Abstract
Down syndrome (DS), one of the most common birth defects and the most widespread genetic cause of intellectual disabilities, is caused by extra genetic material on chromosome 21 (HSA21). The increased genomic dosage of trisomy 21 is thought to be responsible for the distinct DS phenotypes, including an increased risk of developing some types of childhood leukemia and germ cell tumors. Patients with DS, however, have a strikingly lower incidence of many other solid tumors. We hypothesized that the third copy of genes located in HSA21 may have an important role on the protective effect that DS patients show against most types of solid tumors. Focusing on Copy Number Variation (CNV) array data, we have generated frequencies of deleted regions in HSA21 in four different tumor types from which DS patients have been reported to be protected. We describe three different regions of deletion pointing to a set of candidate genes that could explain the inverse comorbidity phenomenon between DS and solid tumors. In particular we found RCAN1 gene in Wilms tumors and a miRNA cluster containing miR-99A, miR-125B2 and miR-LET7C in lung, breast, and melanoma tumors as the main candidates for explaining the inverse comorbidity observed between solid tumors and DS.
Keywords: down syndrome, cancer genomics, Chr. 21p11, RCAN1, BTG3, inverse comorbidity
Introduction
Down syndrome (DS) was first described and named by the British physician John Langdon Down about 150 years ago. However, Professor Jerome Lejeune discovered a century later that the cause of this disorder was the presence of an extra chromosome 21 (HSA21) (Mégarbané et al., 2009). Today, DS is the most widespread cause of genetic intellectual disability, with an approximated prevalence of between 1 in 1000 and 1 in 1100 live births worldwide, and roughly 3000–5000 new cases per year (www.who.int). The Down phenotype is caused by a complete or partial trisomy (TS21) of human chromosome 21 (HSA21) acquired essentially by meiotic non-disjunction events during gametogenesis. Besides the cognitive impairment typical of DS patients, a variable set of associated conditions, such as congenital heart disease, vision problems, hearing loss, or decreased immune system activity, has been linked to the disease (Roizen and Patterson, 2003). In the past two decades, substantial advances in exploring the human genome, together with the exponential growth of bioinformatics, have permitted a better understanding of the functional links between the extra copy of HSA21 and the variations in the different phenotypes of DS individuals (Letourneau and Antonarakis, 2012; Letourneau et al., 2014). The sequencing of HSA21 and the experimental research done with models of DS have allowed the scientific community to connect specific genomic regions and sets of genes to different clinical conditions and syndrome phenotypes. Initially, a total of 225 genes were identified (Hattori et al., 2000), and 2 years later the gene content was updated to 329 genes (Kapranov et al., 2002). Nowadays, based on data provided by large-scale studies, the HSA21 gene number is estimated to be around 534 from a total of 2176 gene transcripts (Scarpato et al., 2014).
New epidemiological insights attempting to define direct and inverse comorbidities between complex disorders have started to reveal the complicated connections that DS and cancer may share. This epidemiological evidence points to both a higher- and a lower-than-expected probability of developing some tumors in patients with DS (Ibáñez et al., 2010; Tabarés-Seisdedos et al., 2011; Tabarés-Seisdedos and Rubenstein, 2013). Catalá-López et al. (2014) conducted a meta-analysis based on published epidemiological studies reporting comorbidities between cancer and CNS disorders, gathering data for 17,090 DS patients. This study showed an increased risk for developing leukemia and testicular cancer in DS patients. Other studies in different ethnographic populations have evidenced a lower incidence of most tumors in patients with DS (Hasle et al., 2000; Boker et al., 2001; Nižetić and Groet, 2012). In particular, breast cancer incidence shows a considerable decrease in DS patients compared with age-matched euploid individuals.
Interestingly, the observed protection against many solid tumors in DS patients has opened a window of opportunity in the search for tumor suppressor genes located on chromosome 21 that would amplify their effect in a dosage-dependent way. Over the last few years, and in parallel to the above, a great amount of research has focused on the associations between genomic alterations, like somatic copy number variations (CNVs), and cancer (Rubio-Moscardo et al., 2005; Climent et al., 2007; Jönsson et al., 2012; Hieronymus et al., 2014). The result of such analyses is a noteworthy amount of open access microarray data available at public repositories such as NCBI Gene Expression Omnibus (GEO, at http://www.ncbi.nlm.nih.gov/geo/), or EBI (ArrayExpress http://www.ebi.ac.uk/arrayexpress/).
In the present perspective, we first approached DS as a genetic disorder caused by the extra copy of genomic DNA on chromosome 21. Our aim was to collect previously published CNV array data from cancers for which there exists an observed decreased incidence in DS as compared to that for non-DS patients. We then screened for the most common structural alterations present in chromosome 21. This might lead to identifying regions containing genes potentially responsible for the protection against solid tumor development in DS individuals. Our underlying hypothesis was that copy gains of tumor suppressor genes in chromosome 21 of DS patients would be responsible for the protection phenomenon observed between DS and most types of solid tumors. Therefore, we expected to find those genes in the maximum frequency deletion regions of tumor samples coming from non-DS patients. To our knowledge, our work presents the first attempt at defining tumor suppressor candidate genes using this approach. We selected four tumor types described as low incidence tumors in DS by epidemiological studies: breast cancer (359 samples), lung cancer (78 samples), melanoma (34 samples) and Wilms tumor (18 samples). Breast and lung cancer showed a standardized incidence ratio (SIR) of 0.4 and 0.24, respectively, between DS and age-matched euploid population cohorts (Hasle et al., 2000; Patja et al., 2006). The SIR value for the skin cancer group, in which melanoma was included, was 0.25. Kidney cancer, including Wilms tumor, showed a SIR value of 0.84 (Hasle et al., 2000; Patja et al., 2006), and no cases of DS were found in a study that included 5854 Wilms tumor patients (Olson et al., 1995).
Materials and methods
Array CGH analysis
We analyzed data from multiple platforms to generate both the disease-specific frequencies of amplification and deletion, as well as the frequency summaries for four tumor types described as low incidence tumors in DS by epidemiological studies based on previously published data (Table 1). Raw Array CGH data files were downloaded from Gene Expression Omnibus, and normalization and conversion to log2 values of intensity were performed. Probe annotations from assemblies older than hg19 were remapped to hg19 using Lift Genome Annotation tool, software (http://genome.ucsc.edu/cgi-bin/hgLiftOver) which converts coordinates and genome annotation files between assemblies. To define amplification and deletion regions, we used the R-package CGHcall, which applies the Circular Binary Segmentation (CBS) algorithm (Hsu et al., 2011) it seeks out gain and loss segments by recursively dividing the genome until it identifies segments that have probe distributions different from their neighbors. This was followed by a CGHcall algorithm producing an objective and effective classification of the segmented data into copy number states. Once we had computed platform-specific data for amplification and deletion, we transformed them into multiplatform-comparable data. To do so, we defined 5000 anchor positions along chromosome 21, and performed an estimation of the amplification and deletion value for each of these anchors in every sample for every platform using custom R code (see Supplementary data). Once we had the amplification and deletion values for the anchors, we calculated the frequencies of amplification and deletion for the set of all diseases.
Table 1.
Datasets used for the copy number (CN), gene expression (Exp) and microRNA expression (miR) downloaded from Gene Expression Omnibus (GEO).
| Tumor type | GEO | Platform | Data | References |
|---|---|---|---|---|
| Breast | GSE22133 | SWEGENE_BAC_33K_Full | CN | Jönsson et al., 2012 |
| Melanoma | GSE45354 | Agilent-021924 SurePrint G3 Human CGH Microarray 8 × 60 K | CN | |
| Lung | GSE29065 | SWEGENE_BAC_32K_Full | CN | Staaf et al., 2013 |
| Wilms | GSE28397 | Custom Agilent 2 × 105 K Human Genomic microarray | CN | |
| Breast | GSE22820 | Agilent-014850 Whole Human Genome Microarray 4x44K G4112F | Exp | Liu et al., 2011 |
| Melanoma | GSE46517 | Affy metrix U133A microarray chip | Exp | Kabbarah et al., 2010 |
| Lung | GSE19804 | Affy metrix Human Genome U133 Plus 2.0 Array | Exp | Lu et al., 2010 |
| Wilms | GSE4530 | Homo sapiens 4.8K 02-01 amplified cDN A | Exp | |
| Breast | GSE48088 | [miRNA-2_0] Affy metrix Multispecies miRNA-2_0 Array | miR | Peña-Chilet et al., 2014 |
| Melanoma | GSE35579 | CRUK/Melton lab-Human melanoma-71 -v2 -microRN A expression | miR | Xu et al., 2012 |
| Lung | GSE51855 | Agilent-015508 Human miRNA Microarray | miR | Arima et al., 2014 |
| Wilms | GSE38419 | Febit Homo Sapiens miRBase 13.0 | miR | Schmitt et al., 2012 |
Expression array analysis
We downloaded raw data from GEO, selecting the datasets describing gene expression from both tumor samples, as well as from normal tissue, in order to perform differential expression analysis (see Table 1 below). Data and raw signal intensities were checked for quality, and a quantile normalization was done. A multiple t-test was performed to search for differentially expressed genes located on chromosome 21, and the p-values were corrected for FDR.
miRNA expression array analysis
For the miRNA differential expression analysis, we retrieved raw data from studies that included tumor samples and healthy control tissues. We applied quantile normalization and carried out a differential expression analysis using the SAM method implemented in the R package samr.
Results and discussion
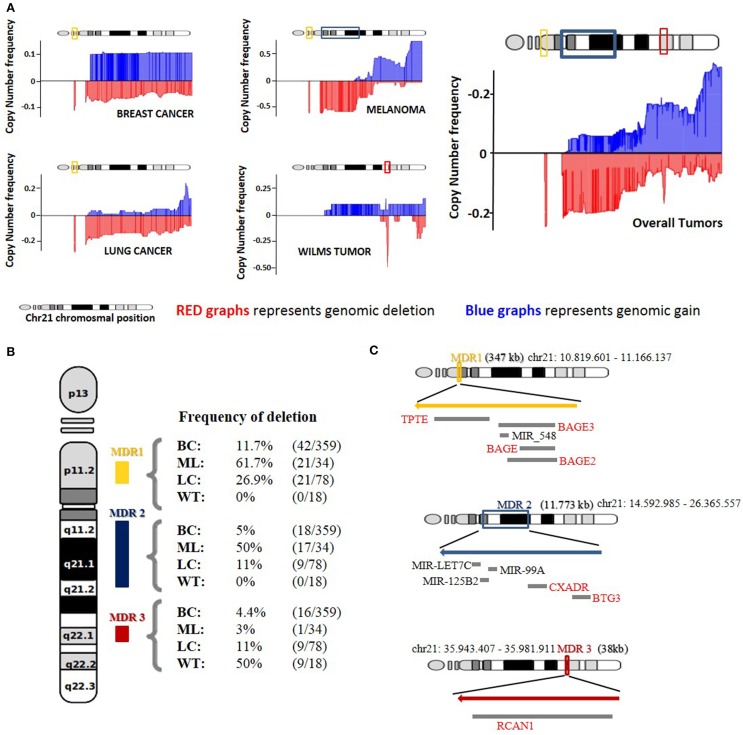
Direct comorbidities between DS and cancer have been the subject of several studies that have shed light onto the primary molecular causes of these associations (Catalá-López et al., 2014; Letourneau et al., 2014). Nevertheless, inverse comorbidities are mainly sketched thanks to epidemiological research, and lack molecular explanations (Tabarés-Seisdedos et al., 2011). To better understand how DS patients could be protected from some types of solid tumors, we have analyzed the copy number profiles of chromosome 21 from four cancer types observed with a lower-than-expected incidence in DS patients by epidemiological data (Nižetić and Groet, 2012; Catalá-López et al., 2014). We generated a mean amplification and deletion profile for the four cancer types and observed three principal maximum deletion regions in chromosome 21. We selected as candidate genes those present in the maximum frequency of deletion regions (MDR) that were further found downregulated in at least 2 of the expression datasets analyzed (Figure 1).
Figure 1.
Copy number analysis of Chromosome 21. (A) Representation of genomic gain (blue) and deletion (red) frequencies on four different tumor types and median average of all tumors together. (B) Deletion frequency for the three major deleted regions (MDR) in breast cancer (BC), melanoma (ML), lung tumors (LT), and Wilms Tumor (WT) on HSA21. (C) Representation of the three MDR based on genomic localization and size with those genes and miRNAs that showed downregulation in the gene expression correlation analysis.
Maximum frequency deletion region 1 (MDR1) is observed at cytoband 21p11.2 and spans from position 10,704,090–11,166,137. Strikingly, this region is the maximum deletion region for 3 of the 4 cancer types included in the analysis. Lung and breast cancers and melanoma show deletion frequencies of 26.9, 11.7, and 61.7%, respectively. Wilms tumor does not present deletion in this region. MDR1 contains the genes TPTE and a cluster of different isoforms of the BAGE gene. The BAGE gene has been reported to be a tumor rejection antigen recognized by cytotoxic T cells (Nagel et al., 2003). An impaired expression of the BAGE gene, due to CNVs, could lead to a reduction in immune system targeting and recognition of cancerous cells capacity. The TPTE gene has been described as a transmembrane tyrosine phosphatase related to the PTEN gene and may play a role in signal transduction pathways. PTEN is a validated tumor suppressor gene with phosphatase activity that inhibits the PI3K pathway (Davidson et al., 2010). The actual TPTE in vivo function is still unclear, but a hypothetical role in PI3K pathway inhibition could explain how an overexpression of this gene in cells carrying an extra-copy of chromosome 21 could protect from cancer cell proliferation and why MDR1 is the most common deleted region in the three cancer types previously described.
Data regarding the CNVs for BAGE and TPTE agree with the results of the differential expression analysis carried out for breast and lung cancer, where both genes are downregulated. The reduction of the genetic product of those genes could confer advantages in the tumor proliferation process. Moreover, MDR1 contains a micro RNA, miR_548, which recently has been reported to play a possible role in tumor suppression through the control of gene FHIT action in human cancer (Hu et al., 2014). We were not able to detect a downregulation of miR_548 in any of the miRNA expression datasets.
Maximum frequency deletion region 2 (MDR2) is a wide deletion region, mainly found in melanoma, spanning from position 14,592,985 to position 26,365,557 and covering cytobands 21q11.2, 21q21.1 and a segment of 21q21.2. It shows a deletion frequency of 50%, and also appears deleted in breast and lung cancer, displaying a deletion frequency of 5 and 11%, respectively (Figure 1B). MDR2 includes 26 coding genes and 3 miRNAs. Given the high number of genes contained in this region, we focused only on those 2 genes that were also downregulated in at least two of the associated expression datasets.
The BTG3 gene is downregulated both in melanoma and breast cancer, while CXADR is downregulated in lung cancer and melanoma. BTG3 has previously been reported to be downregulated in a wide variety of breast cancer cell lines (Yu et al., 2008) and belongs to a protein family characterized by its antiproliferative properties. Moreover, the low expression of BTG3 has previously been related to the progression of several kinds of cancer, such as adenocarcinoma, oral squamous cell cancer, non-small cell lung cancer, prostate cancer, and hepatocellular carcinoma (Chen et al., 2013; Lv et al., 2013). Additionally, the downregulation of BTG3 is a feature of breast cancer patient samples in the study from Liu et al. (2011). Furthermore, data from the expression analysis suggest that BTG3 downregulation may play an important role in melanoma progression, and to our knowledge, this is a result not previously described in the literature. The molecular function reported for BTG3, along with the large number of solid tumors where its downregulation is observed, indicates that BTG3 may be a good candidate for explaining inverse comorbidity events between DS and cancer.
MDR2 also contains a microRNA cluster containing miR-LET7C,miR-152B2, and miR-99A (Figure 1C). It has been shown that the downregulation of miR-LET7C in prostate cancer is followed by an increase in the androgen receptor (AR) expression levels leading to an increased proliferation of the tumor cells. Additionally, the overexpression of miR-LET7C leads to an impairment of AR molecules entailing a reduction in the proliferative capacity of the cancerous cells (Nadiminty et al., 2012). Moreover, low levels of miR-LET7C have been reported in lung tumors associated with high levels of Ras protein, which activate cell proliferation via the MAPK pathway (Johnson et al., 2005). miR-125B2 has been reported to be downregulated in several tumor types, such as breast, prostate, ovarian and neuroblastoma, among others (Iorio et al., 2005; Ozen et al., 2008), indicating a putative tumor suppressor function. Finally, the downregulation of miR-99A has been linked to increased proliferative capacities in different cancer types (Sun et al., 2011; Chen et al., 2012; Xin et al., 2013). We found miR-99A downregulated in the lung cancer, breast cancer and melanoma datasets, miR-125B2 in breast cancer and melanoma datasets and miR-LET7C in the melanoma and Wilms tumor datasets. Altogether, this could indicate that the extra copy of chromosome 21 in DS patients could lead to an increased expression of the microRNA cluster, leading to a synergic effect over cell proliferation. These miRNAs could be good candidates for explaining the inverse comorbidity observed between solid tumors and DS.
To delve deeper into this notion, we used the known gene targets of the 3 miRNAs to perform a REACTOME (Croft et al., 2014) pathway enrichment analysis. The results of the analysis showed that the set of validated target genes for the three miRNAs is enriched in pathways related to cell proliferation or apoptosis inhibition, like PI3K/AKT activation (REACT_12464), signaling by ERBB2 (REACT_115755) and signaling by NOTCH, Interferon alpha/beta and IFG1R (REACT_299, REACT_25162 and REACT_150210, respectively). These enrichments are consistent with their theoretical role as candidate genes for the inverse comorbidity phenomenon observed between DS and solid tumors.
The last maximum frequency deletion region we describe in our study, MDR3, is a very small region on Wilm's tumors and is deleted in 50% of the samples analyzed. It covers a narrow segment between positions 35,943,407 and 35,981,911, and it includes only one gene, RCAN1 (Figure 1C). The protein encoded by this gene, previously known as DSCR1 (DS critical region gene 1), interacts with calcineurin-inhibiting calcineurin-dependent signaling pathways (Carme Mulero et al., 2010). RCAN1 is located in the minimal candidate region of the DS phenotype, an area of approximately 3 Mb at chromosomal region 21q22 (OMIM 602917), and it is overexpressed in the DS patient's brain. Its overexpression is linked to the formation of neurofibrillary tangles in Alzheimer's disease patients and with the facilitation of neural apoptosis in the DS phenotype (Sun et al., 2014). Comparative sequence analyses of HSA21 and the mouse genome models of DS resulted in the discovery of DSRC1 (RCAN1) as a potential candidate for being a tumor suppressor gene in both lung tumor, as well as melanoma cells, through the suppression of tumor angiogenesis (Minami et al., 2014; Shin et al., 2014). These findings, combined with the observed frequency of deletion shown in Wilms tumor, to our knowledge has not been previously reported. Moreover, the general downregulation trend present in three of the four tumor types analyzed here, lung cancer, breast cancer and Wilms tumor, point to RCAN1 as the best supported link between the Down phenotype and protection from cancer.
In conclusion, although we admit the possible existence of bias as a result of the small number of samples, our genomic approach demonstrates that the analysis of Chromosome 21-deleted regions from specific tumors may allow the prediction of candidate genes to explain why individuals with DS have a reduced risk for developing these tumors. Further functional studies, however, are needed to prove the potential tumor suppressor properties of these candidate genes and miRNA.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants to JC from the Instituto de Salud Carlos III (PI13/01887) and the Ramon y Cajal Program (RYC-2011-07843) from the Secretaria del Estado de Investigación, Desarrollo e Innovación, from Spain.
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fphys.2015.00010/abstract
References
- Arima C., Kajino T., Tamada Y., Imoto S., Shimada Y., Nakatochi M. (2014). Lung adenocarcinoma subtypes definable by lung development-related miRNA expression profiles in association with clinicopathologic features. Carcinogenesis 35, 2224–2231. 10.1093/carcin/bgu127 [DOI] [PubMed] [Google Scholar]
- Boker L. K., Blumstein T., Sadetzki S., Luxenburg O., Litvak I., Akstein E., et al. (2001). Incidence of leukemia and other cancers in Down syndrome subjects in Israel. Int. J. Cancer 93, 741–744. 10.1002/ijc.1383 [DOI] [PubMed] [Google Scholar]
- Carme Mulero M., Orzáez M., Messeguer J., Messeguer A., Pérez-Payá E., Pérez-Riba M. (2010). A fluorescent polarization-based assay for the identification of disruptors of the RCAN1-calcineurin A protein complex. Anal. Biochem. 398, 99–103. 10.1016/j.ab.2009.10.045 [DOI] [PubMed] [Google Scholar]
- Catalá-López F., Suárez-Pinilla M., Suárez-Pinilla P., Valderas J. M., Gómez-Beneyto M., Martinez S., et al. (2014). Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother. Psychosom. 83, 89–105. 10.1159/000356498 [DOI] [PubMed] [Google Scholar]
- Chen X., Chen G., Cao X., Zhou Y., Yang T., Wei S. (2013). Downregulation of BTG3 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 437, 173–178. 10.1016/j.bbrc.2013.06.062 [DOI] [PubMed] [Google Scholar]
- Chen Z., Jin Y., Yu D., Wang A., Mahjabeen I., Wang C., et al. (2012). Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral Oncol. 48, 686–691. 10.1016/j.oraloncology.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Climent J., Dimitrow P., Fridlyand J., Palacios J., Siebert R., Albertson D. G., et al. (2007). Deletion of chromosome 11q predicts response to anthracycline-based chemotherapy in early breast cancer. Cancer Res. 67, 818–826. [DOI] [PubMed] [Google Scholar]
- Croft D., Mundo A. F., Haw R., Milacic M., Weiser J., Wu G., et al. (2014). The Reactome pathway knowledgebase. Nucleic Acids Res. 42, D472–D477. 10.1093/nar/gkt1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L., Maccario H., Perera N. M., Yang X., Spinelli L., Tibarewal P., et al. (2010). Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene 29, 687–697. 10.1038/onc.2009.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasle H., Clemmensen I. H., Mikkelsen M. (2000). Risks of leukaemia and solid tumours in individuals with down's syndrome. Lancet 355, 165–169. 10.1016/S0140-6736(99)05264-2 [DOI] [PubMed] [Google Scholar]
- Hattori M., Fujiyama A., Taylor T. D., Watanabe H., Yada T., Park H. S., et al. (2000). Chromosome 21 mapping and sequencing consortium. The DNA sequence of human chromosome 21. Nature 405, 311–319. 10.1038/35012518 [DOI] [PubMed] [Google Scholar]
- Hieronymus H., Schultz N., Gopalan A., Carver B. S., Chang M. T., Xiao Y., et al. (2014). Copy number alteration burden predicts prostate cancer relapse. Proc. Natl. Acad. Sci. U.S.A. 11, 11139–11144. 10.1073/pnas.1411446111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F. H., Chen H. I., Tsai M. H., Lai L. C., Huang C. C., Tu S. H., et al. (2011). A model-based circular binary segmentation algorithm for the analysis of array CGH data. BMC Res. Notes 4:394. 10.1186/1756-0500-4-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Ying X., Wang J., Piriyapongsa J., Jordan I. K., Sheng J., et al. (2014). Identification of a tumor-suppressive human-specific microRNA within the FHIT tumor-suppressor gene. Cancer Res. 74, 2283–2294. 10.1158/0008-5472.CAN-13-3279 [DOI] [PubMed] [Google Scholar]
- Ibáñez K., Boullosa C., Tabarés-Seisdedos R., Baudot A., Valencia A. (2010). Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 10:e1004173. 10.1371/journal.pgen.1004173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio M. V., Ferracin M., Liu C. G., Veronese A., Spizzo R., Sabbioni S., et al. (2005). MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070. 10.1158/0008-5472.CAN-05-1783 [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., et al. (2005). RAS is regulated by the let-7 microRNA family. Cell 120, 635–647. 10.1016/j.cell.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Jönsson G., Staaf J., Vallon-Christersson J., Ringnér M., Holm K., Hegardt C., et al. (2012). Genomic subtypes of breast cancer identified by array-comparative genomic hybridization display distinct molecular and clinical characteristics. Breast Cancer Res. 12, R42. 10.1186/bcr2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbarah O., Nogueira C., Feng B., Nazarian R. M., Bosenberg M., Wu M., et al. (2010). Integrative genome comparison of primary and metastatic melanomas. PLoS ONE 5:e10770. 10.1371/journal.pone.0010770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranov P., Cawley S. E., Drenkow J., Bekiranov S., Strausberg R. L., Fodor S. P., et al. (2002). Large-scale transcriptional activity in chromosomes 21 and 22. Science 296, 916–919. 10.1126/science.1068597 [DOI] [PubMed] [Google Scholar]
- Letourneau A., Antonarakis S. E. (2012). Genomic determinants in the phenotypic variability of Down syndrome. Prog. Brain Res. 197, 15–28. 10.1016/B978-0-444-54299-1.00002-9 [DOI] [PubMed] [Google Scholar]
- Letourneau A., Santoni F. A., Bonilla X., Sailani M. R., Gonzalez D., Kind J., et al. (2014). Domains of genome-wide gene expression dysregulation in Down's syndrome. Nature. 508, 345–350. 10.1038/nature13200 [DOI] [PubMed] [Google Scholar]
- Liu R. Z., Graham K., Glubrecht D. D., Germain D. R., Mackey J. R., Godbout R. (2011). Association of FABP5 expression with poor survival in triple-negative breast cancer: implication for retinoic acid therapy. Am. J. Pathol. 178, 997–1008. 10.1016/j.ajpath.2010.11.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. P., Tsai M. H., Lee J. M., Hsu C. P., Chen P. C., Lin C. W., et al. (2010). Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol. Biomarkers Prev. 19, 2590–2597. 10.1158/1055-9965.EPI-10-0332 [DOI] [PubMed] [Google Scholar]
- Lv Z., Zou H., Peng K., Wang J., Ding Y., Li Y., et al. (2013). The suppressive role and aberrent promoter methylation of BTG3 in the progression of hepatocellular carcinoma. PLoS ONE 8:e77473. 10.1371/journal.pone.0077473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégarbané A., Ravel A., Mircher C., Sturtz F., Grattau Y., Rethoré M. O., et al. (2009). The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet. Med. 11, 611–616. 10.1097/GIM.0b013e3181b2e34c [DOI] [PubMed] [Google Scholar]
- Minami T., Horiuchi K., Miura M., Abid M. R., Takabe W., Noguchi N., et al. (2014). Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J. Biol. Chem. 279, 50537–50554. 10.1074/jbc.M406454200 [DOI] [PubMed] [Google Scholar]
- Nadiminty N., Tummala R., Lou W., Zhu Y., Zhang J., Chen X., et al. (2012). MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J. Biol. Chem. 287, 1527–1537. 10.1074/jbc.M111.278705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel H., Laskawi R., Eiffert H., Schlott T. (2003). Analysis of the tumour suppressor,genes, FHIT and WT-1, and the tumour rejection genes, BAGE, GAGE-1/2, HAGE,MAGE-1, and MAGE-3, in benign and malignant neoplasms of the salivary glands. Mol. Pathol. 56, 226–231. 10.1136/mp.56.4.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nižetić D., Groet J. (2012) Tumorigenesis in Down's syndrome: big lessons from a small chromosome. Nat. Rev. Cancer 12, 721–732. 10.1038/nrc3355 [DOI] [PubMed] [Google Scholar]
- Olson J. M., Hamilton A., Breslow N. E. (1995). Non-11p constitutional chromosome abnormalities in Wilms' tumour patients. Med. Pediatr. Oncol. 24, 305–309. 10.1002/mpo.2950240507 [DOI] [PubMed] [Google Scholar]
- Ozen M., Creighton C. J., Ozdemir M., Ittmann M. (2008). Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 27, 1788–1793. 10.1038/sj.onc.1210809 [DOI] [PubMed] [Google Scholar]
- Patja K., Pukkala E., Sund R., Iivanainen M., Kaski M. (2006). Cancer incidence of persons with Down syndrome in Finland: a population-based study. Int. J. Cancer 118, 1769–1772. 10.1002/ijc.21518 [DOI] [PubMed] [Google Scholar]
- Peña-Chilet M., Martínez M. T., Pérez-Fidalgo J. A., Peiró-Chova L., Oltra S. S., Tormo E., et al. (2014). MicroRNA profile in very young women with breast cancer. BMC Cancer 14:529. 10.1186/1471-2407-14-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizen N. J., Patterson D. (2003). Down's syndrome. Lancet 361, 1281–1289. 10.1016/S0140-6736(03)12987-X [DOI] [PubMed] [Google Scholar]
- Rubio-Moscardo F., Climent J., Siebert R., Piris M. A., Martín-Subero J. I., Nieländer I., et al. (2005). Mantle-cell lymphoma genotypes identified with CGH to BAC microarrays define a leukemic subgroup of disease and predict patient outcome. Blood 105, 4445–4454. 10.1182/blood-2004-10-3907 [DOI] [PubMed] [Google Scholar]
- Scarpato M., Esposito R., Evangelista D., Aprile M., Ambrosio M. R., Angelini C., et al. (2014). Analysis of expression on human chromosome 21, ALE-HSA21: a pilot integrated web resource. Database (Oxford) 2014:bau009. 10.1093/database/bau009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J., Backes C., Nourkami-Tutdibi N., Leidinger P., Deutscher S., Beier M., et al. (2012). Treatment-independent miRNA signature in blood of Wilms tumor patients. BMC Genomics. 13:379. 10.1186/1471-2164-13-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Lee J. C., Baek K. H. (2014). A single extra copy of Dscr1 improves survival of mice developing spontaneous lung tumors through suppression of tumor angiogenesis. Cancer Lett. 342, 70–81. 10.1016/j.canlet.2013.08.047 [DOI] [PubMed] [Google Scholar]
- Staaf J., Isaksson S., Karlsson A., Jönsson M., Johansson L., Jönsson P., et al. (2013). Landscape of somatic allelic imbalances and copy number alterations in human lung carcinoma. Int. J. Cancer. 132, 2020–2031. 10.1002/ijc.27879 [DOI] [PubMed] [Google Scholar]
- Sun D., Lee Y. S., Malhotra A., Kim H. K., Matecic M., Evans C., et al. (2011). miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 71, 1313–1324. 10.1158/0008-5472.CAN-10-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Wu Y., Herculano B., Song W. (2014). RCAN1 overexpression exacerbates calcium overloading-induced neuronal apoptosis. PLoS ONE 9:e95471. 10.1371/journal.pone.0095471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarés-Seisdedos R., Dumont N., Baudot A., Valderas J. M., Climent J., Valencia A., et al. (2011). No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 12, 604–608. 10.1016/S1470-2045(11)70041-9 [DOI] [PubMed] [Google Scholar]
- Tabarés-Seisdedos R., Rubenstein J. L. (2013). Inverse cancer comorbidity: a serendipitous opportunity to gain insight into CNS disorders. Nat. Rev. Neurosci. 14, 293–304. 10.1038/nrn3464 [DOI] [PubMed] [Google Scholar]
- Xin J. X., Yue Z., Zhang S., Jiang Z. H., Wang P. Y., Li Y. J., et al. (2013). miR-99 inhibits cervical carcinoma cell proliferation by targeting TRIB2. Oncol. Lett. 6, 1025–1030. 10.3892/ol.2013.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Brenn T., Brown E. R., Doherty V., Melton D. W. (2012). Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br. J. Cancer 103, 553–561. 10.1038/bjc.2011.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zhang Y., Qi Z., Kurtycz D., Vacano G., Patterson D. (2008). Methylation-mediated downregulation of the B-cell translocation gene 3 (BTG3) in breast cancer cells. Gene Expr. 14, 173–182. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.