Abstract
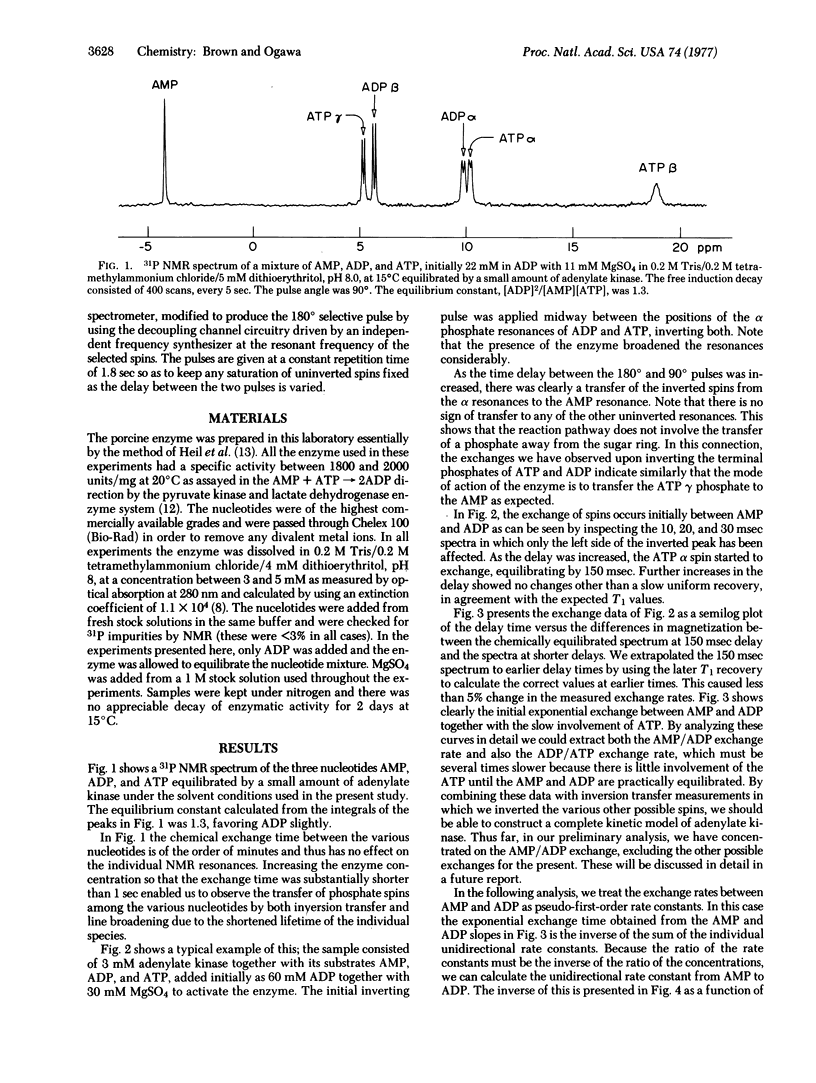
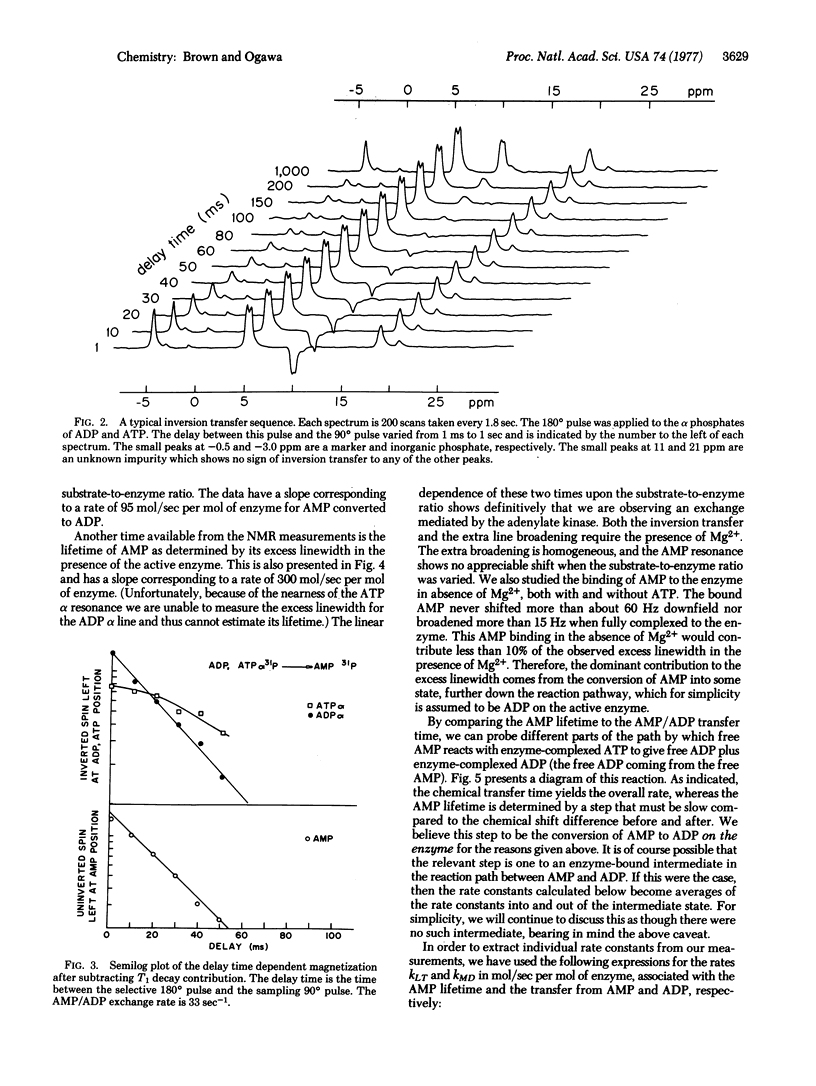
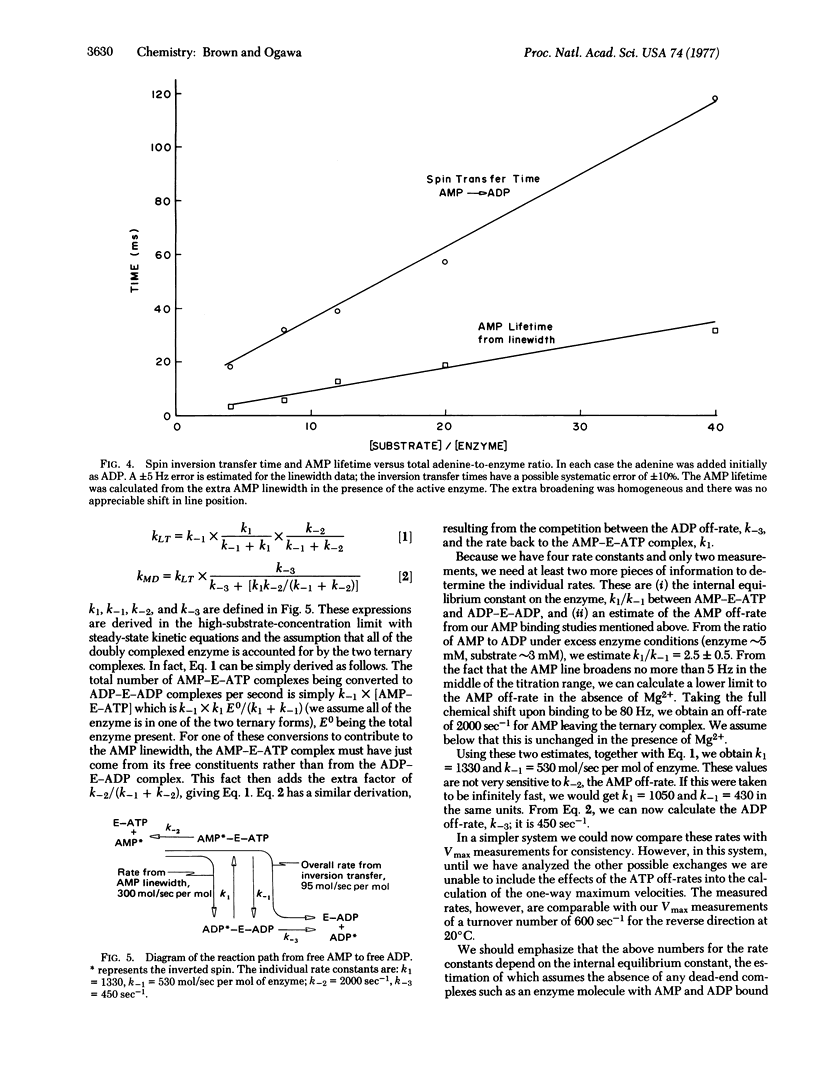
By using 31P double-resonance nucelar magnetic resonance, preliminary kinetic measurements were made on the equilibrium reaction 2ADP in equilibrium ATP +AMP catalyzed by adenylate kinase. The double-resonance method used consisted of selectively inverting the spin population in a particular chemical environment and observing the transfer of the inverted spin to another chemical environment. The chemical transfer time between AMP and ADP, free in solution, was proportional to the substrate-to-enzyme ratio, and a transfer rate of 95 mol of AMP/see per mol of enzyme was obtained. In the same series of experiments, the life-time of AMP in solution was determined from the extra broadening of its resonance line due to the active enzyme. This gave a rate 3.2 times faster than the overall transfer rate given above. From these rates and other nuclear magnetic resonance measurements wehave calculated the individual rate constants between the ternary complexes, AMP-enzyme-ATP and ADP-enzyme-ADP. In addition, we obtained one of the ADP off-rates from this latter complex. The rates on the enzyme are approximately 1250 and 500mol/sec per mol of enzyme for the forward and reverse directions, respectively. The ADP has an off-rate of 450 sec (-1).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn M., Leigh J. S., Jr, Reed G. H. Mapping active sites of phosphoryl-transferring enzymes by magnetic resonance methods. Cold Spring Harb Symp Quant Biol. 1972;36:533–540. doi: 10.1101/sqb.1972.036.01.067. [DOI] [PubMed] [Google Scholar]
- Glickson J. D., Dadok J., Marshall G. R. Proton magnetic double-resonance study of angiotensin II (Asn1Val5) in aqueous solution employing correlation spectroscopy. Assignment of peptide NH resonances and transfer of saturation from water. Biochemistry. 1974 Jan 1;13(1):11–14. doi: 10.1021/bi00698a002. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Redfield A. G. Double nuclear magnetic resonance observation of electron exchange between ferri- and ferrocytochrome c. Science. 1970 Sep 18;169(3951):1204–1206. doi: 10.1126/science.169.3951.1204. [DOI] [PubMed] [Google Scholar]
- Heil A., Müller G., Noda L., Pinder T., Schirmer H., Schirmer I., von Zabern I. The amino-acid sequence of sarcine adenylate kinase from skeletal muscle. Eur J Biochem. 1974 Mar 15;43(1):131–144. doi: 10.1111/j.1432-1033.1974.tb03393.x. [DOI] [PubMed] [Google Scholar]
- McDonald G. G., Cohn M. Proton magnetic resonance spectra or porcine muscle adenylate kinase and substrate complexes. J Biol Chem. 1975 Sep 10;250(17):6947–6954. [PubMed] [Google Scholar]
- Price N. C., Reed G. H., Cohn M. Magnetic resonance studies of substrate and inhibitor binding to porcine muscle adenylate kinase. Biochemistry. 1973 Aug 14;12(17):3322–3327. doi: 10.1021/bi00741a026. [DOI] [PubMed] [Google Scholar]
- Schulz G. E., Elzinga M., Marx F., Schrimer R. H. Three dimensional structure of adenyl kinase. Nature. 1974 Jul 12;250(462):120–123. doi: 10.1038/250120a0. [DOI] [PubMed] [Google Scholar]


