Abstract
Myocardial perfusion imaging (MPI) to diagnose coronary artery disease (CAD) is best performed in patients with intermediate pretest likelihood of disease; unfortunately, pretest likelihood is often overestimated, resulting in the inappropriate use of perfusion imaging. A good functional capacity often predicts low risk, and MPI for diagnosing CAD should be reserved for individuals with poor exercise capacity, abnormal resting electrocardiography, or an intermediate or high probability of CAD. With respect to anatomy-based testing, coronary CT angiography has a good negative predictive value, but stenosis severity correlates poorly with ischemia. Therefore decision making with respect to revascularization may be limited when a purely noninvasive anatomical test is used. Regarding perfusion imaging, the diagnostic accuracies of SPECT, PET, and cardiac magnetic resonance are similar, though fewer studies are available with cardiac magnetic resonance. PET coronary flow reserve may offer a negative predictive value sufficiently high to exclude severe CAD such that patients with mild to moderate reversible perfusion defects can forego invasive angiography. In addition, combined anatomical and perfusion-based imaging may eventually offer a definitive evaluation for diagnosing CAD, even in higher risk patients. Any remarkable findings on single-photon emission computed tomography and PET MPI studies are valuable for prognostication. Furthermore, assessment of myocardial blood flow with PET is particularly powerful for prognostication as it reflects the end result of many processes that lead to atherosclerosis. Decision making with respect to revascularization is limited for cardiac MRI and PET MPI. In contrast, retrospective radionuclide studies have identified an ischemic threshold, but randomized trials are needed. In patients with at least moderately reduced left ventricular systolic function, viable myocardium as assessed by PET or MRI, appears to identify patients who benefit from revascularization, but well-executed randomized trials are lacking.
Introduction
Several noninvasive imaging options are available for the assessment of suspected or known coronary artery disease (CAD) and for prognostication. These include coronary CT angiography (CCTA), SPECT, PET, and cardiac magnetic resonance (CMR). Stress echocardiography with myocardial perfusion imaging (MPI) is not commonly performed in the United States, as discussed elsewhere.1 In this review, we address 3 fundamental questions that most clinicians might often get asked:
Who needs imaging and what are the advantages of the various testing options?
How do the imaging modalities perform in risk stratification?
How do the results of individual tests guide decision making with respect to revascularization vs medical therapy?
With respect to the first question, the importance of accurate pretest risk assessment is addressed, and the advantages of each modality are framed within the context of anatomical or perfusion-based imaging. Newer techniques including coronary flow reserve (CFR) with PET and combined anatomical and perfusion-based imaging are emphasized. Regarding risk stratification and prognostication, the prognostic value of SPECT, CMR, and more recent studies with CCTA are discussed. Abnormal findings on PET CFR are usually a manifestation of macrovascular disease, microvascular disease, or a combination of both; the prognostic value of PET-based quantification of CFR is highlighted. Finally, studies that incorporate imaging results to identify patients who benefit from revascularization are discussed with the caveat that a well-executed randomized trial with imaging-guided revascularization vs medical therapy is lacking.
Diagnosis of Obstructive CAD
When is MPI Not Indicated?
In addition to further refinement of risk, a diagnostic test must more effectively classify a patient’s risk such that downstream treatment is affected and subsequent morbidity and mortality attenuated. For patients at low risk of adverse cardiac events, initial imaging thus has low yield. Very few of these patients will have significantly discordant clinical and imaging results such that differential treatment has a major effect on outcome. Unfortunately, pretest risk assessment is frequently overestimated, and many of these patients undergo up-front MPI, leading to its overutilization.
In contemporary practice, patients are more likely to be treated for hypertension, hyperlipidemia, and diabetes mellitus. Moreover, over the years, patients will have varying success in treatment of these comorbidities. These temporal changes were illustrated in a study where pretest probability of CAD increased from 40.1%–49.2% from 1991–2009, yet the number of tests with abnormal findings on SPECT MPI decreased from 40.9%–8.7%.2 During this time, use of aspirin, antihypertensive drugs, and lipid-lowering medications increased. Consequently, MPI may be frequently performed in patients labeled as having intermediate risk of disease but who may in fact be at low risk. As stress testing with MPI is best performed in patients with an intermediate probability of developing obstructive CAD, further study is needed to elucidate the current disconnect between pretest probability and subsequent demonstration of ischemia.
Exercise Results to Identify the Low-Risk Patient
In patients without known CAD who are able to exercise and have a normal baseline electrocardiography (ECG), exercise treadmill testing (ETT) is a reasonable initial test. The following 2 questions arise: Do any of these individuals benefit from perfusion imaging? In patients who can achieve an acceptable peak heart rate and exercise capacity, which test results should prompt follow-up imaging? With regard to the first question, a large single-center study showed that only 2.9% of exercising patients without typical angina had abnormal findings on SPECT MPI.2 In the WOMEN trial, there was no difference in adverse cardiac events between women who underwent ETT vs ETT and MPI (1.7% vs 2.3%, P = 0.59).3 It is noteworthy that the expected event rate was lower than predicted, again highlighting the frequent overestimation of pretest risk. Patients with excellent functional capacity are unlikely to benefit from further risk stratification with imaging. In a study of patients able to achieve more than 10 estimated metabolic equivalents, very few patients had evidence of ischemia, and the annualized cardiac event rate was 0.4%.4
Thus, most patients referred for testing to diagnose CAD who are able to adequately exercise are at low risk and should undergo ETT alone. The second question concerns the ETT results that should prompt imaging. Patients with very abnormal findings on tests frequently directly undergo invasive coronary angiography (ICA), but the appropriate management of inconclusive tests has been less well defined. A recent study addressed this issue by evaluating the yield of downstream MPI after ETT. Among patients with rapid recovery of ECG changes, none of the patients had positive results on imaging studies. Conversely, 21% had abnormal imaging findings following an ETT with typical angina but no ECG changes.5 In conclusion, the yield of subsequent MPI is highest in patients whose ETT results change the probability of CAD from low to intermediate or high.
What Imaging Study Should You Order?
For truly intermediate-risk patients, noninvasive imaging has a well-established role to diagnose CAD in many different clinical scenarios.6 As the options available to the clinician have increased, several questions concerning which test to order have become important: Is anatomical or perfusion-based testing preferable? Is one imaging modality better than another is? Finally, what is the role for combined anatomical and perfusion-based testing?
Anatomy-Based Approach
Regarding anatomy-based testing, in a summary of published studies examining the diagnostic accuracy of CCTA, the sensitivity and specificity per patient has been reported to be 94%–97% and 83%–90%, respectively.7 In patients with no history of CAD, CCTA has an excellent negative predictive value. A meta-analysis that compared 64-slice multidetector computed tomography coronary angiography with ICA reported sensitivity, specificity, positive predictive values, and negative predictive values with 95% confidence intervals (CI) of 97.5% (CI: 0.96–0.99), 91% (CI: 0.88–0.94), 93%, and 96.5%, respectively.8 CCTA can reliably exclude obstructive CAD, but the reported positive predictive value is lower. The ACCURACY trial reported a sensitivity of 93.8% and a specificity of 81.8% for detecting a stenosis of more than 70%.9 In this trial, CCTA misidentified stenosis severity more than half the time.
Stenosis severity assessment by CCTA allows identification of patients with high-risk diseases such as left main stenosis and multivessel disease. However, there is wide variability in the relationship between CCTA-based stenosis severity and myocardial ischemia. Only 30%–50% of individuals with a CCTA-based stenosis severity of more than 50% manifest ischemia detectable by PET or SPECT.10–12 Even though normal findings on CCTA essentially rule our obstructive epicardial CAD, when a stenosis of more than 50% is detected, a second noninvasive imaging test may be necessary for the definitive diagnosis of ischemia.
Perfusion-Based Approach
More than 50% of patients who undergo cardiac catheterization have had a stress test with MPI.13 In stable symptomatic patients, PET and SPECT MPI have high diagnostic accuracy for the detection of significant epicardial CAD. When compared with SPECT, PET MPI offers technical advantages including reliable attenuation correction and improved counts with a decreased radiation dose. Consequently, PET may significantly improve diagnostic accuracy compared with SPECT. However, in expert laboratories, SPECT MPI that routinely incorporates attenuation correction and ECG gating may be equally accurate.
In a meta-analysis of 177 studies (108 SPECT MPI, 4 PET MPI, and 5 both PET and SPECT), the sensitivity of PET was 92.6% and the specificity was 81.3%. SPECT had a lower sensitivity at 88.3% but a similar specificity of 75.8%.14 Another meta-analysis also compared these 2 modalities.15 The sensitivities and specificities with Rubidium-82 PET were 90% (CI: 0.88–0.92) and 88% (CI 0.85–0.91), respectively, whereas SPECT results were 85% (CI: 0.82–0.87) and 85% (CI: 0.82–0.87), respectively. In both these reports, PET outperformed SPECT, but the differences were small. Moreover, not all patients underwent coronary angiography, and visual assessment of stenosis severity was used as the reference standard. Angiography with fractional flow reserve (FFR) is considered the gold standard, and FFR-guided therapy has been shown to improve outcomes.16,17 A study that compares PET and SPECT using FFR in all patients as the reference standard, ideally in a randomized controlled trial, may demonstrate the diagnostic superiority of PET, but it has not yet been done. Currently, for the diagnosis of obstructive CAD in most patients, there appears to be an equipoise between expertly performed SPECT and PET.
Myocardial Blood Flow With PET MPI
When compared with SPECT, PET also has the advantage of quantitative analysis of myocardial blood flow and calculation of CFR with pharmacologic stress. MPI with SPECT depends on flow heterogeneity, and sensitivity may be decreased because counts can normalize to a region also subtended by a diseased coronary artery. Therefore, in diagnosing CAD, PET with analysis of myocardial blood flow has the potential to affect clinical practice in 2 important ways. First, normal myocardial blood flow may offer such a high negative predictive value to exclude obstructive CAD that further invasive testing is not needed, even in higher risk patients. Second, abnormal CFR may improve classification of ischemia. With SPECT, transient ischemic dilation of the left ventricle or decrease in ejection fraction during stress may suggest multivessel disease.18 Similarly, PET with CFR may even more accurately determine myocardium at risk. For example, while a perfusion defect may implicate single-vessel CAD, abnormal findings on CFR may correctly reclassify the patient as having multivessel CAD.
With regard to the first question, small studies have assessed the incremental diagnostic value of decreased CFR. One study using a cutoff of CFR <2 in patients undergoing adenosine-stress N13-ammonia-PET demonstrated improved accuracy. However, this improved accuracy was primarily due to increased sensitivity (79%–96%) and improved negative predictive value (59%–89%).19 Likewise, in another larger study of 136 patients with evidence of less than 10% ischemic myocardium, preserved CFR (>1.93) had a good negative predictive value (97%) to exclude high-risk CAD on angiography.20 Thus, CFR may have a role in the future to select which patients with mild to moderately abnormal perfusion defects should undergo ICA.
The ability of reduced global CFR to predict multivessel CAD has been less promising. One study showed that CFR was more predictive than summed stress score for multivessel CAD, but a CFR <1 had only a 50% positive predictive value.21 Moreover, a previously mentioned study also showed only a modest contribution of CFR to predict high-risk CAD.20 Even though studies have heretofore been small, reduced CFR as a predictor of multivessel CAD may continue to disappoint. CFR reflects blood flow across the coronary vasculature and may be decreased with diffuse nonobstructive coronary atherosclerosis and microvascular disease, including impaired vasodilator response of the resistance vessels. Consequently, CFR reflects a final common pathway of many pathologies that lead to atherosclerosis across the coronary vascular bed and may therefore be relatively nonspecific at predicting obstructive epicardial coronary stenosis.
Cardiac MR to Diagnose CAD
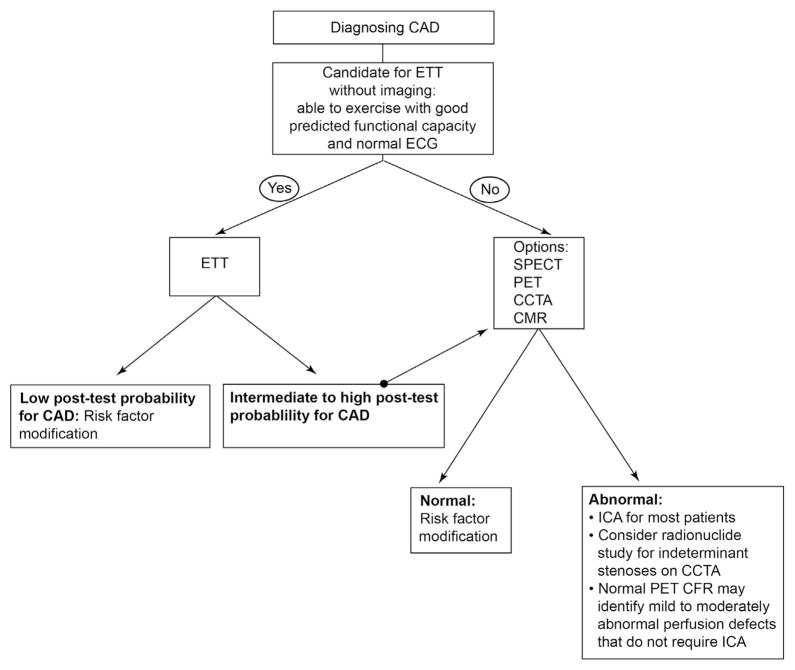
When compared with CCTA, SPECT, and PET, fewer studies have been performed with CMR imaging to diagnose CAD. Moreover, an assessment of distal coronary arteries with MR is technically challenging.22 The primary applications of CMR imaging are for assessment of myocardial perfusion and viability. CMR perfusion based on first pass of contrast at rest and stress can be used to derive a myocardial perfusion reserve. In a study of 84 patients who underwent rest and vasodilator stress imaging, myocardial perfusion reserve using CMR was found to have a sensitivity of 88%, specificity of 90%, and diagnostic accuracy of 89% when compared with invasive angiography.23 On direct comparison with SPECT MPI, CMR perfusion imaging has equivalent sensitivity and specificity for detection of angiographically significant stenosis.24 CMR perfusion imaging has also been shown to have excellent agreement with PET MPI for detection of obstructive epicardial CAD.25 Therefore, there also appears to be an equipoise between CMR, SPECT, and PET for diagnosing CAD, although the published literature for CMR is much smaller. A simplified approach highlighting appropriate imaging and respective advantages of each modality to diagnose CAD is presented (Fig. 1).
Figure 1.
Simplified approach to the use of imaging to diagnose coronary artery disease (CAD). ETT = exercise treadmill testing; SPECT = single-photon emission computed tomography; PET = positron emission tomography; CCTA = coronary CT angiography; MRI = magnetic resonance imaging; ICA = invasive coronary angiography.
Combined Anatomical and Perfusion-Based Imaging
CCTA is expanding with optimization of CTA-based MPI techniques. As an example, CCTA can provide a noninvasive assessment of flow reserve. A meta-analysis of 13 studies performed from 1995–2011 compared CCTA perfusion with other modalities.26 The sensitivity, specificity, positive predictive values, and negative predictive values are shown in Table 1. Although the technology is promising, CCTA perfusion does not perform well enough to represent an acceptable combined anatomical and perfusion-based assessment. More recent studies have also applied CCTA to measure FFR, although the results are still preliminary.27–29 Finally, there may be a role for combined cardiac PET and CT angiography with PET for identification of perfusion defects and CCTA for identification of epicardial obstruction, thus providing a complete anatomical and perfusion-based assessment.30
Table 1.
Comparison of Computed Tomography Perfusion (CTP) vs Radionuclide Myocardial Perfusion Imaging (MPI), Invasive Coronary Angiography (ICA), and Perfusion With Magnetic Resonance Imaging (MRI)
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Per vessel: CTP vs radionuclide MPI | 87% (n = 206) | 69% (n = 190) | 72% (n = 235) | 83% (n = 161) |
| Per vessel: CTP vs ICA | 84% (n = 198) | 73% (n = 267) | 69% (n = 241) | 86% (n = 224) |
| Per vessel: CTP vs MRI | 94% (n = 92) | 85% (n = 82) | 88% (n = 99) | 93% (n = 75) |
PPV = positive predictive value; NPV = negative predictive value. (Adapted with permission from Tashakkor et al.26)
Decision Making for Diagnosis of CAD
Patients with ECG abnormalities, poor exercise capacity, or intermediate to high pretest likelihood of disease would be best served by noninvasive imaging (Fig. 1). Additionally, patients with high-risk markers on ETT may benefit from noninvasive imaging. A negative result on an ETT, which signals low posttest likelihood of the disease, may be managed with lifestyle changes and potential medical therapy for risk factors; on the contrary, patients with a positive ETT would benefit from invasive angiography. Patients with equivocal results and those with intermediate posttest likelihood should be referred for noninvasive MPI with SPECT or PET. CMR may be a suitable alternative in centers where perfusion imaging with CMR is routinely performed. Patients who are not able to exercise or who have an abnormal resting ECG would benefit from noninvasive imaging. Once the decision to employ a noninvasive strategy is made, a CCTA can be used to rule out hemodynamically significant obstructive CAD; however, this would be most useful in young patients with a low likelihood of coronary calcium, which can interfere with accurate assessment of stenosis severity. MPI with SPECT, PET and, in select centers, CMR can be used for the identification of patients with high-risk disease. Normal findings on a stress test can be followed by risk factor modification with lifestyle changes and medical therapy. On the contrary, a positive test with inducible ischemia should prompt further decision making in terms of whether an invasive angiogram or aggressive medical therapy will be employed.
Risk Stratification
Prognostic Value of CCTA
With respect to prognostication, a meta-analysis of 18 studies evaluated 9592 patients over a median duration of 20 months and showed that normal findings on CCTA portend an excellent prognosis.31 In individuals with normal findings on CCTA, the risk for major adverse cardiac events was similar to the baseline risk of healthy patients, and in patients with an abnormal findings on CCTA, the relative risk was higher than 40 times that of those with normal findings on CCTA. Similar results were reported in a study analyzing data from a multicenter registry.32 After adjusting for risk factors, medications, and revascularization, patients with stenosis of more than 50% had a higher annualized incidence of death or myocardial infarction (MI) with a hazard ratio of 2.77 among whites, 6.25 among Africans, and 4.84 among East Asians. In a study of 1127 low- to intermediate-risk patients, results were similar, with the risk of death increasing in proportion to the number of vessels with plaque and with stenosis severity.33 Thus, even though the evidence base is not as robust as for SPECT, CCTA provides effective risk stratification.
Prognostic Value of SPECT
In patients with known or suspected CAD, SPECT MPI has provided incremental prognostic value for decades. This enhanced risk stratification has been demonstrated in patients with low, intermediate, and high risk.34–36 A review of 14 trials with 12,000 patients with stable chest pain demonstrated that normal technetium 99m-sestamibi SPECT is associated with a low cardiac event rate of 0.6% per year.37 A meta-analysis of 19 studies (n = 39,173) found that a normal- or low-risk stress MPI was associated with an annual cardiac event rate of only 0.6% (25th–75th percentile = 0.5%–0.9%).38 Even though the presence, extent, and severity of perfusion abnormalities are strongly associated with increasing patient risk, normal findings on stress MPI, even in the presence of angiographically documented CAD, are associated with only a 1% annual risk of cardiac events.39,40 More recently, net reclassification improvement (NRI) has been used to illustrate the clinical importance of risk stratification. NRI expresses how test results change risk category and thus provide more of a clinical context to risk stratification. Studies evaluating NRI with SPECT ischemia, PET ischemia and scar, and PET CFR are shown in Table 2.41–43 It is noteworthy that NRI appears more robust with more categories, but clinical relevance requires that a change in risk category affects downstream treatment.
Table 2.
SPECT and PET Studies Illustrating That NRI Depends Not Only on Test Characteristic But Also on Other Variables in the Model and the Number of Risk Categories
| Test Characteristic | PET CFR (n = 2783)43 | PET Ischemia or Scar (n = 6037)42 | SPECT Ischemia (n = 4575)41 |
|---|---|---|---|
| Model | Clinical variables, rest and stress LVEF, ischemia, and scar | Clinical variables | Duke treadmill score and pretest CAD likelihood |
| Risk categories | 3 | 3 | 4 |
| NRI | 9.8% | 11.6% | 35.8% |
Adapted with permission from Cremer and Hachamovitch.44
In addition, rest and stress ejection fraction, left ventricular volumes, and transient ischemic dilation can be assessed with SPECT MPI. These additional data provide further prognostication. In a study of 1680 patients who underwent dual-isotope (rest thallium 201/Stress Tc99m Sestamibi) MPI, patients with a left ventricular ejection fraction (LVEF) greater than 45% had less than 1% mortality rate per year. In comparison, patients with a LVEF less than 45% had a mortality rate of 9.2% per year, even with only mild to moderate perfusion abnormalities. Furthermore, inclusion of left ventricular end-systolic volume (ESV) improved risk prediction: patients with (ESV > 70 mL) had more events compared with those with ESV less than 70 mL.45 These results highlight that the reason a test is ordered (to assess for ischemia) is often distinct from the findings of most prognostic significance (LVEF and volumes).
Prognostic Value of PET
PET MPI allows further refinement of prognostication. In a study of 1432 patients who underwent Rb82-PET imaging, patients without ischemia had an annualized event rate of 0.7% for all-cause death compared with the 11% rate among patients with ischemia that affected more than 20% of the myocardium.46 Furthermore, with PET, LVEF can be measured at peak stress, and as discussed, myocardial blood flow can be quantified. Abnormal CFR measured by both N13-ammonia and by Rb82 has been associated with adverse cardiac events.43,47–49 A CFR <2 has been associated with a 3.3-fold increased hazard of all-cause death, and a CFR <1.5 was associated with a 5.6-fold increase in risk for all-cause death. It is of particular clinical importance that diabetic patients without CAD but with impaired CFR have an annual cardiac mortality that is similar to diabetics with known CAD (2.9% vs 2.8%).50 This observation illustrates that CFR may have particular prognostic importance as it represents the final common pathway of many diseases that cause coronary atherosclerosis.
Prognostic Value of Cardiac MR
Perfusion abnormalities seen on vasodilator stress CMR imaging or wall motion abnormalities seen using dobutamine CMR imaging also provide incremental value over clinical risk factors. In a study of 493 patients, an abnormal perfusion study was associated with an event rate of 12.2% at 2 years.51 A more recent meta-analysis of 56 studies with 25,497 patients who underwent either resting CMR or rest and stress CMR imaging found that wall motion abnormalities, stress-induced perfusion abnormalities, and low LVEF were associated with increased risk of adverse events including all-cause death.52 Thus, CMR imaging also provides effective risk stratification, but again, this literature is less well developed when compared with that for stress testing with radionuclides.
Choice of an Imaging Test for Prognostication
Among the tests available for risk stratification and assessment of the future risk of adverse events, SPECT has the most data available and additional parameters that could be measured by SPECT MPI including LVEF, and LV volume may provide incremental value for prognostication. With PET MPI, quantification of LVEF, LVEF reserve, myocardial blood flow, and CFR can be incorporated into the assessment to improve the prognostic value. CMR perfusion imaging provides equivalent prognostic value compared with SPECT and PET but the incremental benefit of MRI-based flow reserve has not been evaluated. CCTA may be a useful test for prognostication but additional testing becomes necessary to obtain rest and stress myocardial perfusion and flow reserve measurements to improve the prognostic value of CCTA.
Noninvasive Imaging to Select Patients for Revascularization
CCTA and Revascularization
A recent multicenter study examined invasive angiography, subsequent revascularization, and all-cause mortality in 15,207 intermediate-likelihood patients without known CAD who underwent CCTA over 2.3 ± 1.2 years.53 Invasive angiography rates for patients with no CAD to mild CAD visualized by CCTA were low (2.5% and 8.3%), and these patients had similarly low rates of revascularization (0.3% and 2.5%). Relative hazard for death was 0.61 for revascularization vs nonrevascularization (P = 0.047). However, patients with obstructive CAD were not randomized, and medically treated patients are often not treated aggressively outside of randomized trials.54 As a result, this study does not unequivocally establish a mortality benefit for CCTA-guided revascularization.
Radionuclide MPI and Revascularization
Among patients with no ischemia or mild ischemia by SPECT MPI, medical therapy and revascularization provide equivalent survival benefit; however, in retrospective studies, revascularization is associated with improved survival compared with medical therapy in patients with moderate to severe ischemia (>10% ischemia).55 Results from retrospective studies have been similar in diabetics and in the elderly. In diabetic patients, coronary artery bypass grafting was associated with improved survival among patients with moderate to severe ischemia as detected by SPECT MPI.56 Among elderly patients without known CAD, a benefit from revascularization was observed only in patients with ischemic myocardium ≥15%.57
A single-site study that included patients with and without CAD examined the relationship between extent of ischemia and benefit derived from SPECT imaging. In a large cohort of 13,969 patients who were followed up for an average of 8.7 years, inducible ischemia identified patients with improved survival with early revascularization, and this relationship was observed among patients without prior CAD and in patients with prior CAD (but without prior MI). However, revascularization was not beneficial in the case of patients with prior MI and with a fixed perfusion defect in more than 10% of myocardium.58 These studies support using SPECT MPI to identify patients with inducible ischemia who may benefit from revascularization. However, nuclear substudies of randomized controlled trials have been conflicting and generally underpowered.59–61 There is evidence for improved survival with revascularization in patients with evidence of ischemia on Rb82 PET imaging from preliminary studies presented recently.62 It is noteworthy that the magnitude of reversible perfusion defect on PET to demonstrate a survival benefit was less than that previously reported with SPECT. However, randomized data to support this approach are lacking, and the ISCHEMIA trial has been designed to more definitively answer this question by only enrolling patients with moderate to severe ischemia. After undergoing a blinded CCTA, patients will be randomized to medical therapy alone or revascularization plus medical therapy.63
FDG-PET MPI and Revascularization
The extent and severity of hibernating myocardium detected and quantified by 18F-fluorodeoxyglucose (FDG)-PET may also select patients that would benefit from revascularization. In PARR-2, patients with LV dysfunction were randomized to a strategy of viability imaging with FDG-PET guided revascularization vs standard care. There was no improvement in outcomes in the FDG-PET patients.64 However, a significant number of patients in the PARR-2 trial were not revascularized according to recommendations based on FDG-PET results. In a subsequent substudy of the PARR-2 trial, patients with severe LV systolic dysfunction who underwent revascularization according to the extent of viable myocardium, revascularization was associated with improved outcomes.65 In a more recent retrospective study of 648 patients who underwent FDG-PET with RB82 for perfusion, revascularization was superior to medical therapy with respect to survival among patients with more than 10% hibernating myocardium.66 Thus, in patients with reduced left ventricular systolic function, current evidence supports revascularization on the basis of myocardial viability as assessed with PET, but this hypothesis needs to be confirmed in a randomized trial.
Cardiac MR for Revascularization
Delayed-enhancement imaging is a highly accurate and reproducible technique for myocardial viability assessment, and several studies have shown that the presence of delayed enhancement is associated with adverse clinical outcomes in patients with and without CAD.67–69 Among patients who undergo revascularization, functional recovery is associated with the extent of scar as assessed by delayed enhancement.70 Patients with viable myocardium also have improved survival with revascularization whereas patients with nonviable myocardium do not realize a survival benefit with revascularization.71,72 In a recent single-center study of 450 patients with CAD and systolic dysfunction, left ventricular end-systolic volume index quantified by CMR provided incremental prognostic value, and patients with increasing left ventricular end-systolic volume index had a survival benefit with revascularization.71 In conclusion, retrospective data support using viability assessment with CMR to select patients with poor left ventricular systolic function for revascularization, but the studies are small and randomized trials are needed. CMR data demonstrating an ischemic threshold that identifies patients who benefit from revascularization is altogether lacking.
Clinical Decision Making for Revascularization
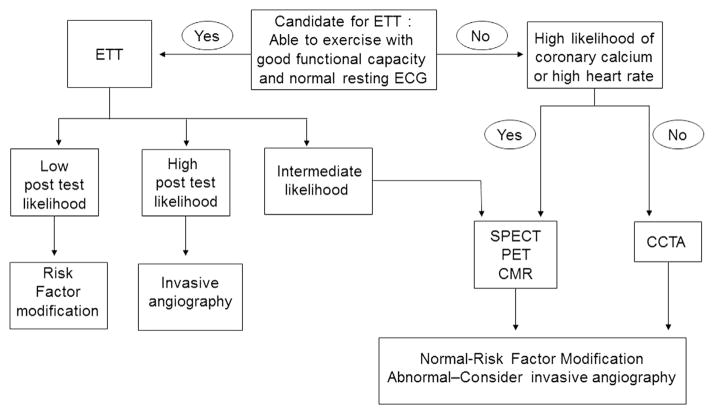
The extent and severity of myocardial ischemia and myocardial viability can serve as useful guides for deciding between revascularization and medical therapy. Retrospective studies of SPECT MPI have shown that patients with moderate or severe ischemia derive benefit from revascularization. There is less information available from PET perfusion imaging but preliminary evidence suggests that the threshold for ischemic myocardium that would prompt revascularization to provide mortality benefit may be lower for PET compared with that for SPECT.62 FDG-PET–based assessment of myocardial viability can also serve as a guide for revascularization. There is evidence from both a prospective clinical trial and a few retrospective studies that demonstrates the benefit of revascularization when there is significant amount of viable myocardium. Similar data are now becoming available using CMR for the assessment of myocardial availability for the identification of patients who might most likely benefit from revascularization. In choosing a clinical test for the assessment of myocardial ischemia, the options vary between SPECT, PET, and CMR (Fig. 2). The incremental benefit of being able to quantify the extent of scar and hibernating myocardium by PET-FDG imaging and CMR imaging would most profoundly affect decision making between revascularization and medical therapy in patients with a reduced LVEF. In a patient with a reduced LVEF, the ideal options would be PET or CMR imaging as the amount of viable myocardium can be accurately quantified using these techniques. Patients with a significant amount of viable myocardium would benefit from revascularization; however, patients with large amounts of scar or a dilated ventricle would be better served with medical therapy. Among patients with a normal or mildly reduced LVEF, SPECT, PET, CMR, and CCTA may be considered. If there is a significant amount of ischemia (≥10%) on perfusion imaging with SPECT or PET, revascularization would be recommended. Patients without significant myocardial ischemia by SPECT or PET may not benefit from revascularization and would be ideally managed with maximal medical therapy. Among patients who undergo CCTA, the presence of stenosis >70% may indicate the need for revascularization; however, among those with stenosis severity between 50% and 70%, one might consider MPI to determine if the stenosis detected by CCTA is associated with ischemia. In select centers where CCTA is used for MPI, quantification of ischemia along with detection of obstructive CAD may allow identification of patients who would benefit from revascularization. Although CMR imaging is acceptable as an option for MPI and for detection of ischemia, there are no studies that directly demonstrate a survival benefit in patients where CMR imaging has been used as a guide for revascularization; furthermore, there are no CMR-based perfusion studies that describe a threshold of ischemia that might identify patients who would derive the most benefit from revascularization as opposed to maximal medical therapy.
Figure 2.
Simplified approach to the use of imaging to select patients for revascularization. CAD = coronary artery disease; EF = ejection fraction; SPECT = single-photon emission computed tomography; PET = positron emission tomography; CCTA = coronary CT angiography; CMR = cardiac magnetic resonance; LVESVi = left ventricular end-systolic volume index.
Conclusions
The value of any imaging test is defined according to yield and effect. With respect to the former, the frequency of abnormal results should justify testing, and imaging results should provide prognostic information beyond what is available from clinical risk factors and other tests. However, in addition to this incremental prognostication, the results of an imaging test should lead to differential treatment that affect outcome. Within this framework, the use of MPI to diagnose CAD is not justified in low-risk patients. Most patients with preserved exercise capacity are at low risk, and MPI for suspected CAD should be reserved for patients whose ETT suggests an intermediate or high probability of CAD.
Imaging for suspected CAD is most valuable in truly intermediate-risk patients and can be categorized as anatomical or perfusion based. In anatomy-based testing, CCTA provides excellent negative predictive value to exclude CAD. With perfusion-based testing, there is equipoise between SPECT, PET, and CMR to diagnose CAD. Combined anatomical and perfusion-based testing offers the potential for high accuracy; however, its application is limited to specialized imaging centers at this time. Such an approach would represent a paradigm shift from imaging as a gatekeeper to imaging as a complete evaluation.
SPECT has a long history of effective risk stratification, and more recently, normal findings on CCTA have been associated with an excellent short-term prognosis. CFR, as it reflects the final common pathway of many pathophysiological processes that affect the macrovasculature and microvasculature, is tremendously powerful for prognostication. Several retrospective SPECT studies support an ischemic threshold to identify patients who realize a survival benefit with revascularization. In patients with a reduced ejection fraction, PET and MRI may likewise identify patients who should undergo revascularization (Fig. 2). Currently, there is lack of evidence from randomized trials. The ISCHEMIA trial, whose results should be forthcoming, is designed to test the concept of an ischemic threshold to identify patients who will benefit from revascularization when it is added to optimal medical therapy.
References
- 1.Thomas JD. Myocardial contrast echocardiography perfusion imaging: Still waiting after all these years. J Am Coll Cardiol. 2013;62(15):1362–1364. doi: 10.1016/j.jacc.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 2.Rozanski A, Gransar H, Hayes SW, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61(10):1054–1065. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 3.Shaw LJ, Mieres JH, Hendel RH, et al. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: Results from the what is the optimal method for ischemia evaluation in women (WOMEN) trial. Circulation. 2011;124 (11):1239–1249. doi: 10.1161/CIRCULATIONAHA.111.029660. [DOI] [PubMed] [Google Scholar]
- 4.Bourque JM, Charlton GT, Holland BH, et al. Prognosis in patients achieving ≥10 METS on exercise stress testing: Was SPECT imaging useful? J Nucl Cardiol. 2010;18(2):230–237. doi: 10.1007/s12350-010-9323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christman MP, Marcio Sommer Bittencourt MD, Edward Hulten MM, et al. The yield of downstream tests after exercise treadmill testing: A prospective cohort study. J Am Coll Cardiol. 2014;63(13):1264–1274. doi: 10.1016/j.jacc.2013.11.052. http://dx.doi.org/10.1016/j.jacc.2013.11.052. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendel RC, Berman DS, Di Carli MF, et al. Appropriate use criteria for cardiac radionuclide imaging. J Am Coll Cardiol. 2009;53(23):2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Min JK, Shaw LJ. Noninvasive diagnostic and prognostic assessment of individuals with suspected coronary artery disease: Coronary computed tomographic angiography perspective. Circulation. 2008;1(3):270–281. doi: 10.1161/CIRCIMAGING.108.823807. [DOI] [PubMed] [Google Scholar]
- 8.Abdulla J, Abildstrom SZ, Gotzsche O, et al. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: A systematic review and meta-analysis. Eur Heart J. 2007;28(24):3042–3050. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Di Carli M, Dorbala S, Curillova Z, et al. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol. 2007;14(6):799–809. doi: 10.1016/j.nuclcard.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Gaemperli O, Schepis T, Valenta I, et al. Functionally relevant coronary artery disease: Comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology. 2008;248(2):414–423. doi: 10.1148/radiol.2482071307. [DOI] [PubMed] [Google Scholar]
- 12.Schuijf JD, Wijns W, Jukema JW, et al. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48(12):2508–2514. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 13.Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: A report from the cath PCI registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012;60(20):2017–2031. doi: 10.1016/j.jacc.2012.08.966. [DOI] [PubMed] [Google Scholar]
- 14.Parker MW, Iskandar A, Limone B, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: A bivariate meta-analysis. Circulation. 2012;5(6):700–707. doi: 10.1161/CIRCIMAGING.112.978270. [DOI] [PubMed] [Google Scholar]
- 15.McArdle BAM, Dowsley TF, deKemp RA, et al. Does rubidium-82 have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease. J Am Coll Cardiol. 2012;60(18):1828–1837. doi: 10.1016/j.jacc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Tonino PAL, De Bruyne B, Pijls NHJ, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 17.De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 18.Berman DS, Kang X, Slomka PJ, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14(4):521–528. doi: 10.1016/j.nuclcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Fiechter M, Ghadri JR, Gebhard C, et al. Diagnostic value of 13N-ammonia myocardial perfusion PET: Added value of myocardial flow reserve. J Nucl Med. 2012;53(8):1230–1234. doi: 10.2967/jnumed.111.101840. [DOI] [PubMed] [Google Scholar]
- 20.Naya M, Murthy VL, Taqueti VR, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med. 2014;55(2):248–255. doi: 10.2967/jnumed.113.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziadi MC, Dekemp RA, Williams K, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19(4):670–680. doi: 10.1007/s12350-011-9506-5. [DOI] [PubMed] [Google Scholar]
- 22.Poon M, Fuster V, Fayad Z. Cardiac magnetic resonance imaging: A “one-stop-shop” evaluation of myocardial dysfunction. Curr Opin Cardiol. 2002;17(6):663. doi: 10.1097/00001573-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108(4):432–437. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet. 2012;379(9814):453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwitter J, Nanz D, Kneifel S, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: A comparison with positron emission tomography and coronary angiography. Circulation. 2001;103(18):2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 26.Tashakkor AY, Nicolaou S, Leipsic J, et al. The emerging role of cardiac computed tomography for the assessment of coronary perfusion: A systematic review and meta-analysis. Can J Cardiol. 2012;28(4):413–422. doi: 10.1016/j.cjca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Koo B-K, Erglis A, Doh J-H, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms: Results from the prospective multi-center DISCOVER-FLOW (diagnosis of ischemia-causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol. 2011;58(19):1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 28.Choi J-H, Min JK, Labounty TM, et al. Intracoronary transluminal attenuation gradient in coronary CT angiography for determining coronary artery stenosis. J Am Coll Cardiol Imaging. 2011;4(11):1149–1157. doi: 10.1016/j.jcmg.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Wong DTL, Ko BS, Cameron JD, et al. Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: A comparison with fractional flow reserve. J Am Coll Cardiol. 2013;61(12):1271–1279. doi: 10.1016/j.jacc.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 30.Kajander S, Joutsiniemi E, Saraste M, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122(6):603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 31.Hulten EA, Carbonaro S, Petrillo SP, et al. Prognostic value of cardiac computed tomography angiography: A systematic review and meta-analysis. J Am Coll Cardiol. 2011;57(10):1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Hulten E, Villines TC, Cheezum MK, et al. Usefulness of coronary computed tomography angiography to predict mortality and myocardial infarction among Caucasian, African and East Asian ethnicities (from the CONFIRM [Coronary CT angiography evaluation for clinical outcomes: An international multicenter] registry) Am J Cardiol. 2013;111(4):479–485. doi: 10.1016/j.amjcard.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 34.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: Incremental prognostic value and use in risk stratification. Circulation. 1996;93 (5):905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 35.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97(6):535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 36.Hachamovitch R, Hayes SW, Friedman JD, et al. Stress myocardial perfusion single-photon emission computed tomography is clinically effective and cost effective in risk stratification of patients with a high likelihood of coronary artery disease (CAD) but no known CAD. J Am Coll Cardiol. 2004;43(2):200–208. doi: 10.1016/j.jacc.2003.07.043. [DOI] [PubMed] [Google Scholar]
- 37.Iskander S, Iskandrian AE. Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol. 1998;32(1):57–62. doi: 10.1016/s0735-1097(98)00177-6. [DOI] [PubMed] [Google Scholar]
- 38.Shaw L, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11(2):171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Berman DS, Shaw LJ, Hachamovitch R, et al. Comparative use of radionuclide stress testing, coronary artery calcium scanning, and non-invasive coronary angiography for diagnostic and prognostic cardiac assessment. Semin Nucl Med. 2007;37(1):2–16. doi: 10.1053/j.semnuclmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Gibbons RJ, Smith SC, Antman E, et al. Clinical practice guidelines: Part II: Evolutionary changes in a continuous quality improvement project. Circulation. 2003;107(24):3101–3107. doi: 10.1161/01.CIR.0000079017.53579.9C. [DOI] [PubMed] [Google Scholar]
- 41.Shaw LJ, Wilson PW, Hachamovitch R, et al. Improved near-term coronary artery disease risk classification with gated stress myocardial perfusion SPECT. J Am Coll Cardiol. 2010;3(11):1139–1148. doi: 10.1016/j.jcmg.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Dorbala S, Di Carli MF, Beanlands RS, et al. Prognostic value of stress myocardial perfusion positron emission tomography: Results from a multicenter observational registry. J Am Coll Cardiol. 2013;61(2):176–184. doi: 10.1016/j.jacc.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cremer PC, Hachamovitch R. Assessing the prognostic implications of myocardial perfusion studies: Identification of patients at risk vs patient who may benefit from revascularization. Curr Cardiol Rep. 2014;16:472–479. doi: 10.1007/s11886-014-0472-9. [DOI] [PubMed] [Google Scholar]
- 45.Sharir T, Germano G, Kavanagh PB, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999;100(10):1035–1042. doi: 10.1161/01.cir.100.10.1035. [DOI] [PubMed] [Google Scholar]
- 46.Dorbala S, Hachamovitch R, Curillova Z, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. J Am Coll Cardiol Imaging. 2009;2:846–854. doi: 10.1016/j.jcmg.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13 N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Anal Chem. 2009;54(2):150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 48.Fukushima K, Javadi MS, Higuchi T, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52(5):726–732. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 49.Ziadi MC, Williams KA, Guo A, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 50.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126(15):1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: Adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115(13):1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 52.Aidi El H, Adams A, Moons KGM, et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease—a systematic review of prognostic studies. J Am Coll Cardiol. 2014;63(11):1031–1045. doi: 10.1016/j.jacc.2013.11.048. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Shaw LJ, Hausleiter J, Achenbach S, et al. Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: Results from the multicenter CONFIRM (Coronary CT angiography evaluation for clinical outcomes: An international multi-center) registry. J Am Coll Cardiol. 2012;60(20):2103–2114. doi: 10.1016/j.jacc.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 54.Hachamovitch R, Nutter B, Hlatky MA, et al. Patient management after noninvasive cardiac imaging results from SPARC (Study of myocardial perfusion and coronary anatomy imaging roles in coronary artery disease) J Am Coll Cardiol. 2012;59(5):462–474. doi: 10.1016/j.jacc.2011.09.066. [DOI] [PubMed] [Google Scholar]
- 55.Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 56.Sorajja P, Chareonthaitawee P, Rajagopalan N, et al. Improved survival in asymptomatic diabetic patients with high-risk SPECT imaging treated with coronary artery bypass grafting. Circulation. 2005;112:311–316. doi: 10.1161/CIRCULATIONAHA.104.525022. [DOI] [PubMed] [Google Scholar]
- 57.Hachamovitch R, Kang X, Amanullah AM, et al. Prognostic implications of myocardial perfusion single-photon emission computed tomography in the elderly. Circulation. 2009;120(22):2197–2206. doi: 10.1161/CIRCULATIONAHA.108.817387. [DOI] [PubMed] [Google Scholar]
- 58.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32(8):1012–1024. doi: 10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 59.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 60.Shaw LJ, Weintraub WS, Maron DJ, et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J. 2012;164(2):243–250. doi: 10.1016/j.ahj.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Shaw LJ, Cerqueira MD, Brooks MM, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: Results from the bypass angioplasty revascularization investigation 2 diabetes (BARI 2D) trial. J Nucl Cardiol. 2012;19(4):658–669. doi: 10.1007/s12350-012-9548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taqueti V, Hachamovitch R, Murthy V, et al. Interaction of left ventricular ischemia and coronary vasomotor dysfunction on the survival benefit of revascularization and medical therapy in patients undergoing stress myocardial perfusion positron emission tomography. Presented at 2014 ACC; Washington D.C. March 29, 2014. [Google Scholar]
- 63. [Accessed March 8, 2014]; https://ischemiatrial.org.
- 64.Beanlands RSB, Nichol G, Huszti E, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease. J Am Coll Cardiol. 2007;50(20):2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 65.D’Egidio G, Nichol G, Williams KA, et al. Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: A substudy of the PARR-2 trial. J Am Coll Cardiol Imaging. 2009;2(9):1060–1068. doi: 10.1016/j.jcmg.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Ling LF, Marwick TH, Flores DR, et al. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: Inducible ischemia versus hibernating myocardium. Circulation. 2013;6(3):363–372. doi: 10.1161/CIRCIMAGING.112.000138. [DOI] [PubMed] [Google Scholar]
- 67.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113(23):2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 68.Kwong RY, Sattar H, Wu H, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011–1020. doi: 10.1161/CIRCULATIONAHA.107.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwon DH, Halley CM, Carrigan TP, et al. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: A delayed hyperenhancement cardiac magnetic resonance study. J Am Coll Cardiol Imaging. 2009;2(1):34–44. doi: 10.1016/j.jcmg.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 71.Gerber BL, Rousseau MF, Ahn SA, et al. Prognostic value of myocardial viability by delayed-enhanced magnetic resonance in patients with coronary artery disease and low ejection fraction impact of revascularization therapy. J Am Coll Cardiol. 2012;59(9):825–835. doi: 10.1016/j.jacc.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 72.Kwon DH, Hachamovitch R, Popovic ZB, et al. Survival in patients with severe ischemic cardiomyopathy undergoing revascularization versus medical therapy: Association with end-systolic volume and viability. Circulation. 2012;126(11 suppl 1):S3–S8. doi: 10.1161/CIRCULATIONAHA.111.084434. [DOI] [PubMed] [Google Scholar]