SCN (the site of the central circadian pacemaker) are functional from P4 in mice [28]. We show that long-duration light pulses induce Fos expression in the SCN at P0 in wild-type mice (Figure 7E). The need for long-duration stimuli may reflect the lower light sensitivity of the ipRGCs at birth (Figure 4; see Properties of the Inner-Retinal Light Response) and may account for the discrepancy with previous data. Accordingly, in rats, photic induction of SCN Fos is also achieved on the day of birth [29–31]. Importantly, in melanopsin knockout mice, Fos induction was not detectable in the early neonate (Figures 6A and 6B) even though normal axonal projections of melanopsin-expressing retinal ganglion cells to the SCN were observed (Figures 7A and 7B). Light-evoked Fos induction was detected in Opn4–/– mice at P14 (Figures 6C and 6D), when the rod and cone photoreceptor cells have matured and provide a supplementary photic drive to the SCN. The results show that the melanopsin-dependent non-image-forming system is photosensitive and functional before the image-forming pathway assumes any photic capabilities.
Overproduction of ipRGCs in the Neonate
We found that a high proportion of neurons in the ganglion-cell layer were light responsive in the neonatal retina. At P0–1, 13.7% of the cells responded to light stimulation, and this percentage was reduced to 5.4% at P4–5. In the adult retina (via the same technique), 2.7% of the cells in the ganglion-cell layer were light responsive, and over 50% of the light responses were due to functional coupling between ipRGCs and other cells in the inner retina [15]. Application of a gap-junction blocker to neonatal retina determined that the contribution of cell-to-cell coupling to the total number of light-responsive cells was only 33% at P0–1 and 44% at P4–5 (Figure 3A). This would suggest that coupling could not account for the high level of neonatal light responsiveness.
In the developing retina, there is a significant overproduction of ganglion cells until just before birth [26], and this is followed by a phase of cell death from P2 [37] and peaking at P4–6 [36, 37]. In C3H/He mice, 70% of the ganglion cells are lost between birth and adulthood [35]. We show that melanopsin-expressing RGCs are substantially overproduced during early development (Figures 3B and 3C), with a 65% loss of melanopsin-positive cells between P0 and adult, closely mirroring the pattern of total ganglion-cell loss. An increase in the overall ganglion-cell population at P0 should not alter the proportion of cells responding to light; however, we saw a 5-fold difference in the percentage of light-responsive cells between birth and adulthood. The Ca2+ imaging technique permits sampling of only the innermost region of the ganglion-cell layer (closest to the nerve-fiber layer) and therefore may be subject to sampling bias. The results suggest that the high number of light-responsive neurons that we detected in the ganglion-cell layer of neonatal mice may reflect an overproduction of melanopsin-positive ganglion cells. Although it would appear that this is a general feature of ganglion-cell development, it is possible that the high level of neonatal photosensitivity serves a specific purpose (see Significance of Neonatal Photosensitivity).
Properties of the Inner-Retinal Light Response
We observed a developmental increase in light sensitivity of the ipRGCs (Figure 4). Lower sensitivity in the early neonate could be attributed to several factors. First, there may be delayed or reduced expression of the photopigment protein. However, melanopsin RNA [25] and protein (Figures 3B and 3C) [38] are expressed in the neonatal retina. Second, insufficient chromophore may be present. Melanopsin associates with cis-isoforms of retinal, but recent evidence suggests that it may have the capability to function as a bistable pigment and to regenerate its chromophore from all-trans isomers [17, 18]. Although 11-cis retinaldehyde is rate limiting for rod and cone light responsiveness in the early neonatal retina [6, 41], all-trans retinyl esters are present [6], which could facilitate ipRGC light responses. Finally, the reduced light sensitivity at birth may be due to immature expression of a component or components of the phototransduction pathway or to general Ca2+ signaling changes.
A characteristic of the circadian system is its ability to integrate light information over time [42, 43]. The site of photon integration could occur within the photoreceptors and/or in the neural input pathway to SCN. In the present study, we show that ipRGCs themselves, unlike the classical rod and cone photoreceptor cells [44], are able to integrate light information over a period of minutes (Figure 5). This would suggest that the phototransduction pathway in classical photoreceptors and ipRGCs is distinct. Although the native ipRGC phototransduction pathway is yet to be deciphered, recent studies have alluded to an invertebrate-like cascade involving a pertussis-toxin-insensitive G protein coupled to a phospholipase C pathway [18].
Significance of Neonatal Photosensitivity
There are several reasons why neonates may require the capacity for non-image-forming photosensitivity. The presence of these cells may have a functional significance. Early studies have suggested that the maternal circadian system is responsible for coordinating the timing of the neonatal clock [45, 46]. However, the present data suggest that innate responses from ipRGCs may also play a role in non-image-forming irradiance detection in the developing infant. Photic information could be useful for avoidance behavior to prevent exposure of pups to predators. In primates, there is evidence to suggest that non-image-forming light responsiveness is important in early neonates [47, 48].
The emergence of photosensitivity of the ipRGCs may play a role in the development of the SCN and other non-image-forming brain regions just as rod- and cone-mediated visual experience affects development of the visual cortex [49]. Furthermore, because ipRGCs may form indirect connections (via coupling) with ganglion cells that project to “visual” brain centers and indeed may even directly project to the lateral geniculate nucleus [50], these inner-retinal photoreceptors could drive activity-dependent development of both the non-image-forming and image-forming cortical pathways in the early neonate.
Experimental Procedures
Calcium Imaging
Wild-type (C3H/He, wild-type at the rd locus) or melanopsin knockout (Opn4–/– in mixed C57BL/6 and 129/SvJ background) mice at P0 to P5 were enucleated. Isolated retinae were incubated in Ringer solution (119 mM NaCl, 2.5 mM KCl, 1 mM KH2PO4, 1.3 mM MgCl2, 2.5 mM CaCl2, 26.2 mM NaHCO3, and 11 mM D-glucose; equilibrated with 95% O2/5% CO2; [pH 7.4]) containing 10 μM FURA-2AM (Molecular Probes) for 1 hr and subsequently perfused with fresh Ringer. The fluorescence imaging technique and data analysis have been previously described [15]. In brief, the Ca2+-sensitive indicator was excited with ultraviolet light and emitted fluorescence of 510 ± 15 nm was captured with an intensified CCD camera (IPentamax, Princeton Instruments). The drift in the baseline fluorescence was corrected by curve-fitting. Rolling averaging and subtraction were employed for spatial analysis of retinal photosensitivity. Data are presented as mean ± standard error of the mean (SEM) and were analyzed with t tests.
Melanopsin Immunohistochemistry
Wild-type mice (P0, P4, P14, and adult) were enucleated, and the eyes were placed in 4% paraformaldehyde at 4°C for 4 days before washing in 0.1% azide in phosphate buffered saline (PBS). Retinae were dissected, washed in PBS, and incubated with the primary melanopsin antibody (Provencio type UF006; 1:5000 at 4°C for 4 days). After they were rinsed in 0.01 M PBS containing 0.3% Triton-X 100 buffer, retinae were incubated with Alexa fluor 568 nm conjugated to goat anti-rabbit IgG at a dilution of 1:200 for 2 hr. The retinae were mounted onto glass slides, coverslipped with PBS, sealed, and visualized under a fluorescence microscope (Zeiss Axioplan 2). Melanopsin-expressing cells were counted in 12 regions (0.2 mm2) across the retina. Data were analyzed with one-factor analysis of variance (ANOVA) followed by post-hoc Fishers LSD test.
X-gal Labeling
Opn4–/– (tau-LacZ+/+) animals at P0 were anesthetized and then perfused intracardially with 2 ml cold PBS followed by 4 ml 4% paraformaldehyde in PBS. The retina was isolated, placed on a slide, and incubated in staining solution (100 mM phosphate buffer at [pH 7.4], 2 mM MgCl2, 0.01% Na-desoxycholate, 0.02% [Octylphenoxy] polyethoxyethanol [IGEPAL], 5 mM K-ferricyanide, 5 mM K-ferrocyanide, and X-gal [1 mg/ml]; 24 to 30 hr at 37°C in darkness). The whole head was placed in 30% sucrose solution and sectioned with a cryostat to 50 μm thickness, and the sections were incubated in the staining solution (6 to 30 hr at room temperature in darkness) [13].
Fos Induction in the SCN
Wild-type and Opn4–/– mice at P0–1, P4–5, and P14 (kept on a 12:12 light-dark cycle) were given a 30 min or 90 min white-light pulse of 2 mW/cm2 at 4 hr after lights off. Sham pulsed mice were handled in a similar manner but no light pulse was given. Ninety minutes after the beginning of light treatment, animals were deeply anesthetized with a lethal dose of sodium pentobarbitone and perfused transcardially. Brains were dissected, postfixed overnight, and cryoprotected (30% sucrose). Serial coronal brain sections (40 μm) containing the SCN were processed for Fos immunohistochemistry with the primary rabbit anti-Fos antibody (Calbiochem; 1:20,000 at 4°C for 72 hr). The secondary antibody binding and avidin biotin amplification were carried out with the Vectastain ABC Elite kit. Sections were visualized by incubation with 3,-3′ diaminobenzidine-nickel ammonium sulfate (DAB-Ni) and 0.001% H2O2. Images of the SCN sections were captured with a Spot Digital Camera (Diagnostic Instruments), and the integrated optical density of Fos was determined with a computerized image-analysis system [51]. Control slices, for which the primary or secondary antibodies were replaced with normal serum, did not show any label. T tests were used for statistical analysis.
Pharmacology
One hundred micromolars of L-amino-4-phosphonobutyrate (L-AP4; Tocris); 250 μM DL-amino-4-phosphonobutyrate (DL-AP4; Tocris); 10 μM carbenoxolone (Sigma); and 1 mM cis-2,3-piperidinedicarboxylic acid (PDA; Sigma) were added to the retinal perfusate where indicated.
Supplementary Material
Acknowledgments
This work was supported by The Wellcome Trust and in part by NSBRI through NASA NCC 9-58. We thank Dr. Iggy Provencio for providing the melanopsin antibody.
Footnotes
Supplemental Data
Supplemental Data include three movies and are available with this article online at http://www.current-biology.com/cgi/content/full/15/12/1099/DC1/.
References
- 1.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young RW. Cell differentiation in the retina of the mouse. Anat. Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 3.Fei Y. Development of the cone photoreceptor mosaic in the mouse retina revealed by fluorescent cones in transgenic mice. Mol. Vis. 2003;9:31–42. [PubMed] [Google Scholar]
- 4.Bibb LC, Holt JK, Tarttelin EE, Hodges MD, Gregory-Evans K, Rutherford A, Lucas RJ, Sowden JC, Gregory-Evans CY. Temporal and spatial expression patterns of the CRX transcription factor and its downstream targets. Critical differences during human and mouse eye development. Hum. Mol. Genet. 2001;10:1571–1579. doi: 10.1093/hmg/10.15.1571. [DOI] [PubMed] [Google Scholar]
- 5.Olney JW. An electron microscopic study of synapse formation, receptorouter segment development, and other aspects of developing mouse retina. Invest. Ophthalmol. 1968;7:250–268. [PubMed] [Google Scholar]
- 6.Carter-Dawson L, Alvarez RA, Fong S-L, Liou GI, Sperling HG, Bridges CDB. Rhodopsin, 11-cis vitamin A, and interstitial retinol-binding protein (IRBP) during reti nal development in normal and rd mutant mice. Dev. Biol. 1986;116:431–438. doi: 10.1016/0012-1606(86)90144-2. [DOI] [PubMed] [Google Scholar]
- 7.Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 8.Wong RO. Retinal waves and visual system development. Annu. Rev. Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 9.Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40:1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- 11.Wong WT, Wong RO. Rapid dendritic movements during synapse formation and rearrangement. Curr. Opin. Neurobiol. 2000;10:118–124. doi: 10.1016/s0959-4388(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 12.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 13.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provencio I, Cooper HM, Foster RG. Retinal projections in mice with inherited retinal degeneration: Implications for circadian photoentrainment. J. Comp. Neurol. 1998;395:417–439. doi: 10.1002/(sici)1096-9861(19980615)395:4<417::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr. Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 16.Provencio I, Rollag MD, Castrucci AM. Photo-receptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 17.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 18.Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 19.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosen sitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 20.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 21.Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 22.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 23.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 25.Tarttelin EE, Bellingham J, Bibb LC, Foster RG, Hankins MW, Gregory-Evans K, Gregory-Evans CY, Wells DJ, Lucas RJ. Expression of opsin genes early in ocular development of humans and mice. Exp. Eye Res. 2003;76:393–396. doi: 10.1016/s0014-4835(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 26.Drager UC. Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc. R. Soc. Lond. B. Biol. Sci. 1985;224:57–77. doi: 10.1098/rspb.1985.0021. [DOI] [PubMed] [Google Scholar]
- 27.Speh JC, Moore RY. Retinohypothalamic tract development in the hamster and rat. Brain Res. Dev. Brain Res. 1993;76:171–181. doi: 10.1016/0165-3806(93)90205-o. [DOI] [PubMed] [Google Scholar]
- 28.Munoz Llamosas M, Huerta JJ, Cernuda-Cernuda R, Garcia-Fernandez JM. Ontogeny of a photic response in the retina and suprachiasmatic nucleus in the mouse. Brain Res. Dev. Brain Res. 2000;120:1–6. doi: 10.1016/s0165-3806(99)00175-3. [DOI] [PubMed] [Google Scholar]
- 29.Hannibal J, Fahrenkrug J. Melanopsin containing retinal ganglion cells are light responsive from birth. Neurore-port. 2004;15:2317–2320. doi: 10.1097/00001756-200410250-00003. [DOI] [PubMed] [Google Scholar]
- 30.Leard LE, Macdonald ES, Heller HC, Kilduff TS. Ontogeny of photic-induced c-fos mRNA expression in rat suprachiasmatic nuclei. Neuroreport. 1994;5:2683–2687. doi: 10.1097/00001756-199412000-00069. [DOI] [PubMed] [Google Scholar]
- 31.Weaver DR, Reppert SM. Definition of the developmental transition from dopaminergic to photic regulation of c-fos gene expression in the rat suprachiasmatic nucleus. Brain Res. Mol. Brain Res. 1995;33:136–148. doi: 10.1016/0169-328x(95)00117-b. [DOI] [PubMed] [Google Scholar]
- 32.Gunhan E, van der List D, Chalupa LM. Ectopic photoreceptors and cone bipolar cells in the developing and mature retina. J. Neurosci. 2003;23:1383–1389. doi: 10.1523/JNEUROSCI.23-04-01383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araki M, Hanihara T, Saito T. Histochemical observations on unique rod-like cells in the developing retina of the normal rat. J. Neurocytol. 1988;17:179–188. doi: 10.1007/BF01674205. [DOI] [PubMed] [Google Scholar]
- 34.Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J. Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strom RC, Williams RW. Cell production and cell death in the generation of variation in neuron number. J. Neu rosci. 1998;18:9948–9953. doi: 10.1523/JNEUROSCI.18-23-09948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden R, Pinto LH. Developmental genetics of the retina: Evidence that the pearl mutation in the mouse affects the time course of natural cell death in the ganglion cell layer. Exp. Brain Res. 1985;60:79–86. doi: 10.1007/BF00237021. [DOI] [PubMed] [Google Scholar]
- 37.Young RW. Cell death during differentiation of the retina in the mouse. J. Comp. Neurol. 1984;229:362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- 38.Fahrenkrug J, Nielsen HS, Hannibal J. Expression of melanopsin during development of the rat retina. Neuroreport. 2004;15:781–784. doi: 10.1097/00001756-200404090-00008. [DOI] [PubMed] [Google Scholar]
- 39.Pequignot MO, Provost AC, Salle S, Taupin P, Sainton KM, Marchant D, Martinou JC, Ameisen JC, Jais JP, Abitbol M. Major role of BAX in apoptosis during retinal development and in establishment of a functional post-natal retina. Dev. Dyn. 2003;228:231–238. doi: 10.1002/dvdy.10376. [DOI] [PubMed] [Google Scholar]
- 40.Aronin N, Sagar SM, Sharp FR, Schwartz WJ. Light regulates expression of a Fos-related protein in rat suprachiasmatic nuclei. Proc. Natl. Acad. Sci. USA. 1990;87:5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratto GM, Robinson DW, Yan B, McNaughton PA. Development of the light response in neonatal mammalian rods. Nature. 1991;351:654–657. doi: 10.1038/351654a0. [DOI] [PubMed] [Google Scholar]
- 42.Dkhissi-Benyahya O, Sicard B, Cooper HM. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: Temporal summa tion and reciprocity. J. Neurosci. 2000;20:7790–7797. doi: 10.1523/JNEUROSCI.20-20-07790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson DE, Takahashi JS. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;277:R1351–R1361. doi: 10.1152/ajpregu.1999.277.5.R1351. [DOI] [PubMed] [Google Scholar]
- 44.Baumgardt E. Threshold quantal problems. In: Jameson D, Hurvich LM, editors. Hand book of Sensory Physiology. VII. Springer; Berlin: 1972. pp. 29–55. [Google Scholar]
- 45.Reppert SM, Schwartz WJ. Maternal coordination of the fetal biological clock in utero. Science. 1983;220:969–971. doi: 10.1126/science.6844923. [DOI] [PubMed] [Google Scholar]
- 46.Reppert SM, Schwartz WJ. Maternal suprachiasmatic nuclei are necessary for maternal coordination of the developing circadian system. J. Neurosci. 1986;6:2724–2729. doi: 10.1523/JNEUROSCI.06-09-02724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao H, Rivkees SA. The biological clock of very premature primate infants is responsive to light. Proc. Natl. Acad. Sci. USA. 1999;96:2426–2429. doi: 10.1073/pnas.96.5.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mann NP, Haddow R, Stokes L, Goodley S, Rutter N. Effect of night and day on preterm infants in a newborn nursery: Randomised trial. Br. Med. J. (Clin. Res. Ed.) 1986;293:1265–1267. doi: 10.1136/bmj.293.6557.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr. Opin. Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 50.Dacey DM, Liao HW, Peterson BB, Robsinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 51.Rieux C, Carney R, Lupi D, Dkhissi-Benyahya O, Jansen K, Chounlamountri N, Foster RG, Cooper HM. Analysis of immunohistochemical label of Fos protein in the suprachiasmatic nucleus: Comparison of different methods of quantification. J. Biol. Rhythms. 2002;17:121–136. doi: 10.1177/074873002129002410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.