Abstract
As antimicrobial resistance increases, understanding the current epidemiology of bloodstream infections (BSIs) in hematopoietic stem cell transplant (HSCT) recipients is essential to guide empirical antimicrobial therapy. We therefore reviewed microbial etiologies, timing and outcomes of BSIs in patients who were transplanted from September 2007–December 2011. Vancomycin-resistant enterococci (VRE) were the most common pathogens in allogeneic HSCT recipients and 4th most common after autologous transplantation. VRE did not cause any of 101 BSIs in neutropenic patients who were not receiving antibacterials, but caused 32 (55%) of 58 BSIs in neutropenic patients receiving a broad-spectrum β-lactam agent (P<0.001). Rates of septic shock and seven-day mortality were 5% and 0% for streptococcal bacteremia, 12% and 18% for VRE bacteremia, and 20% and 14% for Gram-negative bacteremia. In conclusion, VRE bacteremia was the most common BSI in allogeneic HSCT recipients, occurred primarily in neutropenic patients receiving broad-spectrum β-lactams, and was associated with poor outcomes.
Keywords: vancomycin-resistant enterococci, bloodstream infections, hematopoietic stem cell transplant recipients
INTRODUCTION
Bloodstream infections (BSIs) are common causes of morbidity and mortality in hematopoietic stem cell transplant (HSCT) recipients [1–3]. The administration of timely antimicrobial therapy that targets likely pathogens is critical in preventing morbidity and mortality due to BSIs in this population, particularly in neutropenic patients [3,4]. The guidelines of the Infectious Diseases Society of America recommend that patients with fever and neutropenia, including HSCT recipients, be given empirical therapy with an anti-pseudomonal β-lactam agent [5]. These recommendations are based on randomized trials that were conducted more than ten years ago, when the prevalence of multidrug-resistant (MDR) bacteria was low [6–9]. Given the recent emergence of MDR bacteria as prominent causes of healthcare-associated infections [10], characterization of the changing pattern of isolates that cause BSI after HSCT is essential to ensure the adequacy of recommended empirical regimens.
To this end, we reviewed all BSIs in allogeneic and autologous HSCT recipients at our center to determine the most common etiologies, timing, and outcomes. We then focused on neutropenic HSCT recipients, and compared BSI etiologies in patients who were not already receiving antibacterial therapy to those in patients already receiving a broad-spectrum β-lactam agent. Finally, we reviewed outcomes of BSIs and compared outcomes by pathogen.
MATERIALS AND METHODS
Patients and Procedures
We reviewed all BSIs that occurred from the start of conditioning until one year after transplantation in adults who received a HSCT at New York-Presbyterian Hospital/Weill Cornell Medical Center from September 2007 to December 2011. Patients who had received a prior allogeneic transplant or an autologous transplant within the previous year were excluded. We also excluded BSI episodes that occurred after malignancy relapse or after a subsequent HSCT.
During the study period, HSCT recipients were placed in private rooms on a dedicated transplant unit and infection control precautions, as recommended by the Centers for Disease Control and Prevention (CDC), were followed. Active surveillance to detect asymptomatic colonization with MDR bacteria was not routinely performed. Prophylactic levofloxacin was administered before neutrophil engraftment only to patients with multiple myeloma. Trimethoprim-sulfamethoxazole (TMP/SMX) was given to allogeneic HSCT recipients after neutrophil engraftment for at least six months after transplantation or until discontinuation of immunosuppressive drug therapy. Prophylactic fluconazole was administered until neutrophil engraftment and continued for at least six months in allogeneic HSCT recipients. For initial work-up of fever and neutropenia, blood cultures (one aerobic and one anaerobic bottle per set) were obtained from peripheral blood and each central venous catheter lumen. Subsequent blood cultures were drawn daily for persistent fever.
BacT/ALERT® 3D was the automated blood culture system used during the study period. Species identification and antimicrobial susceptibility testing of bloodstream isolates were routinely performed by Vitek II (BioMérieux Inc., Durham, NC), according to Clinical and Laboratory Standards Institute recommendations [11].
Piperacillin-tazobactam (PTZ) was the primary agent used for empirical treatment of fever and neutropenia. Meropenem was administered to patients with a penicillin allergy other than anaphylaxis, with a history of recent infection with an extended-spectrum β-lactamase (ESBL)-producing organism, or with fever that persisted while receiving PTZ. Empirical vancomycin was only administered in the setting of a suspected skin, soft tissue or catheter infection, severe sepsis, or history of recent infection with methicillin-resistant Staphylococcus aureus (MRSA). Broad-spectrum Gram-negative coverage was generally continued until both fever and neutropenia resolved.
Definitions and Data Collection
A BSI episode was defined as growth of organism(s) from a blood culture from a patient who did not have a prior blood culture yielding the same organism(s) within the previous 30 days. BSI onset was defined as the date of blood culture collection. Common skin commensals (coagulase-negative staphylococci, Bacillus and Corynebacterium spp. other than C. jeikeium) were only considered causes of BSI if isolated from at least two sets of blood cultures collected on the same day or on consecutive days. A BSI was designated polymicrobial if more than one organism was identified from blood cultures collected on the same day or on consecutive days. Neutropenia was defined as an absolute neutrophil count ≤ 500 cells/μL.
We reviewed all BSI episodes to determine the causative organism(s), antimicrobial susceptibilities, and seven-day mortality (death within 7 days of BSI onset). BSI etiologies in allogeneic and autologous HSCT recipients were analyzed separately. We also determined whether severe sepsis or septic shock occurred within two calendar days of BSI onset, using Society of Critical Care Medicine definitions [12]. All BSIs during neutropenia underwent further review to determine BSI etiologies in patients who were not receiving antibacterials and those in patients who were already receiving a broad-spectrum β-lactam agent for at least two days (breakthrough BSIs).
After finding that vancomycin-resistant enterococci (VRE) were the most common causes of BSIs in allogeneic HSCT recipients, we performed two post-hoc analyses in this population. First, we calculated the one-year cumulative incidence of VRE bacteremia. Second, we identified patient and transplant characteristics that were independently associated with developing VRE bacteremia.
Statistical Analysis
Proportions were compared using two-tailed Chi-square or Fisher’s exact tests and P ≤ 0.05 was considered statistically significant. Continuous variables were expressed as median values and compared by the Wilcoxon rank-sum test. Cumulative incidences were expressed as proportions with 95% confidence intervals (CIs) and as Kaplan-Meier curves with censoring for malignancy relapse, subsequent HSCT, mortality and loss to follow-up. A Cox proportional hazards model was used to determine patient and transplant-related characteristics that were associated with developing VRE bacteremia. All variables with a P value ≤ 0.2 in univariate analysis were entered into a multivariate model. STATA, version 12.0 (StataCorp, College Station, TX) was used for statistical analysis.
RESULTS
Allogeneic HSCT Recipients
BSI Etiologies
There were 251 allogeneic HSCTs performed during the study period. Of these, 11 were excluded because the patient had a prior allogeneic transplant and two because the patient had an autologous transplant within the previous year, leaving 238 eligible allogeneic HSCTs.
Overall, 243 isolates caused 216 BSI episodes (Table I). Fifty-four percent of isolates were Gram-positive bacteria, 43% were Gram-negative bacteria and 3% were Candida species. Enterococci were the most common pathogens, and VRE accounted for 19% of all bloodstream isolates. The next most common pathogens were coagulase-negative staphylococci (14% of isolates), Klebsiella pneumoniae (13%), Escherichia coli (8%), and Staphylococcus aureus and Pseudomonas aeruginosa (7% each).
Table I.
Isolates causing bloodstream infections (BSIs) within 1 year after HSCT.
| Organism | Allogeneic n (% of total) | Autologous n (% of total) |
|---|---|---|
| Total bloodstream isolates | 243 | 109 |
| Gram-positive bacteria1 | 131 (54) | 57 (52) |
| Enterococci | 55 (23) | 15 (14) |
| Enterococcus faecium | 48 (20) | 12 (11) |
| VRE2 | 45 (19) | 11 (10) |
| Coagulase-negative staphylococci3 | 33 (14) | 5 (5) |
| Viridans group streptococci4 | 12 (5) | 18 (17) |
| Staphylococcus aureus | 18 (7) | 10 (9) |
| MSSA | 10 | 3 |
| MRSA | 8 | 7 |
| Gram-negative bacteria5 | 105 (43) | 50 (46) |
| Enterobacteriaceae | 72 (30) | 41 (38) |
| Klebsiella pneumoniae | 31 (13) | 18 (17) |
| Escherichia coli | 20 (8) | 19 (17) |
| Klebsiella oxytoca | 7 (3) | 1 (1) |
| Enterobacter cloacae | 6 (2) | 1 (1) |
| Pseudomonas aeruginosa | 18 (7) | 5 (5) |
| Stenotrophomonas maltophilia | 7 (3) | 0 |
| Acinetobacter baumannii | 5 (2) | 0 |
| Candida spp.6 | 7 (3) | 2 (2) |
HSCT, hematopoietic stem cell transplant; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Other Gram-positive isolates: Clostridium spp., Streptococcus agalactiae, Streptococcus bovis and Streptococcus pneumoniae (n = 3, of each); Corynebacterium jeikeium, Corynebacterium striatum and Leuconostoc spp. (n = 2, of each); Abiotrophia spp., Bacillus spp., Rhodococcus spp. and Streptococcus pyogenes (n = 1, of each).
All VRE isolates were Enterococcus faecium, except for one, which was E. durans.
Coagulase-negative staphylococci were only considered causes of BSI if isolated from two sets of blood cultures that were collected on the same day or consecutive days.
The 19 viridans group streptococci that were identified to the species level were S. mitis (n = 7), S. oralis (n = 5), S. sanguinis and S. salivarius (n = 3, of each) and S. constellatus (n = 1).
Other Gram-negative isolates: Morganella morganii (n = 3); Moraxella catarrhalis and Serratia marcescens (n = 2, of each); Acinetobacter lwoffii, Aeromonas hydrophila, Bacteroides ureolyticus, Capnocytophaga spp., Citrobacter freundii, Enterobacter aerogenes, Fusobacterium varium, Pantoea spp., Raoultella planticola and Sphingomonas paucimobilis (n = 1, of each).
Candida species: C. parapsilosis (n = 3); C. glabrata and C. krusei (n = 2, of each); C. albicans and C. kefyr (n = 1, of each).
VRE Bacteremia: Timing, Incidence, and Risk Factors
The median time from allogeneic transplantation until the first BSI episode was 9 days (interquartile range [IQR] 5–36). However, the median time until the first episode of VRE bacteremia was 18 days (IQR 11–44; P = 0.02). Of the 45 episodes of VRE bacteremia, 27 (60%) were within 30 days after transplantation, 10 (22%) were between 31–100 days after transplantation, and 8 (18%) were between 101–365 days after transplantation. Twenty-four episodes (62%) occurred before neutrophil engraftment and an additional six (13%) occurred during a period of neutropenia subsequent to engraftment. Of the 15 episodes that did not occur during neutropenia, all but three were in patients with gastrointestinal graft-versus-host disease (GVHD).
VRE bacteremia occurred within one year after transplantation in 39 (16.4%; 95% CI 12.2%–21.6%) of 238 allogeneic HSCT recipients (Figure 1). There were no statistically significant trends in incidence by year of transplantation. In univariate analysis, receipt of cord blood, receipt of peripheral blood stem cells from a mismatched or syngeneic donor, and increased number of days until neutrophil engraftment were associated with VRE bacteremia (Table II). Of these, only receipt of mismatched peripheral blood stem cells (HR 3.76, 95% CI 1.08–13.12, P = 0.04) and increased number of days until neutrophil engraftment (HR 1.06 per day, 95% CI 1.02–1.10, P = 0.005) were independent risk factors in multivariate analysis.
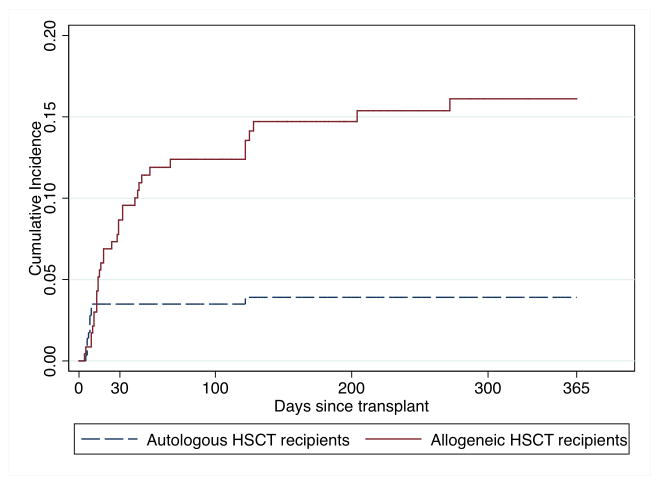
Figure 1.
Cumulative incidence of VRE bacteremia in allogeneic and autologous HSCT recipients.
Table II.
Patient and transplant characteristics and associations with VRE bacteremia in allogeneic HSCT recipients.
| Characteristic | VRE bacteremia n (%) | No VRE bacteremia n (%) | Univariate HRa (95% CI) | P | Multivariate HRa (95% CI) | P |
|---|---|---|---|---|---|---|
| Total number of patients | 39 | 199 | ||||
| Year of transplant | ||||||
| September 2007 – December 2008 | 12 (31) | 39 (20) | 1.0 (Ref) | N/A | 1.0 (Ref) | N/A |
| 2009 | 8 (21) | 45 (23) | 0.56 (0.22–1.44) | 0.23 | 0.48 (0.17–1.40) | 0.18 |
| 2010 | 8 (21) | 48 (24) | 0.52 (0.19–1.40) | 0.19 | 0.52 (0.17–1.54) | 0.24 |
| 2011 | 11 (28) | 67 (34) | 0.54 (0.23–1.27) | 0.23 | 0.47 (.017–1.31) | 0.15 |
| Age at transplant, median (IQR) | 56 (44–63) | 56 (45–63) | 0.99 (0.97–1.02) | 0.45 | ||
| Male gender | 20 (51) | 106 (53) | 1.21 (0.61–2.39) | 0.59 | ||
| Underlying disease | ||||||
| Acute myeloid leukemia (AML) | 18 (46) | 109 (55) | 1.0 (Ref) | N/A | ||
| Leukemia other than AML | 5 (13) | 26 (13) | 1.44 (0.52–3.95) | 0.48 | ||
| Myelodysplastic syndrome | 5 (13) | 23 (12) | 1.22 (0.40–3.67) | 0.73 | ||
| Lymphoma | 6 (15) | 23 (12) | 1.50 (0.54–4.12) | 0.43 | ||
| Other | 5 (13) | 18 (9) | 1.91 (0.69–5.26) | 0.21 | ||
| Stem cell source | ||||||
| Matched related donor | 10 (26) | 76 (38) | 1.0 (Ref) | N/A | 1.0 (Ref) | N/A |
| Matched unrelated donor | 16 (41) | 92 (46) | 1.39 (0.57–3.35) | 0.47 | 1.56 (0.63–3.87) | 0.34 |
| Cord blood | 8 (21) | 25 (13) | 2.93 (1.10–7.82) | 0.03 | 2.62 (0.83–8.28) | 0.10 |
| Mismatched peripheral blood | 4 (10) | 5 (3) | 5.64 (1.69–18.76) | 0.005 | 3.76 (1.08–13.12) | 0.04 |
| Syngeneic | 1 (3) | 1 (1) | 8.45 (1.04–68.45) | 0.046 | 8.17 (0.98–68.46) | 0.053 |
| Myeloablative conditioning regimen | 13 (33) | 60 (30) | 1.20 (0.59–2.42) | 0.62 | ||
| ASBMT RFI Classification | ||||||
| Low risk | 13 (33) | 93 (47) | 1.0 (Ref) | N/A | ||
| Intermediate or high risk | 21 (54) | 87 (44) | 1.54 (0.74–3.23) | 0.25 | ||
| Unable to determine | 5 (13) | 19 (10) | 1.95 (0.69–5.55) | 0.21 | ||
| Use of ATG or alemtuzumab | 16 (41) | 64 (32) | 1.55 (0.79–3.05) | 0.21 | ||
| Days until neutrophil engraftment, median (IQR) | 16 (12–23) | 16 (13–19) | 1.06 (1.03–1.10) | <0.001 | 1.06 (1.02–1.10) | 0.005 |
ASBMT, American Society for Blood and Marrow Transplantation; ATG, anti-thymocyte globulin; CI, confidence interval; HR, hazard ratio; HSCT, hematopoietic stem cell transplant; IQR, interquartile range; N/A, not applicable; Ref, reference; VRE, vancomycin-resistant enterococci.
A Cox proportional hazards model was used. Only variables with P ≤ 0.2 in univariate analysis were included in the multivariate analysis.
Autologous HSCT Recipients
BSI etiologies
There were 287 autologous HSCTs performed during the study period and all were eligible for study inclusion. Overall, 109 isolates caused 90 BSI episodes (Table I). The proportions of BSI isolates that were Gram-positive bacteria, Gram-negative bacteria and fungi were similar to those in allogeneic HSCT recipients. Unlike after allogeneic transplantation, viridans group streptococci, Escherichia coli and Klebsiella pneumoniae were the most common pathogens (17% of isolates, each). VRE were next most common, accounting for 10% of isolates.
VRE bacteremia: Timing and Incidence
The median time from autologous transplantation until the first BSI episode was 5 days (IQR 1–7) and the median time until VRE bacteremia was 8 days (IQR 6–9; P = 0.01). Of the 11 episodes of VRE bacteremia, 10 (91%) occurred within 9 days after transplantation in the setting of neutropenia. The one-year cumulative incidence of VRE bacteremia in autologous HSCT recipients was 3.8% (95% CI 2.2–6.7%; 11 out of 287 patients; Figure 1).
BSIs During Neutropenia
There were 101 BSIs in neutropenic patients who were not receiving antibacterials and none of these were caused by VRE. Klebsiella pneumoniae, Escherichia coli and viridans group streptococci were the most common pathogens (Table III). In contrast, VRE caused 32 (55%) of 58 breakthrough BSIs in neutropenic patients who were already receiving PTZ or meropenem (P < 0.001).
Table III.
Microbial etiologies of bloodstream infections (BSIs) in neutropenic patients who were not receiving antibacterials and in neutropenic patients who were receiving piperacillin-tazobactam or meropenem (breakthrough).
| Causative organism(s) | BSIs in patients not receiving antibacterials1 (n = 101) | Breakthrough BSIs2 (n = 58) | P |
|---|---|---|---|
| Enterococcus faecium | 0 | 33 (31 VRE) | <0.001 |
| Klebsiella pneumoniae | 20 | 4 | 0.03 |
| Escherichia coli | 16 | 1 | 0.006 |
| Viridans group streptococci (VGS) | 14 | 0 | 0.003 |
| Pseudomonas aeruginosa | 8 | 2 | 0.33 |
| Staphylococcus aureus | 7 (4 MRSA) | 2 (both MRSA) | 0.49 |
| Coagulase-negative staphylococci (CoNS) | 6 | 2 | 0.71 |
| Moraxella catarrhalis | 2 | 0 | 0.53 |
| Acinetobacter baumannii | 0 | 2 | 0.13 |
| Stenotrophomonas maltophilia | 0 | 2 | 0.13 |
| Polymicrobial | 20 | 5 | 0.06 |
| Streptococci – Enterobacteriaceae | 7 | 0 | 0.048 |
| Multiple Enterobacteriaceae | 5 | 1 | 0.42 |
| Staphylococci – Enterobacteriaceae | 3 | 0 | 0.30 |
| Streptococci – Enterococcus faecalis (VSE) | 2 | 0 | 0.53 |
| Multiple VRE | 0 | 1 | 0.37 |
MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; VSE, vancomycin-susceptible enterococci.
Other etiologies of monomicrobial BSIs in patients not receiving antibacterials were Aeromonas hydrophila, Citrobacter freundii, Clostridium spp., Enterobacter aerogenes, Enterobacter cloacae, Fusobacterium varium, Klebsiella oxytoca and Rhodococcus spp. (n = 1, of each). Other etiologies of polymicrobial BSIs in patients who were not receiving antibacterials were VGS – anaerobic Gram-negative rod, Enterobacteriaceae – anaerobic Gram-negative rod, and S. aureus – VGS – Sphingomonas paucimobilis (n = 1, of each).
Other etiologies of monomicrobial breakthrough BSIs were Acinetobacter lwoffii, Clostridium spp., Corynebacterium jeikeium, Enterococcus faecalis (VSE) and Klebsiella oxytoca (n = 1, of each). Other etiologies of polymicrobial breakthrough BSIs were multiple CoNS, CoNS – Stenotrophomonas maltophilia, and Corynebacterium spp. – Candida glabrata (n = 1, of each).
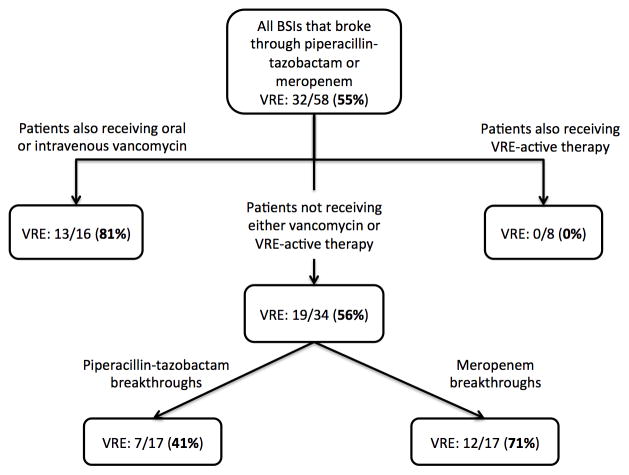
The proportion of breakthrough BSIs that were caused by VRE, stratified by antibacterials received by the patient at the time of BSI onset, is displayed in Figure 2. VRE bacteremia did not occur in patients already receiving VRE-active therapy. In patients already receiving PTZ or meropenem and vancomycin (oral or intravenous), 13 (81%) of 16 BSI episodes were caused by VRE. Of 34 breakthrough BSIs in patients who were not receiving vancomycin or VRE-active therapy, a larger proportion of meropenem breakthroughs were caused by VRE (71%) than PTZ breakthroughs (41%; P = 0.08). These proportions were similar in allogeneic and autologous HSCT recipients.
Figure 2.
Proportion of breakthrough bloodstream infections (BSIs) that were caused by VRE, stratified by antibacterials being received at the time of BSI onset.
There were 28 organisms that were initially reported as Gram-positive cocci in chains and/or pairs when stained directly from blood culture bottles from neutropenic patients who were not receiving antibacterials. Twenty-six (93%) of these organisms were subsequently identified as streptococci and none were VRE (P < 0.001). In contrast, of 36 organisms that were initially reported as Gram-positive cocci in chains and/or pairs in neutropenic patients who were already receiving PTZ or meropenem, 32 (89%) were subsequently identified as VRE and none were streptococci (P < 0.001).
Outcomes
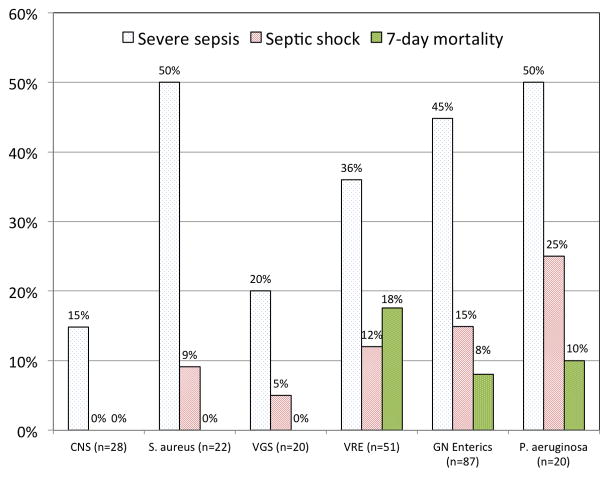
The proportions of bacteremias that were associated with severe sepsis, septic shock and seven-day mortality, stratified by causative pathogens, are shown in Figure 3. Severe sepsis developed within two days of bacteremia onset at the same rate (36%) in VRE bacteremias and bacteremias caused by viridans group streptococci (VGS) or Staphylococcus aureus. There was a trend towards a higher rate of severe sepsis in monomicrobial Gram-negative bacteremias (49%; P = 0.13). Septic shock developed in 12% of bacteremias caused by VRE, compared to 7% of bacteremias caused by VGS or Staphylococcus aureus (P = 0.44), and 20% of monomicrobial Gram-negative bacteremias (P = 0.22). Septic shock did not occur in coagulase-negative staphylococcal bacteremias.
Figure 3.
Proportion of monomicrobial bacteremias associated with severe sepsis and septic shock within two days of bacteremia onset and seven-day mortality, by organism. CNS, coagulase-negative staphylococci; VGS, viridans group streptococci; VRE, vancomycin-resistant enterococci; GN, Gram-negative.
The seven-day mortality rate for VRE bacteremia was 18%, compared to 0% for bacteremias caused by staphylococci or VGS (P < 0.001), and 14% for monomicrobial Gram-negative bacteremia (P = 0.55). Outcomes were similar in neutropenic and non-neutropenic patients. Four patients with VRE bacteremia died before antimicrobial susceptibility data were available and never received VRE-active therapy. In all other VRE bacteremia episodes, there was a median of 35 hours from blood culture collection until initiation of VRE-active agents.
DISCUSSION
We found that VRE were the most common causes of BSI in allogeneic HSCT recipients. Nearly one out of six allogeneic HSCT recipients developed VRE bacteremia within one year after transplantation; whereas, the incidence in autologous HSCT recipients was approximately 4%. Allogeneic HSCT recipients who received mismatched peripheral blood stem cells or had longer durations of neutropenia were at increased risk for VRE bacteremia. VRE bacteremia occurred primarily during neutropenia as a breakthrough infection in patients who were already receiving broad-spectrum β-lactam agents. In fact, VRE caused the majority of breakthrough BSIs in neutropenic patients. In contrast, VRE bacteremia did not occur in neutropenic patients who were not receiving antibacterials. VRE bacteremia was associated with rates of severe sepsis and septic shock that were comparable to those of bacteremias caused by VGS and Staphylococcus aureus, and with a seven-day mortality rate that resembled that of Gram-negative bacteremia.
VRE have been increasingly recognized as common causes of BSIs in allogeneic HSCT recipients [13–16]. Two recent studies in this population, one from a center where vancomycin was given empirically for fever and neutropenia, and the other from a center that used prophylactic vancomycin during neutropenia after myeloablative transplants, demonstrated cumulative incidences of VRE bacteremia of 7% and 11%, respectively [14,15]. Despite not routinely using vancomycin prophylactically or empirically, we identified an even higher incidence of VRE bacteremia after allogeneic transplantation (16%). To our knowledge, the 50 patients who developed VRE bacteremia in this study comprise the largest reported cohort of VRE bacteremia in adult HSCT recipients.
The high incidence of VRE bacteremia after allogeneic transplantation begs the question of if and when empirical VRE-active therapy should be initiated in response to fever and/or sepsis in HSCT recipients at institutions where VRE are prevalent. VRE have traditionally been characterized as organisms of low virulence that do not possess exotoxin or endotoxin [17]. Thus, unlike Gram-negative bacteremia, immediate treatment of VRE bacteremia may not be necessary. However, our data suggest that VRE bacteremia can be associated with severe presentations in HSCT recipients, as rates of septic shock and seven-day mortality were high. These findings confirm those of other reports of a rapidly deteriorating clinical course after VRE bacteremia in HSCT recipients [14,16]. Given the association between delay in effective antimicrobial therapy and increased mortality in patients with severe sepsis and septic shock [18,19], the empirical use of VRE-active therapy while awaiting blood culture results may be beneficial in high-risk settings.
The potential benefit of empirically initiating VRE-active therapy must be carefully weighed against the potential harms of this approach. Increased use of VRE-active antimicrobials, such as daptomycin and linezolid, has been associated with the emergence of resistance to these agents, leaving few therapeutic options [20–22]. The addition of empirical VRE-active therapy also poses a risk of added toxicity. Linezolid has been associated with impaired neutrophil recovery [23], daptomycin carries a risk of rhabdomyolysis [24] and quinupristin-dalfopristin is not well tolerated [25].
Considering this delicate risk/benefit balance, a rational approach would be to only administer empirical VRE-active therapy to HSCT recipients who are at high risk for VRE bacteremia. We identified that receipt of mismatched stem cells and prolonged duration of neutropenia are independent risk factors for VRE bacteremia. However, the more clinically useful finding was that patients with neutropenia who are receiving broad-spectrum β-lactam agents comprise a high-risk population, particularly those receiving meropenem or also receiving vancomycin. We also showed that neutropenic patients who were not receiving antibacterials did not develop VRE bacteremia, and thus empirical VRE-active therapy is not warranted in this setting. The finding that VRE bacteremia occurred at a later time than bacteremias of other etiologies reflects the observation that VRE bacteremia did not occur with the initial episode of neutropenic fever, but occurred after exposure to broad-spectrum antibacterial agents.
These findings also have implications for the management of neutropenic HSCT recipients with growth of Gram-positive cocci in pairs and/or chains from blood culture bottles. If the patient was already receiving a broad-spectrum β-lactam at the time of culture collection, these bacteria likely represent VRE, and VRE-active therapy should be initiated. However, the same report in a neutropenic patient who was not already receiving antibacterials likely indicates streptococcal bacteremia, and vancomycin should be initiated.
Although VRE were the most frequent causes of breakthrough BSIs in neutropenic patients, breakthrough Gram-negative bacteremias also occurred and were associated with high rates of septic shock (60%) and seven-day mortality (33%). Thus, in addition to considering empirical VRE-active therapy in this setting, the antibacterial regimen should be modified to cover potential breakthrough Gram-negative pathogens.
This study has several limitations. Given that few autologous HSCT recipients developed VRE bacteremia, we did not assess risk factors in this population. Our findings may not be applicable to other centers with different bacterial flora and antimicrobial prescribing practices. This study was conducted in a hospital where one-half of all inpatient enterococcal isolates were vancomycin-resistant. The incidence of VRE bacteremia after transplantation in institutions with a lower rate of vancomycin resistance among enterococci is likely much lower. Cephalosporins were rarely used as empirical therapy at our center and fluoroquinolone prophylaxis was only used in patients with myeloma. Thus, we do not know if VRE would be the dominant cause of BSI in neutropenic patients who were already receiving cephalosporins, as it was with those receiving PTZ or meropenem, or how often VRE bacteremia would occur in allogeneic HSCT recipients who receive fluoroquinolone prophylaxis. Given the limitations of applying CDC surveillance definitions of healthcare-associated infections to neutropenic patients [26], we did not determine the source of each episode of VRE bacteremia. However, given that enterococci reside in the intestines and nearly all episodes occurred in patients with neutropenia or GI GVHD, we presume that translocation from the gastrointestinal tract was the source in most cases.
We did not routinely perform surveillance cultures of the gastrointestinal tract to assess for asymptomatic VRE colonization. Other studies have demonstrated that gastrointestinal VRE colonization prior to allogeneic HSCT is common and is a risk factor for post-transplant VRE bacteremia, with rates of 14–34% in colonized patients and 2–6% in patients without colonization [13–15]. However, pre-transplant VRE colonization does not definitively predict subsequent VRE bacteremia. Over 40% of patients in Kamboj et al. who developed VRE bacteremia within 30 days after allogeneic HSCT were not colonized with VRE on pre-transplant screening cultures [14]. The high VRE bacteremia rates in this study imply that many patients were likely colonized pre-transplant, yet none developed VRE bacteremia unless exposed to antibacterials. Further studies are needed to create a more precise algorithm for when to administer empirical VRE-active therapy in neutropenic HSCT recipients that would incorporate colonization status, antibacterial exposures and host and transplant-related factors. Until such models are developed, we recommend that empirical treatment with daptomycin or linezolid should be considered in febrile neutropenic HSCT recipients who are receiving broad-spectrum β-lactam agents pending results of blood cultures.
We propose a multifactorial model to explain the emergence of VRE as a major cause of bacteremia in HSCT recipients. First, VRE have become increasingly common healthcare-associated pathogens and survive for long periods of time on environmental surfaces in the hospital [17,28,29]. Thus, exposure to VRE is common. However, in order for VRE bacteremia to occur, it not only needs to be present in the gastrointestinal tract, but it needs to be the dominant member of the intestinal flora [29]. Exposure to broad-spectrum antimicrobial agents with activity against Gram-negative and anaerobic bacteria is an important step that facilitates intestinal domination with VRE, and leads to VRE bacteremia [30,31].
In our cohort, VRE bacteremia typically occurred before neutrophil engraftment, in a setting of intestinal mucosal disruption from pre-transplant conditioning, or in patients with gastrointestinal GVHD, many of whom had qualitative neutrophil dysfunction because of corticosteroids [32]. These findings suggest that the presence of intestinal mucosal disruption and either quantitative or qualitative impairment of neutrophil function are additional factors that contribute to a high-risk setting for VRE bacteremia in HSCT recipients.
In conclusion, VRE were the most common causes of BSIs in HSCT recipients and VRE bacteremia was associated with high rates of severe sepsis, septic shock and seven-day mortality. VRE caused the majority of BSIs in neutropenic patients who were already receiving broad-spectrum β-lactam agents, but was never a cause of BSI in neutropenic patients who were not already receiving antibacterials. These findings should assist clinicians in deciding on when to administer empirical VRE-active therapy. Continued surveillance of BSI etiologies is needed to ensure adequate empirical antimicrobial therapy in HSCT recipients.
Acknowledgments
This study was partially supported by a grant (to M.J.S.) from the Clinical and Translational Science Center at Weill Cornell Medical College (KL2TR000458) and was presented, in part, at IDWeek 2012™, October 17–21, 2012, Abstract #537.
Footnotes
Financial disclosure: The authors have nothing to disclose.
References
- 1.Almyroudis NG, Fuller A, Jakubowski A, et al. Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7:11–17. doi: 10.1111/j.1399-3062.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 2.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Bloodstream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40:63–70. doi: 10.1038/sj.bmt.1705690. [DOI] [PubMed] [Google Scholar]
- 3.Mikulska M, Del Bono V, Bruzzi P, et al. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection. 2012;40:271–278. doi: 10.1007/s15010-011-0229-y. [DOI] [PubMed] [Google Scholar]
- 4.Trecarichi EM, Tumbarello M, Spanu T, et al. Incidence and clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect. 2009;58:299–307. doi: 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 6.Pizzo PA, Hathorn JW, Hiemenz J, et al. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986;315:552–558. doi: 10.1056/NEJM198608283150905. [DOI] [PubMed] [Google Scholar]
- 7.Leyland MJ, Bayston KF, Cohen J, et al. A comparative study of imipenem versus piperacillin plus gentamicin in the initial management of febrile neutropenic patients with haematological malignancies. J Antimicrob Chemother. 1992;30:843–854. doi: 10.1093/jac/30.6.843. [DOI] [PubMed] [Google Scholar]
- 8.Cometta A, Calandra T, Gaya H, et al. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto Infection Program. Antimicrob Agents Chemother. 1996;40:1108–1115. doi: 10.1128/aac.40.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Favero A, Menichetti F, Martino P, et al. A multicenter, double-blind, placebo-controlled trial comparing piperacillin-tazobactam with and without amikacin as empiric therapy for febrile neutropenia. Clin Infect Dis. 2011;33:1295–1301. doi: 10.1086/322646. [DOI] [PubMed] [Google Scholar]
- 10.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 11.Institute CaLS. Performance standards for antimicrobial susceptibility testing; 20th information supplement (June 2010 update) Clinical and Laboratory Standards Institute; Wayne, PA: 2010. M100-S20 June 2010 Update. [Google Scholar]
- 12.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock DM, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant Enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13:615–621. doi: 10.1016/j.bbmt.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 14.Kamboj M, Chung D, Seo SK, et al. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant. 2010;16:1576–1581. doi: 10.1016/j.bbmt.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vydra J, Shanley RM, George I, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:764–770. doi: 10.1093/cid/cis550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avery R, Kalaycio M, Pohlman B, et al. Early vancomycin-resistant Enterococcus (VRE) bacteremia after allogeneic bone marrow transplantation is associated with a rapidly deteriorating clinical course. Bone Marrow Transplant. 2005;35:497–499. doi: 10.1038/sj.bmt.1704821. [DOI] [PubMed] [Google Scholar]
- 17.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 20.Kamboj M, Cohen N, Gilhuley K, Babady NE, Seo SK, Sepkowitz KA. Emergence of daptomycin-resistant VRE: experience of a single institution. Infect Control Hosp Epidemiol. 2011;32:391–394. doi: 10.1086/659152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez E, Gomez-Gil R, Borobia AM, et al. Improving linezolid use decreases the incidence of resistance among Gram-positive microorganisms. Int J Antimicrob Agents. 2013;41:174–178. doi: 10.1016/j.ijantimicag.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Wudhikarn K, Gingrich RD, de Magalhaes Silverman M. Daptomycin nonsusceptible enterococci in hematologic malignancy and hematopoietic stem cell transplant patients: an emerging threat. Ann Hematol. 2013;92:129–131. doi: 10.1007/s00277-012-1539-6. [DOI] [PubMed] [Google Scholar]
- 23.Jaksic B, Martinelli G, Perez-Oteyza J, Hartman CS, Leonard LB, Tack KJ. Efficacy and safety of linezolid compared with vancomycin in a randomized, double-blind study of febrile neutropenic patients with cancer. Clin Infect Dis. 2006;42:597–607. doi: 10.1086/500139. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos S, Ball AM, Liewer SE, Martin CA, Winstead PS, Murphy BS. Rhabdomyolysis during therapy with daptomycin. Clin Infect Dis. 2006;42:e108–110. doi: 10.1086/504379. [DOI] [PubMed] [Google Scholar]
- 25.Lamb HM, Figgitt DP, Faulds D. Quinupristin/dalfopristin: a review of its use in the management of serious gram-positive infections. Drugs. 1999;58:1061–1097. doi: 10.2165/00003495-199958060-00008. [DOI] [PubMed] [Google Scholar]
- 26.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey AM, Zilberberg MD. Secular trends of hospitalization with vancomycin-resistant Enterococcus infection in the United States, 2000–2006. Infect Control Hosp Epidemiol. 2009;30:184–186. doi: 10.1086/593956. [DOI] [PubMed] [Google Scholar]
- 28.Bradley CR, Fraise AP. Heat and chemical resistance of enterococci. J Hosp Infect. 1996;34:191–196. doi: 10.1016/s0195-6701(96)90065-1. [DOI] [PubMed] [Google Scholar]
- 29.Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med. 2000;343:1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]