Abstract
BACKGROUND
The health and economic burden from liver disease in the United States is substantial and rising. The objective of this study was to characterize temporal trends in mortality from chronic liver disease and liver cancer and the incidence of associated risk factors using population-based data over the past 30 years.
METHODS
Population-based mortality data were obtained from the National Vital Statistics System, and population estimates were derived from the national census for US adults (aged >45 years). Crude death rates (CDRs), age-adjusted death rates (ADRs), and average annual percentage change (AAPC) statistics were calculated.
RESULTS
In total, 690,414 deaths (1.1%) were attributable to chronic liver disease, whereas 331,393 deaths (0.5%) were attributable to liver cancer between 1981 and 2010. The incidence of liver cancer was estimated at 7.1 cases per 100,000 population. Mortality rates from chronic liver disease and liver cancer increased substantially over the past 3 decades, with ADRs of 23.7 and 16.6 per 100,000 population in 2010, respectively. The AAPC from 2006 to 2010 demonstrated an increased ADR for chronic liver disease (AAPC, 1.5%; 95% confidence interval, 0.3%–2.8%) and liver cancer (AAPC, 2.6%; 95% confidence interval, 2.4%–2.7%).
CONCLUSIONS
A comprehensive approach that involves primary and secondary prevention, increased access to treatment, and more funding for liver-related research is needed to address the high death rates associated with chronic liver disease and liver cancer in the United States.
Keywords: liver cancer, chronic liver disease, epidemiology, mortality
INTRODUCTION
The health and economic burden associated with chronic liver disease and liver cancer in the United States is substantial.1,2 An estimated 150,000 new patients are diagnosed with chronic liver disease each year in the United States, and nearly 20% of these patients have cirrhosis at presentation.3 The increase in cirrhosis, along with rises in the prevalence of other risk factors, has resulted in a growing incidence of primary liver cancer.4 Once diagnosed, primary liver cancer generally carries a guarded prognosis with an estimated 5-year survival of only 25% to 40% and a median overall survival of 1 to 60 months, depending on the stage of disease at presentation.2,5–11 The most common form of primary liver cancer, hepatocellular carcinoma (HCC), is the third leading cause of cancer death in the United States despite a relatively low incidence rate of <5 per 100,000 population.12,13 Preventable risk factors for primary liver cancer include alcohol abuse and infection with hepatitis B virus (HBV) and hepatitis C virus (HCV), and up to 50% of liver cancers in the United States are still attributable to HBV or HCV.12,14–16 Increasing rates of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) also have been observed as worldwide rates of obesity continue to surge.15,17,18 In turn, obesity, NAFLD, and NASH have been implicated in the development of both chronic liver disease and liver cancer, and observational studies have demonstrated a correlation between rising rates of obesity and chronic liver disease.18–22
National efforts to impact the mortality burden associated with liver diseases have not received enough attention.23 To our knowledge, no large population-based study has explicitly examined the mortality of liver-related diseases in relation to the changing incidence of obesity, chronic liver disease, and cirrhosis at the national level. Therefore, the purpose of the current study was to characterize temporal trends in the disease-specific mortality associated with chronic liver disease and liver cancer. Specifically, using population-based data, we sought to identify and define temporal changes in the associated death rates of certain underlying liver-related risk factors (eg, HBV, HCV, obesity, etc), chronic liver disease, as well as primary liver cancer relative to other major causes of mortality in the United States (eg, cardiovascular, diabetes, etc).
MATERIALS AND METHODS
Deaths and Population Estimates
We obtained population-based mortality data from the National Vital Statistics System (NVSS) as well as population estimates derived from the national census between 1981 and 2010.24 Annual numbers of deaths, defined as the underlying cause of death noted on the death certificate, were recorded. The underlying causes of death were classified according to the Ninth Revision of the International Classification of Diseases (ICD-9) for the years 1981 through 1998 and the 10th Revision of the ICD (ICD-10) for the years 1999 through 2010. Specifically, the annual number of deaths from all causes (ICD-9 code 001-E999, ICD-10 code A00-Y89), viral hepatitis (ICD-9 code 070, ICD-10 code B15-19), all cancer (ICD-9 code 140–208, ICD-10 code C00-C97), liver cancer (ICD-9 code 155, ICD-10 code C22), diabetes (ICD-9 code 250, ICD-10 code E10-E14), major cardiovascular disease (CVD) (ICD-9 codes 390–434 and 436–448, ICD-10 code I00-I78), chronic liver disease and cirrhosis (ICD-9 code 571, ICD-10 codes K70 and K73-K74), and alcoholic liver disease (ICD-9 code 571.0–571.3, ICD-10 code K70) were obtained for analysis and comparison.25,26 Because the overwhelming majority of patients who are diagnosed with liver disease aged >45 years, data were collected for all adults in the United States using this cutoff age. To associate trends of risk factors and potential associations with cause-specific mortality, data on the incidence of HBV, HCV, and liver cancer as well as the prevalence of obesity (defined as a body mass index >30 kg/m2) also were collected from the Centers for Disease Control and Prevention (CDC).16,27,28
Statistical Analysis
Crude death rates (CDRs) and age-adjusted death rates (ADRs) and 95% confidence intervals (CIs) were calculated as cases per 100,000 population. The standard error and the 95% CIs for age-adjusted death rates were calculated based on the method originally described by Keyfitz.29 Yearly cause-specific death rates were calculated and also were as stratified into 5-year periods from 1981 to 2010. CDRs were calculated from the total number of deaths from a particular cause in the given year or 5-year period by using the mid-year resident population. ADRs were calculated by direct standardization methods using the 2000 US population as the standard population.30
To illustrate recent trends, we analyzed the chronological pattern of cause-specific death rates from 2006 to 2010. We used joinpoint regression models to calculate annual percentage change (APC) statistics, which characterize the magnitude and direction of short-term (2006–2010) and long-term (1981–2010) trends in ADR. The same joinpoint regression models also were used to calculate trends in incidence rates of liver cancer between 1999 and 2010.31 Recent epidemiologic studies have used Join-point, a statistical software package (version 4.0.4; Surveillance Research Program, National Cancer Institute, Bethesda, Md) that provides a best-fitting linear regression model for incidence rates over time using the least amount of “joinpoint.”2,23 Through this approach, we calculated the APC and the average APC (AAPC) between 2006 and 2010. Trends were considered statistically significantly if each joinpoint indicated a change in trend with a 95% CI that did not overlap zero (2-sided t test; P <.05) using a Monte-Carlo permutation method. For this study, a maximum of 3 joinpoints (4 line segments) were allowed for each analysis. The Joinpoint Regression Program was used for the joinpoint analysis, whereas other statistical analyses used STATA version 12.0 (Stata-Corp, College Station, Tex).23,31
RESULTS
CDRs From All Causes and Underlying Diseases: 1981 to 2010
In total, 61,744,032 deaths from all causes among individuals aged >45 years in the United States were registered between 1981 and 2010. Of these, 690,414 deaths (1.1%) were attributable to chronic liver disease and cirrhosis, whereas 331,393 deaths (0.5%) were attributable to liver cancer. CDRs among individuals aged >45 years for all causes declined over the study period from 2490.03 deaths per 100,000 population in 1981 to 1882.67 deaths per 100,000 population in 2010. The largest drops in CDR were from the top 2 leading causes of death in the United States—CVD and diabetes. The CDR from major CVD dropped from a high of 1343.57 deaths per 100,000 population in 1981 to a low of 624.69 deaths per 100,000 population in 2010, representing nearly a 54% decrease. Similarly, crude deaths from diabetes declined nearly 14% over the study period, with the highest drop in deaths reported during the late 1990s. Deaths from all cancer followed a similar trend, decreasing 23.8% over the study period (from 565.8 deaths per 100,000 population in 1981 to 457.0 deaths per 100,000 population in 2010). In contrast, the CDR from liver cancer doubled between 1981 (8.0 deaths per 100,000 population) and 2010 (16.3 deaths per 100,000 population). It is noteworthy that, although the CDRs from chronic liver disease and cirrhosis decreased (from 34.7 deaths per 100,000 population in 1981 to 23.8 deaths per 100,000 population in 2010) over the study period, the CDR from viral hepatitis increased (from 0.8 deaths per 100,000 population in 1981 to 5.9 deaths per 100,000 population in 2010). Similarly, deaths from alcoholic liver disease decreased over the study period from a high of 13.5 deaths per 100,000 population in 1981 to a nadir of 9.7 deaths per 100,000 population in 2002/2003.
ADRs for All Causes and Underlying Disease and Trends in Risk Factors: 1981 to 2010
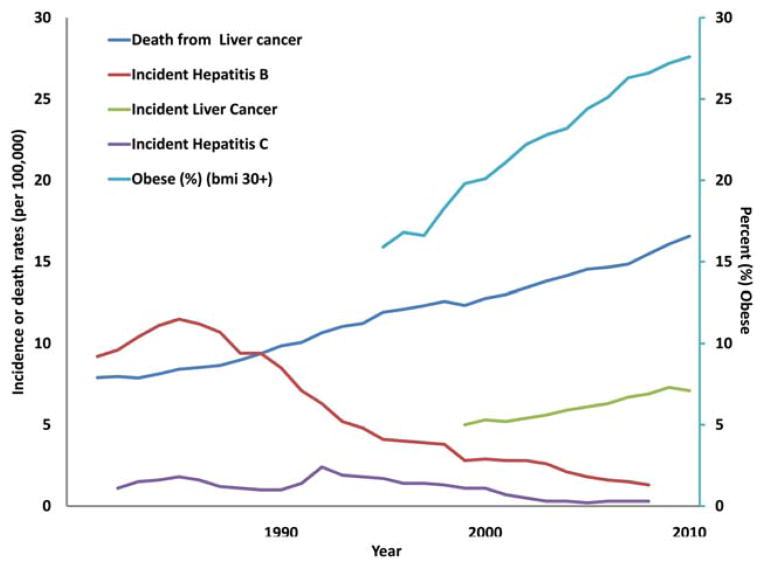
Variations in population structure over time were accounted for by calculating ADRs using the 2000 US population as the standard population. The ADRs from all causes (2624.5 deaths per 100,000 population in 1981 to 1962.7 deaths per 100,000 population in 2010) and from all cancer (560.5 deaths per 100,000 population in 1981 to 476.2 deaths per 100,000 population in 2010) sharply declined over the study period (Table 1). ADRs for chronic liver disease and cirrhosis declined over the study period from a high of 33.5 deaths per 100,000 population in 1981to a nadir of 22.1 deaths per 100,000 population in 2006. In contrast, deaths from liver cancer rose from a low of 7.9 deaths per 100,000 population in 1981 to 16.6 deaths per 100,000 population in 2010. The incidence of liver cancer increased 3.7% between 1999 and 2010 and is currently estimated at 7.1 new cases per 100,000 population (P <.05). It is noteworthy that incidence rates of risk factors for chronic liver disease and cancer were mixed during the study period (Fig. 1). Incident cases of HBV declined after reaching a peak of 11.5 new cases per 100,000 population in 1985 to a low of 1.3 new cases per 100,000 population in 2008. Similarly, incident HCV cases declined after 1992, reaching a low of 0.3 new cases per 100,000 population in 2008. The prevalence of obesity, however, increased nearly 2-fold from 15.9% of the US population in 1995 to 28.1% in 2010.
TABLE 1.
Age-Adjusted Death Rates in Individuals Aged >45 Years According to Underlying Diseases in the United States From 1981 to 2010 per 100,000 Population
| Underlying Disease | Rate per 100,000 Population (95% CI)
|
||
|---|---|---|---|
| 1981–1985 | 1986–1990 | 1991–1995 | |
| All causes | 2592.0 (2590.3–2593.7) | 2504.1 (2502.5–2505.7) | 2376.2 (2374.8–2377.7) |
| Viral hepatitis | 0.8 (0.8–0.8) | 1.2 (1.2–1.3) | 2.3 (2.2–2.3) |
| Cancer | 569.1 (568.4–569.9) | 581.9 (581.1–582.6) | 582.8 (582.1–583.5) |
| Liver cancer | 8.1 (8.0–8.2) | 9.1 (9.0–9.2) | 11.0 (11.1–12.3) |
| Diabetes mellitus | 47.6 (47.4–47.9) | 51.3 (51.1–51.6) | 60.0 (59.7–60.2) |
| Major cardiovascular diseases | 1392.1 (1390.9–1393.4) | 1231.0 (1229.9–1232.2) | 1087.4 (1086.4–1088.4) |
| Chronic liver disease and cirrhosis | 31.1 (30.9–31.3) | 27.5 (27.3–27.7) | 24.5 (24.4–24.7) |
| Alcoholic liver disease | 12.1 (12.0–12.2) | 11.5 (11.3–11.6) | 10.8 (10.7–10.9) |
| Underlying Diseases | Rate per 100,000 Population (95% CI)
|
||
|---|---|---|---|
| 1996–2000 | 2001–2005 | 2006–2010 | |
| All causes | 2304.7 (2303.3–2306.1) | 2195.6 (2194.3–2196.9) | 2009.2 (2008.1–2010.5) |
| Viral hepatitis | 3.9 (3.9–4.0) | 4.6 (4.5–4.6) | 5.8 (5.8–5.9) |
| Cancer | 555.1 (555.4–555.8) | 523.7 (523.1–524.4) | 486.4 (485.8–487.0) |
| Liver cancer | 12.4 (12.3–12.5) | 13.8 (13.7–13.9) | 15.6 (15.5–15.7) |
| Diabetes mellitus | 67.0 (66.8–67.3) | 69.4 (69.2–69.6) | 60.3 (60.1–60.5) |
| Major cardiovascular diseases | 991.9 (991.0–992.8) | 859.3 (858.5–860.1) | 693.7 (693.0–694.4) |
| Chronic liver disease and cirrhosis | 23.2 (23.1–23.4) | 22.9 (22.8–23.1) | 22.9 (22.8–23.0) |
| Alcoholic liver disease | 10.1 (10.0–10.2) | 9.7 (9.6–9.8) | 10.5 (10.4–10.6) |
Abbreviations: CI, confidence interval.
Figure 1.
Trends in the incidence and prevalence of various risk factors for liver cancer are illustrated. BMI indicates body mass index (measured in kg/m2).
Trends in ADRs From All Causes and Underlying Diseases: 1981 to 2010
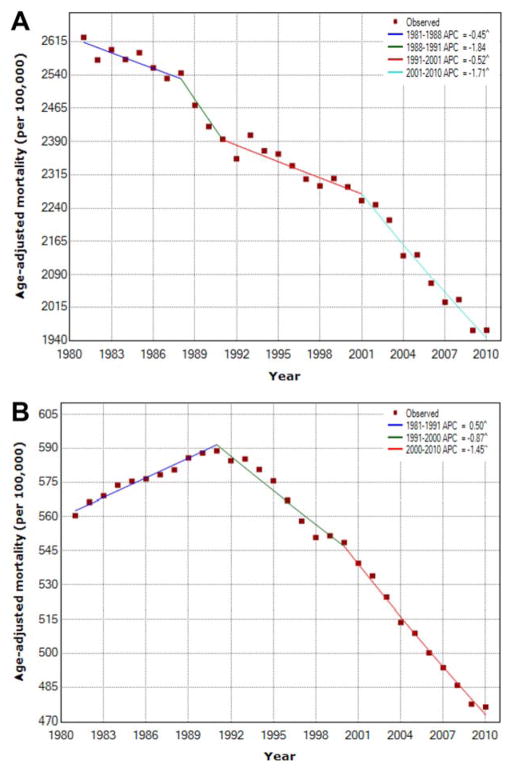
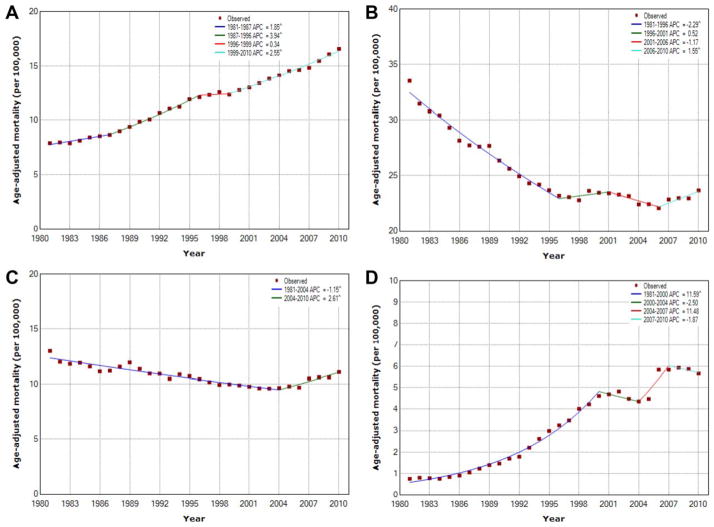
ADRs from all causes, all cancer, major CVD, and diabetes decreased over the study period (Fig. 2). Deaths from CVD experienced the greatest change over the last decade, with a 4.2% reduction in deaths between 2001 and 2010 (Table 2). The steepest decline in deaths from all cancer also occurred during the past decade (2001–2010), with a 1.4% reduction in the ADR during this period. In contrast, the ADR from underlying liver diseases was mixed but generally increased over the study period (Fig. 3). Chronic liver disease and cirrhosis ADRs were mixed, declining by 2.3% until 1996 (95% CI, −2.5% to −2.1%) but witnessing a recent sharp increase from 2006 to 2010 (APC, 1.5%; 95% CI, 0.3%–2.8%). The ADR for liver cancer increased for each study period, with an APC of 2.6% in the most recent period (1999–2010). The ADR from viral hepatitis had the largest increase among mortality causes analyzed, with an 11.6% increase from 1981 to 2000 and an 11.5% increase from 2004 to 2007.
Figure 2.
Trends in age-adjusted death rates are illustrated using Joinpoint analyses from (A) all causes, (B) all cancer. APC indicates annual percentage change. P ≤ .05.
TABLE 2.
Trends in Age-Adjusted Death Rates for Selected Diseases in Individuals Aged >45 Years in the United States From 1981 to 2010 Using Joinpoint Analysis
| Disease | Trend 1
|
Trend 2
|
Trend 3
|
Trend 4
|
AAPC (2006–2010) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Years | APC | Years | APC | Years | APC | Years | APC | ||
| All causes | 1981–1988 | −0.1a | 1988–1991 | −1.8 | 1991–2001 | −0.5a | 2001–2010 | −1.7a | −1.7 a |
| Viral hepatitis | 1881–2000 | 11.6a | 2000–2004 | −2.5 | 2004–2007 | 11.5 | 2007–2010 | −1.9 | 1.3 |
| Cancer | 1981–1991 | 0.5a | 1991–2000 | −0.9a | 2000–2010 | −1.4a | - | −1.4a | |
| Liver cancer | 1981–1987 | 1.8a | 1987–1996 | 3.9a | 1996–1999 | 0.3 | 1999–2010 | 2.6a | 2.6a |
| Diabetes mellitus | 1981–1987 | −0.2 | 1987–1990 | 6.2a | 1990–2002 | 2.1a | 2002–2010 | −2.9a | −2.9a |
| Major cardiovascular disease | 1981–1987 | −2.0a | 1987–1990 | −3.6a | 1990–2001 | −1.8a | 2001–2010 | −4.2a | −4.2a |
| Chronic liver disease and cirrhosis | 1981–1996 | −2.3a | 1996–2001 | 0.5 | 2001–2006 | −1.2 | 2006–2010 | 1.5a | 1.5a |
| Alcoholic liver disease | 1981–2004 | −1.1a | 2004–1010 | 2.6a | - | - | 2.6a | ||
Abbreviations: AAPC, average annual percentage change; APC, annual percentage change.
APC and AAPC are statistically significantly different from zero.
Figure 3.
Trends in age-adjusted death rates are illustrated using Joinpoint analyses from (A) liver cancer (B) chronic liver disease and cirrhosis, (C) alcoholic liver disease, and (D) viral hepatitis. APC indicates annual percentage change. P ≤ .05.
Recent short-term trends evaluated according to the AAPC were conducted for all causes and underlying diseases. The AAPC during the most recent 5 years reflected a statistically significant decline in the ADRs for all causes (AAPC, −1.7%; 95% CI, −1.9% to −1.5%), cancer (AAPC, −1.4%; 95% CI, −1.5% to −1.3%), diabetes mellitus (AAPC, −2.9%; 95% CI, −3.4% to −2.5%), and major CVD (AAPC, −4.2%; 95% CI, −4.4% to −3.9%). However, increasing short-term ADRs were observed for liver diseases such as liver cancer (AAPC, 2.6%; 95% CI, 2.4%–2.7%), chronic liver disease and cirrhosis (AAPC, 1.5%; 95% CI, 0.3%–2.8%), viral hepatitis (AAPC, 1.3%; 95% CI, −1.8% to 4.5%), and alcoholic liver disease (AAPC, 2.6%; 95% CI, 2.4%–2.7%).
DISCUSSION
Large population-based studies are necessary to elucidate the true efficacy of interventions and national initiatives aimed at lowering mortality for a variety of diseases. The current study is important because we used a wide array of population-based data from a variety of sources, including the NVSS, the US national census, as well as the CDC. In doing this, we were able to provide a comprehensive assessment of national trends in the epidemiology of chronic liver disease, liver cancer, and associated liver-specific risk factors in the United States over the past 30 years. Whereas the incidence of liver cancer increased 3.7% between 1999 and 2010, incident cases of HBV and HCV declined. In contrast, the prevalence of obesity nearly doubled, with >25% of the US population categorized as obese in 2010. Perhaps more noteworthy, we observed a decrease in the ADRs from all causes, all cancer, major CVD, and diabetes over the study period but an increase in the ADR of patients with viral hepatitis and liver cancer over that same time. In fact, the AAPC increased by about 1.5% to 2.5% per year between 2006 and 2010 for each of the liver diseases examined, including viral hepatitis, cirrhosis, and liver cancer. Collectively, data from the current study serve to underscore the ongoing fatal impact that liver cancer and other liver-related diseases continue to have in the United States.
Primary liver cancer has traditionally been considered a major health care concern of non-US populations, including Asia and sub-Saharan Africa.12,32,33 In these geographic areas, risk factors, including HBV and HCV, and exposure to environment risk factors, such as aflatoxin, have resulted in a high incidence of liver disease.12,13,32–34 More recently, however, there has been growing recognition that the incidence of primary liver cancer is on the rise in the United States. Altekruse et al noted that the incidence of HCC in 2005 was 4.9 per 100,000 population and has been steadily rising at a rate of 4.5% per year for the last 3 decades.4 In the current study, we similarly noted an increase in liver cancer in the United States with an overall incidence of 7.1 per 100,000 population in 2010. Although the incidence of liver cancer may be relatively low compared with some other diseases, mortality from liver cancer has steadily risen over the past 30 years, with an increase greater than 2-fold in the ADR. In fact, the ADR for liver cancer increased 27.5% in the last 10 years analyzed (2001–2010) (Fig. 3). The reason for this ongoing rise in mortality associated with liver cancer is undoubtedly multifactorial. Although some patients with liver cancer may be candidates for resection, ablation, or transplantation; most patients, because of advanced disease, are not surgical candidates and have more limited therapeutic options (eg, chemoembolization or systemic therapy).35 Other investigators have demonstrated that, although advances in liver transplantation and hepatic resection have improved survival for a select population, the effect on overall disease-specific mortality at the national level has been more limited.4,36 Furthermore, our group has documented variation in choice of therapy for patients with liver cancer and has demonstrated that receipt of therapy depends on a wide variety of factors, including clinical data as well as provider-level and hospital-level factors.37,38 In fact, even referral to a specialist varies considerably, with barriers to treatment preventing up to 50% of patients who have early stage disease from receiving potentially curative therapy.39–41
Thus, as the US population continues to age disproportionately, it seems reasonable to conclude that the increased ADR associated with liver cancer and other chronic liver diseases may be attributable in part to this aging phenomenon. In the current study, however, we specifically assessed both the CDR and the ADR. Although the CDR may have been subject to confounding because of an aging population, we controlled for age in the ADR models. It is noteworthy that, even after controlling for age, we noted increases in the ADR for liver cancer over the last 30 years and for chronic liver disease over the last 5 years. These data suggest that, rather than age, other factors like HBV or HCV exposure may be responsible for the documented increased trend in liver-specific mortality over time. El-Serag previously reported that chronic hepatic viral infections account for approximately 80% of all cases of HCC in the United States.33 By comparison, data from the current study indicate that incident cases of HBV and HCV dropped nearly 8-fold over the last 30 years and are currently estimated to be only 1.3 and 0.3 per 100,000 population, respectively. For example, we noted that the incidence of HBV infection has been on the decline since the mid-1980s, paralleling the introduction and widespread implementation of the HBV vaccine (Fig. 1). In fact, vaccination rates have continued to increase, such that an estimated 93% of US children are currently vaccinated against HBV.42 Despite the reduction in HBV and HCV infection, we observed that mortality from viral hepatitis and chronic liver disease was on the rise. These findings were similar to other recent studies that reported a decrease in HBV and HCV infection yet an increase in hepatic viral-related mortality.14,16,43 Indeed, the CDC predicts that the trend in increasing viral-related mortality will continue, with greater than 170,000 deaths from HBV-related and HCV-related liver diseases predicted between 2010 and 2019.22,44 Consistent with the CDC’s projection, we noted that the ADR from viral hepatitis had the largest increase among mortality causes analyzed, with an 11.6% increase from 1981 to 2000 and an 11.5% increase from 2004 to 2007. Thus, although efforts need to be devoted to primary prevention, attention and energy should also be directed toward secondary prevention efforts among patients with chronic viral hepatitis infection.45
Although much attention has been given to the financial and societal impact caused by rising obesity rates, its impact on liver disease has been less popularized. It has been estimated that obese and diabetic patients are at nearly twice the risk of developing liver cancer than non-obese, nondiabetic patients.46–49 Indeed, although viral hepatitis infection increases the risk of liver cancer more than any other risk factor for any given individual patient, diabetes and obesity are responsible for the greatest population-based attributable risk for liver cancer in the United States.50 A host of previous studies defined the importance of metabolic risk factors, such as obesity and type 2 diabetes, on chronic liver disease and liver cancer.20,51–53 Specifically, obesity is a recognized risk factor in the development of NAFLD, NASH, and primary liver cancer.19–22,46,54 In turn, increasing obesity rates undoubtedly have contributed to the rise in the incidence of NASH and NAFLD, which now affect anywhere between 12% and 46% of the US population.55 Data from our population-based cohort similarly noted a dramatic increase in the incidence of obesity, with obesity tripling over the last 3 decades to the point that nearly 3 in every 10 individuals in the United States in 2010 had a body mass index >30 kg/m2 (Fig. 1). We observed that this dramatic increase in obesity paralleled an increase in chronic liver disease and liver cancer, which was consistent with a previous report by Nordenstedt et al (Fig. 1).56 Because recent trends demonstrate no slowing in the rising epidemic of obesity, the subsequent impact on liver disease will likely continue to worsen.
The current study had several limitations. Although broad in scope, the use of large, population-based data sets inherently lack certain detailed clinical, pathologic, and treatment-related data. Therefore, we could not examine more specific categories of liver disease, and we also were unable to characterize mortality rates according to extent of disease (eg, viral load, stage of liver cancer, etc). The purpose of the study, however, was not to examine patterns of care or outcomes among certain subtypes of patients but, rather, to define broad temporal trends in population-based death rates associated with chronic liver disease and liver cancer in the United States. To this end, the use and synthesis of data from 3 distinct, population-based resources (ie, NVSS, the US national census, and the CDC) was a particular strength of the current study. Another potential limitation involved the reliance on death certificate records for cause of death. Although the cause of death recorded on a death certificate sometimes may be misclassified, death certificates are the standard and accepted means to procure this information.57,58 However, any possible misclassification bias related to the documentation of death on the certificate would likely be nondifferential in nature. In addition, patients with multiple comorbid conditions like HBV and HCV are only reported as having a single, primary cause of death on the death certificate. In turn, information regarding the proportion of patients who might have had overlapping diseases (eg, HCV, HBV, etc) was unavailable.
In conclusion, mortality rates from chronic liver disease and liver cancer have increased substantially over the past 3 decades. In 2010, the ADRs associated with chronic liver disease and liver cancer were 23.67 and 16.57 per 100,000 population, respectively. Although we noted that certain risk factors, such as HBV and HCV, decreased in incidence over time, there was a dramatic rise in the prevalence of other risk factors such as obesity. Although they are still relatively uncommon in the United States, liver diseases, including chronic liver disease and liver cancer, strongly impact health care because of their associated high mortality. A comprehensive approach that involves both primary and secondary prevention, increased access to treatment, and more funding for liver-related research is needed if we hope to address the high death rates associated with chronic liver disease and liver cancer in the United States.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Ejaz is supported by the E. B. Pillsbury Foundation. Dr. Anders reports grants from the National Institutes of Health and grants and other support from Bristol-Myers Squibb during the conduct of the study.
References
- 1.Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Bell BP, Manos MM, Zaman A, et al. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol. 2008;103:2727–2736. doi: 10.1111/j.1572-0241.2008.02071.x. quiz 2737. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morimoto Y, Tanaka Y, Ito T, et al. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:432–440. doi: 10.1007/s00534-002-0842-3. [DOI] [PubMed] [Google Scholar]
- 6.Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008;143:366–374. doi: 10.1016/j.surg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Choi S-B, Kim K-S, Choi J-Y, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez MA, Walters RS, Burke TW, editors. 60 Years of Survival Outcomes at The University of Texas MD Anderson Cancer Center. New York: Springer; 2013. [Google Scholar]
- 9.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in Surveillance, Epidemiology, and End Results registries, 1992–2008. Hepatology. 2012;55:476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 13.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(suppl):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 15.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Viral Hepatitis Surveillance-United States, 2011. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 17.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 18.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 19.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 21.Polesel J, Zucchetto A, Montella M, et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2009;20:353–357. doi: 10.1093/annonc/mdn565. [DOI] [PubMed] [Google Scholar]
- 22.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu D, Katanoda K, Marugame T, Sobue T. A Joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958–2004) Int J Cancer. 2009;124:443–448. doi: 10.1002/ijc.23911. [DOI] [PubMed] [Google Scholar]
- 24.Murphy SL, Xu JQ, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2010;61:1–118. [PubMed] [Google Scholar]
- 25.World Health Organization. International Statistical Classification of Disease and Related Health Problems. 9th revision. Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 26.World Health Organization. International Statistical Classification of Disease and Related Health Problems. 10th revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 27.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2013. [Accessed on January 15, 2014]. Available at: http://www.cdc.gov/uscs. [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 29.Keyfitz N. 3. Sampling variance of standardized mortality rates. Hum Biol. 1966;38:309–317. [PubMed] [Google Scholar]
- 30.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected US population. Healthy People 2010 Stat Notes. 2001;20:1–10. [PubMed] [Google Scholar]
- 31.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris) 2010;58:273–277. doi: 10.1016/j.patbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Hatzaras I, Bischof DA, Fahy B, Cosgrove D, Pawlik TM. Treatment options and surveillance strategies after therapy for hepatocellular carcinoma. Ann Surg Oncol. 2014;21:758–766. doi: 10.1245/s10434-013-3254-5. [DOI] [PubMed] [Google Scholar]
- 36.Capocaccia R, Sant M, Berrino F, Simonetti A, Santi V, Trevisani F. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol. 2007;102:1661–1670. doi: 10.1111/j.1572-0241.2007.01337.x. quiz 1660, 1671. [DOI] [PubMed] [Google Scholar]
- 37.Nathan H, Segev DL, Bridges JF, et al. Influence of nonclinical factors on choice of therapy for early hepatocellular carcinoma. Ann Surg Oncol. 2013;20:448–456. doi: 10.1245/s10434-012-2619-5. [DOI] [PubMed] [Google Scholar]
- 38.Nathan H, Bridges JF, Schulick RD, et al. Understanding surgical decision making in early hepatocellular carcinoma. J Clin Oncol. 2011;29:619–625. doi: 10.1200/JCO.2010.30.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyder O, Dodson RM, Nathan H, et al. Referral patterns and treatment choices for patients with hepatocellular carcinoma: a United States population-based study. J Am Coll Surg. 2013;217:896–906. doi: 10.1016/j.jamcollsurg.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan H, Hyder O, Mayo SC, et al. Surgical Therapy for early hepatocellular carcinoma in the modern era: a 10-year SEER-Medicare analysis. Ann Surg. 2013;258:1022–1027. doi: 10.1097/SLA.0b013e31827da749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stacy S, Hyder O, Cosgrove D, et al. Patterns of consultation and treatment of patients with hepatocellular carcinoma presenting to a large academic medical center in the US. J Gastrointest Surg. 2013;17:1600–1608. doi: 10.1007/s11605-013-2253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannou GN. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154:319–328. doi: 10.7326/0003-4819-154-5-201103010-00006. [DOI] [PubMed] [Google Scholar]
- 43.Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995–2004. Hepatology. 2008;47:1128–1135. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 45.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 46.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 48.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of 1 million United States adults. Diabetes Care. 2012;35:1835–1844. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou XH, Qiao Q, Zethelius B, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53:1867–1876. doi: 10.1007/s00125-010-1796-7. [DOI] [PubMed] [Google Scholar]
- 50.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44(suppl 19):96–101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 52.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 53.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 55.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 56.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(suppl 3):S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manos MM, Leyden WA, Murphy RC, Terrault NA, Bell BP. Limitations of conventionally derived chronic liver disease mortality rates: results of a comprehensive assessment. Hepatology. 2008;47:1150–1157. doi: 10.1002/hep.22181. [DOI] [PubMed] [Google Scholar]
- 58.German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35:126–131. doi: 10.1016/j.canep.2010.09.005. [DOI] [PubMed] [Google Scholar]