Abstract
Fibrosis can be considered as wound healing that never ceases, and activated fibroblasts (myofibroblasts) probably play a critical role in this unabated tissue repair process. In the setting of renal fibrosis, two central questions remain unanswered: Where do activated myofibroblasts come from; and what mechanism or mechanisms keep them activated? The study by Chen and colleagues addresses the role of platelet-derived growth factor receptor (PDGFR) signaling in the activation of myofibroblasts.
Fibrosis can be considered as wound healing that never ceases. In the setting of tissue repair, myofibroblasts appear in conjunction with inflammatory response to provide the required physical and biochemical support to enable regeneration, upon which all repair activities come to a halt with the disappearance of activated myofibroblasts and inflammation. In the kidney, acute injury is associated with such plastic response, whereas in the chronic injury setting, the resolution phase associated with such regenerative process is impaired, resulting in unabated repair that leads to what is referred to as fibrosis. Although many key questions remain unanswered with regard to the mechanism behind organ fibrosis, the kidney is offering an excellent model system to systematically probe them. Two central questions that remain are: Where do activated fibroblasts (myofibroblasts) come from; and what mechanism or mechanisms keep them activated? In the study by Chen and colleagues1 (this issue), the role of platelet-derived growth factor receptor (PDGFR) signaling in the activation of myofibroblasts is addressed.
Chen and colleagues1 demonstrate that altered PDGF – PDGFR signaling is associated with kidney fibrosis, and provide compelling evidence for the role of PDGFR signaling in myofibroblast activation. Although the total number of PDGFRβ /α+ cells (presumably interstitial cells) was not quantified in this study, the authors clearly demonstrate an increase in expression of specific components of the PDGF – PDGFR signaling axis in renal fibrosis, with a robust increase in PDGF-A through PDGF-D and PDGFRβ and PDGFRα. The use of anti-PDGFRα (1E10) and anti-PDGFRβ (2C5) antibodies from ImClone Systems reduces the observed increase in PDGF and PDGFR gene expression in mouse fibrotic kidney and subsequently reduces the activation of PDGFRα and PDGFRβ. By this approach, a marked decrease in the number of α-smooth muscle actin-positive (αSMA +) interstitial myofibroblasts and overall COL1A1 and COL3A1 gene expression is noted. The authors suggest that impaired macrophage recruitment due to diminished PDGFRα /β signaling with the use of anti-PDGFRα /β antibodies, as well as imatinib mesylate (which also targets PDGFRβ signaling), improves renal fibrosis. This study by Chen and colleagues1 is supported by published findings of Wang and colleagues2 which showed that imatinib mesylate might reduce renal fibrosis in unilateral ureteric obstruction in mice, and also by the studies by Lassila and colleagues3 using mice with diabetic nephropathy. Together these studies raise the interesting possibility that targeting PDGFR signaling might offer new therapeutic avenues for renal fibrosis, perhaps by reducing macrophage infiltration.
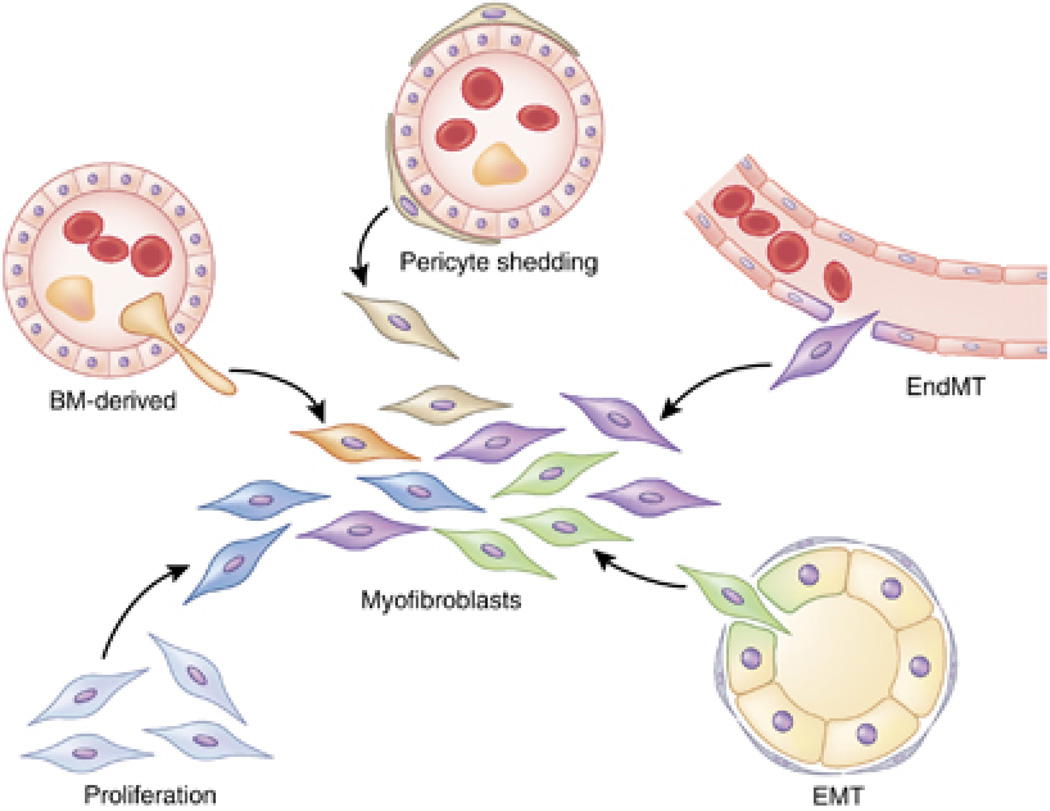
Many different sources of activated fibroblasts/ myofibroblasts/ mesenchymal cells in the setting of renal fibrosis have been proposed (Figure 1), and in some cases mice that allow lineage tracing have also been used.4–6 Nevertheless, the functional role of myofibroblasts and their origin are still largely unknown. In this regard, the study by Chen and colleagues1 does not offer any new insights. A series of assumptions were made to support the notion that myofibroblasts emerge mostly from pericyte differentiation, but compelling genetic, cell-biology, and biochemical data are lacking.
Figure 1. Proposed sources of myofibroblasts in kidney fibrosis.
Activated fibroblasts, or myofibroblasts (center), can originate from proliferation of resident fibroblasts, epithelialto- mesenchymal transition (EMT), endothelial-to-mesenchymal transition (EndMT), and pericyte shedding, as well as from the bone marrow (BM). The origin(s) and mechanism(s) of activation may be multiple, dynamic, and functionally synergistic in renal fibrosis.
What is a pericyte? Comprehensive reviews have summarized findings from electron microscopy imaging, genetic studies, expression profile analyses, and immunolabeling experiments with the hope of providing a clear definition.7,8 The term ‘pericyte’ is often applied to those distinct cells with extended cytoplasmic processes that embrace the capillary basement membrane on the abluminal side of the microvessels. The term ‘vascular smooth muscle cell’ (VSMC) is applied to morphologically similar cells associated with larger blood vessels, perhaps with higher contractile properties and specialized blood-flow-regulating functions. Nevertheless, some ambiguity remains as to the difference between these two terms. The expression of markers including α-SMA, desmin, PDGFRβ, and neuronglial 2 (NG2) varies in pericytes and VSMCs, depending on the vessel type, organ type, and specific pathological insult in a given tissue.7,8 PDGFRβ and NG2 were first described in association with microvessels of the brain, but their expression in other vascular beds may greatly differ.
The tyrosine kinase receptor PDGFRβ, the focus of the study by Chen and colleagues,1 is expressed by fibroblasts, astrocytes, mesangial cells, endothelial cells, macrophages, and cancer cells.1,9–12 Similarly, α SMA is expressed by myofibroblasts and some VSMCs.8,13 The difficulty in defining a specific pericyte marker underscores the challenge the community faces in identifying the origin of pericytes and in identifying them as the origin of other cell types. The use of transgenic mice with broad specificity, such as ColI-GFP transgenic mice (expressing green fluorescent protein under the collagen I promoter), to supplement fate-mapping strategies offers ambiguous answers with regard to the contribution of pericytes to the emergence of myofibroblasts.6,14 Nevertheless, recent studies2,3 and the one by Chen et al.1 identify new functional roles for PDGFRβ + cells in renal fibrosis, in addition to their putative roles in maintaining vessel structural integrity and regulating blood flow.
The idea of ‘pericyte – myofibroblast transition’ is intriguing; however, evidence for this notion does not exist in the study by Chen et al.1 The fact remains that one cannot assume that αSMA is not expressed in VSMCs / pericytes in the adult kidney (a vast body of literature does not support this notion) and that collagen I-expressing cells in the fibrotic kidney are exclusively pericyte-derived. The more plausible explanation is that PDGFRβ + cells in the kidney are of diverse cell types, and during fibrosis, PDGFRβ + cells of many different origins probably contribute to the pathogenesis. Despite the pan-specific nature of PDGFR + cells in fibrosis, Chen and colleagues1 provide elegant and compelling evidence for the role of PDGFR signaling in this setting. Although more studies are required, there is no doubt that Chen and colleagues1 bring more attention to the role of the PDGFR signaling axis in renal fibrosis, and with many agents already available to target this pathway, exciting therapeutic possibilities can be explored for this devastating disease.
Acknowledgements
The work of the authors is supported by a Research Training Grant in Gastroenterology (2T32DK007760-11) at Beth Israel Deaconess Medical Center, as well as by US National Institutes of Health grants DK055001, DK081576, and CA125550 and funds from the Department of Medicine to the Division of Matrix Biology at Beth Israel Deaconess Medical Center.
Footnotes
Disclosure
The authors declared no competing interests.
References
- 1.Chen Y-T, Chang F-C, Wu C-F, et al. Platelet-derived growth factor receptor signaling activates pericyte –myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Wilkes MC, Leof EB, et al. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 3.Lassila M, Jandeleit-Dahm K, Seah KK, et al. Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol. 2005;16:363–373. doi: 10.1681/ASN.2004050392. [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 8.Bergers G, Song S. The role of pericytes in bloodvessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostman A, Heldin CH. PDGF receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- 10.Ishii Y, Matsumoto Y, Watanabe R, et al. Characterization of neuroprogenitor cells expressing the PDGF beta-receptor within the subventricular zone of postnatal mice. Mol Cell Neurosci. 2008;37:507–518. doi: 10.1016/j.mcn.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 13.Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol. 1996;134:67–80. doi: 10.1083/jcb.134.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]