Abstract
Toll-like receptors (TLRs) play essential roles in generating innate immune responses, and are evolutionarily conserved across species. In mammals, TLRs specifically recognize the conserved microbial structural motifs referred to as pathogen-associated molecular patterns (PAMPs). Ligand recognition by TLRs activates signaling cascades that culminate in proinflammatory cytokine production and eventual elimination of invading pathogens. Although TLRs in mammals are expressed predominantly in the immune system, certain TLRs with poorly characterized function are also found in neurons. We recently profiled TLR8 expression during mouse brain development and established its localization in neurons and axons. We uncovered a novel role for TLR8 as a suppressor of neurite outgrowth as well as an inducer of neuronal apoptosis, and found that TLR8 functions in neurons through an NFκB-independent mechanism. These findings add a new layer of complexity for TLR signaling, and expand the realm of mammalian TLR function to the central nervous system (CNS) beyond the originally discovered immune context. Herein, we complement our earlier report with additional data, discuss their biological and mechanistic implications in CNS developmental and pathological processes, and thus further our perspective on TLR signaling and potential physiological roles in mammals.
Keywords: Toll-like receptors, TLR8, MyD88, innate immunity, neuron, axon, neurite outgrowth, apoptosis, NFκB, IκBα, IRAK1, IRAK4
INTRODUCTION
Toll, a type-I transmemberane receptor in Drosophila melanogaster, was found in 1996 to be required for the adult fly to mount an effective immune response against fungal infection.1 Soon after this groundbreaking discovery, mammalian orthologues of Drosophila Toll were identified as Toll-like receptors (TLRs) with similar functions in innate immune responses.2,3 TLRs recognize conserved microbial structures referred to as PAMPs, and thus activate intracellular signaling pathways that induce expression of a broad range of inflammatory cytokines including TNFα and IL-6.4 Several TLRs elicit antiviral responses through induction of type I interferon (IFN) genes.4 Beyond their primary roles in innate immunity, TLR activation modulates differentiation of dendritic cells (DCs) and their surface expression of co-stimulatory molecules, thus contributing to the induction of pathogen-specific adaptive immune responses.4 Recent evidence suggests that TLRs may have even broader roles in controlling immunity, including regulation of T cell responses, antibody production and class-switch recombination.5
Studies of mammalian TLRs have been focused almost exclusively on their roles in immunity. It is interesting to note, however, that Toll was originally identified in Drosophila for its role in establishing embryonic dorso-ventral polarity crucial to body axis specification and subsequent development.6 This role is conserved in a vertebrate model system—Xenopus.7 A variety of additional developmental functions have been ascribed to the Drosophila Toll receptor, among them roles in synapse formation. Toll locally inhibits synaptic initiation of the Drosophila RP3 motoneuron growth cone.8 Misexpression of Toll in the fly musculature disrupted synaptic target recognition between muscles and motoneurons.9 Furthermore, Toll-mediated signaling was found to be critical for several non-neural developmental processes in Drosophila, including muscle development and larval hematopoiesis.10,11 In addition, TIR-1, a post-synaptic protein in C. elegans AWC olfactory neurons containing the Toll/interleukin receptor-I (TIR) homology domain controls asymmetric patterns of odorant receptor expression, called AWC (OFF) and AWC (ON).12 Collectively, these studies indicate that the function of the Toll receptor family is not confined to immunity.
Since the core signaling machinery of Toll pathway is largely conserved between vertebrates and invertebrates,13 one might predict that mammalian TLRs would have a role in embryonic development. Yet, no developmental defect to date has been observed by gross examination of knockout mice of any single TLR family member. Hence, it is generally believed that Toll-controlled establishment of the body axis during embryogenesis is restricted to invertebrates and simpler vertebrates, with mammals having adopted a different mechanism for embryonic patterning.14,15 However, it could be argued that the lack of a role in embryogenesis might reflect functional redundancy among TLRs, as the mammalian TLR family comprises at least 13 genes. The generation of TLR double knockout mice would allow this issue to be clarified, and reveal novel insights that might be masked by the deletion of each receptor individually. TLRs have been implicated in various other developmental processes in mammals. TLR expression and activation were demonstrated in human adipose tissue-derived mesenchymal progenitor cells,16 and during hematopoietic stem cell differentiation.17 Deficiency in myeloid differentiation factor 88 (MyD88), an adaptor protein essential to TLR intracellular signaling, impairs the ability of mesenchymal stem cells (MSCs) to differentiate into osteocytes and chondrocytes under conditions that permit differentiation of their normal counterparts, suggesting that TLR signaling may be required for acquisition of multipotency by MSCs.18 In the CNS, TLR activation was shown to promote cholinergic differentiation from neural progenitors in the developing rat basal forebrain.19 TLRs on neural progenitor cells (NPCs) were recently demonstrated to be directly involved in regulating adult hippocampal neurogenesis.20 These observations have extended the function of TLRs in mammals from the realm of immunity to cellular development more generally. We are particularly interested in TLR roles in normal CNS development and disease.
TLRS IN CNS AND NEURONS
TLRs have been found to exist in a wide range of tissues outside the immune system, including the CNS. The profile of TLR expression in astrocytes is restricted, with human astrocytes preferentially expressing TLR3 upon stimulation.21 Microglia, as resident innate immune cells, are believed to be the principle source of TLR expression in the CNS,22 where they provide an important defense against microbial infection.23 Furthermore, TLRs serve as a bridge linking innate immunity to CNS inflammation associated with various types of brain injury.22,24,25 For example, our previous work demonstrated that injury induced by lipopolysaccharide (LPS) on oligodendrocytes and neurons required the functional presence of TLR4 in microglia cells.26,27 More recently, we demonstrated that Group B Streptococcus induces neuronal apoptosis via microglial activation through a TLR2/MyD88-dependent pathway, illustrating a mechanism underlying neonatal meningitis and neurodegeneration.28 Perhaps the most intriguing observation about TLRs in the CNS is that they can be activated in the absence of microbial infection under conditions of stress, such as autoimmunity, traumatic injury, hypoxia, and neuro-degeneration.22 Such non-infectious stimuli-induced TLR activation clearly links TLRs to the mechanisms underlying a variety of neurological diseases. For example, TLR2 activation in microglia promotes cellular uptake of amyloid β peptide (Aβ42) through enhancing the function of G-protein-coupled receptor mFPR2, indicating that TLR2 may be involved in clearance of Aβ-deposits in the Alzheimer's disease (AD) brain.29 The role of TLR signaling in the pathogenesis of AD is also supported by the finding that mice harboring a Tlr4 mutation (Tlrlps-d) showed markedly increased diffuse and fibrillar Aβ-deposits in their brains, as in human AD.30 Moreover, the importance of TLR signaling in driving disease development in multiple sclerosis (MS) was demonstrated by the observations that TLR3 stimulation suppressed relapsing demyelination in a murine experimental autoimmune encephalomyelitis (EAE) model,31 and that MyD88- or TLR9-deficiency conferred resistance to EAE induction in mice.32 Also, TLR-mediated microglial activation was shown to promote excess cholinergic differentiation in the developing forebrain, and was proposed to be a factor in neurodevelopmental disorders such as autism.19 These observations suggest that TLRs have both pathogen-dependent and -independent roles in the CNS, and are broadly involved in CNS homeostasis, development, and disease processes through glia-mediated mechanisms.22,25
In contrast to the mounting studies on TLR expression in glial cells and its implied broad involvement in CNS pathological processes, evidence on the expression and function of TLRs in neurons is scarce. Recent findings indicate that TLRs are involved in the control of neuronal proliferation and differentiation,20 and are pivotal to the neuronal responses to ischemic brain injury.33 However, little is known about how TLRs function in neurons. TLR expression has been recently documented in various types of neuronal cells. In fact, all TLRs are expressed in NPCs,20 and in cortical neurons.33 TLR2 expression was detected in neurons and other cell types in postischemic brain tissue.34 TLR3 is expressed in human neurons,35 and in neuronal cell lines NT2-N and SH-SY5Y.36,37 TLR4 is expressed by a capsaicin-sensitive subclass of trigeminal sensory neurons,38 dorsal root ganglion (DRG) neurons,39 and neuroblastoma cells.40 MyD88 is present in hypothalamic neurons.41 Particularly noteworthy is the expression of TLR3 in cerebellar Purkinje neurons in human brains affected by viral encephalitis, amyotrophic lateral sclerosis, stroke, and Alzheimer's disease.42 A suggestion that neurons are intrinsically capable of mounting an immune response against microbial challenges comes from data that stimulation in vitro of NT2-N neuronal cells, which express TLR3, with viral infection or a synthetic analogue of viral dsRNA, polyinosinic-polycytidylic acid (polyI:C), induces broad expression of inflammatory genes.37 PolyI:C treatment of human SH-SY5Y neuroblastoma cells induced the hyperphosphorylation of tau protein, a pathological hallmark of Alzheimer's disease.36 Hence, it seems that TLR3 may be involved directly in controlling neuronal responses against viral infection and in the pathological processes of some neurodegenerative diseases. However, caution must accompany the interpretation with regard to how TLR3 exerts its function in neurons because dsRNA can be recognized by intracellular dsRNA-dependent serine/threonine protein kinase (PKR) and helicases (RIG-1 and Mda5) in addition to TLR3.43 Relevant to this issue, our recent work on TLR8 suggests that TLRs in neurons likely function through a mechanism distinct from the one established in immunity.44
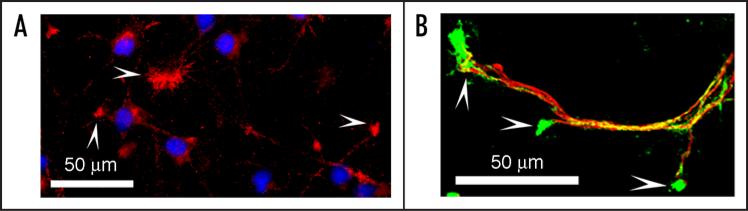
We started our investigation by systematically profiling the expression of TLRs during mouse brain development. TLR8 became our focus because of its abundance and dynamically changing expression during development.44 Of note, TLR8 expression remains detectable in the adult mouse brain, though it is greatly diminished once brain development is completed,44 so that TLR8 is still available to participate in inflammatory processes in the adult brain under appropriate circumstances. The level of TLR8 expression in cultured cortical neurons was much higher than that in immune cells by Western-blot analysis,44 further promoting our interest in TLR8 function within the CNS. Immunohistochemical analysis confirmed TLR8 expression and intracellular localization in neurons and axons (Fig. 1).
Figure 1.
TLR8 expression and localization in cultured cortical neurons. (A) TLR8 (red) is expressed intracellularly in cultured neurons and is enriched in their growth cones (arrowheads). (B) TLR8 (green)- positive punctuate staining is distributed along axons (neurofilament 200 KD, red) and is concentrated in growth cones (arrowheads). Cortical neurons are cultured from embryonic day 17 (E17) mice, fixed at day-in-vitro (DIV) 3 with methanol at -20°C for 10 minutes, followed by staining with anti-TLR8 polyclonal antibody alone (A), or together with anti-neurofilament 200 KD monoclonal antibody (B).
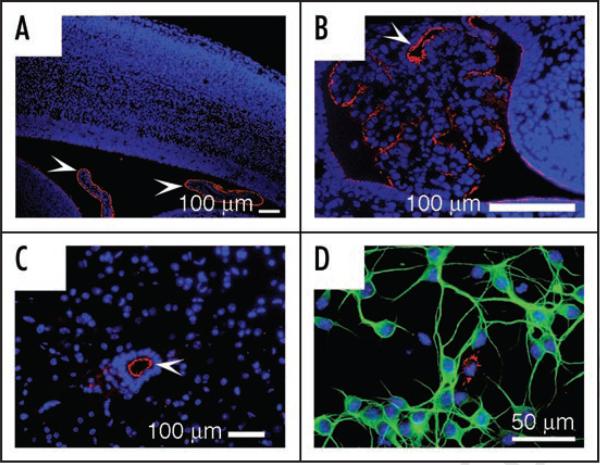
As TLR7 is highly homologous to TLR8, and with it forms a subgroup within the TLR superfamily,45 we also characterized the expression of TLR7 in the CNS. Immunohistochemical analysis revealed that TLR7 is selectively expressed in ependymal cells lining the ventricular walls in the developing and adult CNS (Fig. 2A–C). Consistent with our previous Western-blot result,44 double-immunostaining demonstrated that cultured cortical neurons do not express TLR7 (Fig. 2D). Thus, the expression pattern of TLR7 is distinct from that of TLR8, suggesting that TLR8 may have acquired neuron-specific functions in mice through evolutionary divergence. Importantly, the absence of TLR7 in neurons has allowed us to use resiquimod (R-848), a well-established TLR7/8 agonist,46 to investigate the effects of TLR8 activation in neurons.
Figure 2.
Neurons do not express TLR7. (A-B) Immunostaining with anti-TLR7 polyclonal antibody on sagittal sections of E18 brain shows that TLR7 (red) is expressed only in the choroid plexus (arrowheads) and ependymal cells in the lateral ventricle (A) and fourth ventricle (B). (C) In the adult mouse spinal cord, TLR7 (red) is specifically expressed in ependymal cells (arrowheads) of the central canal. (D) Immunostaining of cortical neuron cultures from E18 mouse confirms that TLR7 is absent in neurons (MAP2, green; cell nuclei DAPI-stained, blue) but rather expressed in a few non-neuronal cells (red).
IS MURINE TLR8 FUNCTIONAL?
TLR8, perhaps the least studied amongst TLRs, was thought to function in a species-specific manner and was considered previously to be non-functional in mice because HEK cells transfected with human but not murine TLR8 were responsive to its agonist, R848,46 and Tlr8-deficient macrophages show unim-paired inflammatory gene expression in response to TLR8 agonists, including R-848 and single-stranded RNA (ssRNA).47 However, TLR8 expression, either constitutively or in response to microbial infections, has been documented in a variety of murine cell types, including embryonic fibroblasts,48 dendritic cells (DCs),49,50 MSCs,18 glia,32,51,52 NPCs,20 cortical neurons,33,44 and granule neurons and axons.53 Further, humans and mice differ significantly in both innate and adaptive immunity mechanisms,54 and species variations in TLR expression between humans and mice with respect to both transcriptional regulation and cell-type specificity, are not uncommon.55 Therefore, it may not be surprising that a given ligand may induce different responses through an orthologous receptor in the two species. For instance, human TLR2, known to be functional, does not efficiently recognize the tri-lauroylated lipopeptide analogue (Lau3CSK4), an effective agonist of murine TLR2.56
Notably, most early experiments on TLR8 function relied solely on monitoring NFκB transactivation and the subsequent induction of cytokine expression. Thus, the possibility that murine TLR8 activation induces NFκB-independent events may have been overlooked. There is evidence that murine TLR8 is functional: TLR8 expression is significantly increased in mouse CNS in response to microbial challenge,51,52 and during the progression of EAE.32 Also, R-848 induces secretion of cytokine IL-12p40 in a subset of murine intestinal dendritic cells (CD172a+iL-DCs) that express TLR8 but not TLR7.50
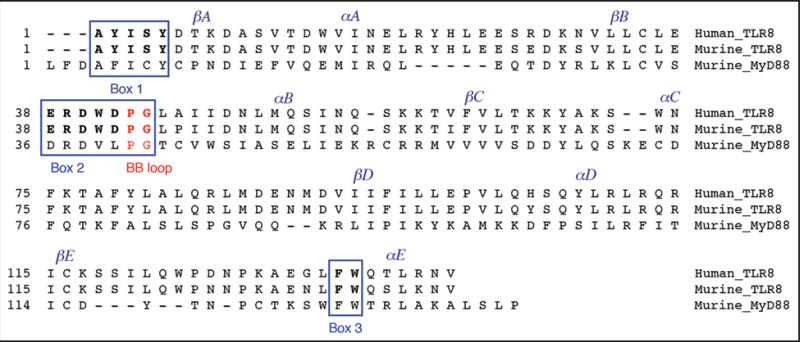
Because the Tlr8 locus in the chicken genome is disrupted by the insertion of chicken repeat-1 (CR1) retroviral-like elements and thus represents a pseudogene,57 we examined whether a similar gene defect might exist in murine Tlr8. On the contrary, the Tlr8 genomic locus is highly conserved between humans and mice. Mutational biases pattern analysis indicated that amongst TLR family members, TLR8 carries minimal variation in the TIR domain, known to be critical for TLR intracellular signaling, over the course of evolution.58 Furthermore, our alignment analysis shows that the amino acid residues within the three motif boxes of the TIR domain are identical in human and murine TLR8 (Fig. 3). These observations suggest that the inability of murine TLR8 to induce NF-κB activation is not due to a structural defect in the TIR domain, a notion that is further supported by the observation that a chimeric molecule composed of murine TLR4 ectodomain and murine TLR8 intracellular domain does activate NFκB in response to LPS stimulation, indicating integrity of the murine TLR8 signaling capability.59 The physical associations between murine TLR7, 8, and 9, and the impact of their interactions on signaling by individual TLRs, have been recently demonstrated, revealing, among other results, that murine TLR8 can regulate TLR7 or TLR9 signaling.60 Direct evidence came from the demonstration that HEK cells transfected with murine TLR8 activated NFκB when stimulated with imidazoquinolines in combination with polyT oligodeoxynucleotides.61 Thus, murine TLR8 appears to require additional ligands for robust NFκB activation. These observations argue that murine TLR8 is indeed functional, even in the context of NFκB activation.
Figure 3.
The amino acid sequences of the TIR domains of human TLR8, murine TLR8, and murine MyD88. The sequences were identified with the SMART (simple modular architecture research tool) program (smart.embl-heidelberg.de/); and the alignment analysis was performed with the MegAlign program (DNASTAR, Inc.) using the ClustalW method. Box 1, Box 2 along with the BB loop, and Box 3 are framed, and residues of the BB loop are shown in red. The residues within the three boxes of human and murine TLR8 are shown in bold. βA, B, C, D, E and αA, B, C, D, E at the top indicate five β-strands and α-helices, respectively, of the secondary structure of the TIR domain. The Genbank accession numbers assigned to the sequences are NP_057694 for human TLR8, NP_573475 for murine TLR8, and NP_034981 for murine MyD88.
The fact that stimulation of murine TLR8 by established agonists, including R-848 and ssRNA, is unable to induce effective NFκB activation has prompted us to speculate that: TLR8 might operate in a cell-type specific manner, and function in biological processes that do not require NFκB activation. Cell-type specific signaling has been observed recently among TLRs. As an example, stimulation of TLR3 with its agonist, polyI:C, activated NFκB and MAPKs in human endothelial cells and synovial fibroblasts, but were unable to do so in dendritic cells and macrophages.62 In support of our hypothesis, we recently observed that TLR8 activation by R-848 in cultured mouse cortical neurons induced morphological responses but did not cause NFκB activation,44 as would have been expected based on the TLR signaling mechanism in immune cells. Specifically, TLR8 activation in neurons inhibited neurite outgrowth, and in a dissociable manner, triggered apoptosis, with no canonical TLR signaling events detected. We demonstrated the specificity of TLR8 activation-induced effects on neurons through (1) stimulation of other PAMPs as controls and (2) specific antibody-mediated partial blockade of the neuronal responses. Our finding substantiated the hypothesis that TLR8 in mice may function in a specific major class of non-immune cells, namely neurons.
HOW DOES TLR8 SIGNAL IN NEURONS?
TLRs, except for TLR3, signal inflammation via a conserved canonical pathway initiated by the association of the adaptor protein MyD88 with TLR intracellular domains through a TIR-mediated interaction.63 Subsequently, IL-1R-associated kinase (IRAK)-1 and IRAK-4 are recruited to the receptor complex. Upon phosphorylation, IRAKs dissociate from MyD88 and bind tumor necrosis receptor factor-associated factor 6 (TRAF6). TRAF6 forms a complex with ubiquitin-conjugating enzyme 13 (Ubc13) and ubiquitin-conjugating enzyme E2 variant 1 (Uev1A), which in turn activates a transforming growth factor-β activated kinase 1 (TAK1). TAK1 in combination with TAK-binding proteins (TABs) activates both MAPKs and IKKs. The IKK complex then catalyzes the phosphorylation of IκBα, leading to its ubiquitin-mediated degradation and the subsequent nuclear translocation of NFκB. In another branch, MAPKs activate the transcription factor AP1, a heterodimer formed by c-jun and c-fos. The transcription factors NFκB and AP-1 control the expression of a broad spectrum of cytokines critical to inflammatory responses.63
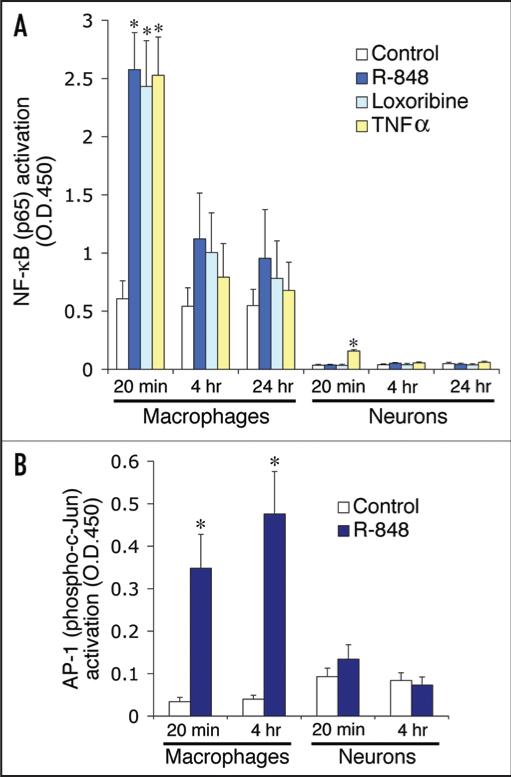
To gain molecular insights into how TLR8 functions in neurons, we explored whether TLR8 signaling would activate the canonical TLR pathway. Contrary to our expectations, none of the characteristic hallmarks of TLR signaling were detected in neurons stimulated with the TLR8 agonist, R-848.44 We have now confirmed by ELISA analysis that neither NFκB nor AP-1 were activated in neurons treated with a TLR8 agonist (Fig. 4). Furthermore, application of a MAPKK inhibitor had no impact on R-848-induced effects on neurons, indicating that MAPK is not required in TLR8 signaling (Fig. 5). In addition, we conducted parallel experiments with neurons cultured from Myd88−/− mice, and found that MyD88 deficiency, while slightly altering cell viability, did not confer resistance against R-848 effects (Fig. 6). Thus, we conclude that TLR8 in neurons does not function through the canonical TLR signaling pathway. Intriguingly, a recent report found that oxygen deprivation in cultured cortical neurons induced cell apoptosis in a TLR2 and 4-dependent manner without causing NFκB activation.33 Therefore, it seems a universal feature that TLR signaling in neurons does not involve NFκB, at least in the context of cell apoptosis.
Figure 4.
TLR8 stimulation in neurons activates neither the transcription factor NFκB (A) nor AP-1 (B). ELISA assays for NFκB (p65) and AP-1 (phospho-c-Jun) transactivation using nuclear extracts from the cortical neurons treated with 100 μM R-848, 500 μM loxoribine, 10 ng/ml TNFα for the indicated times. Note the robust activation of both NFκB and AP-1 detected in macrophages in response to R-848 stimulation. Data is presented as the mean ± SEM (n = 3). *p < 0.05, Student's t-Test.
Figure 5.
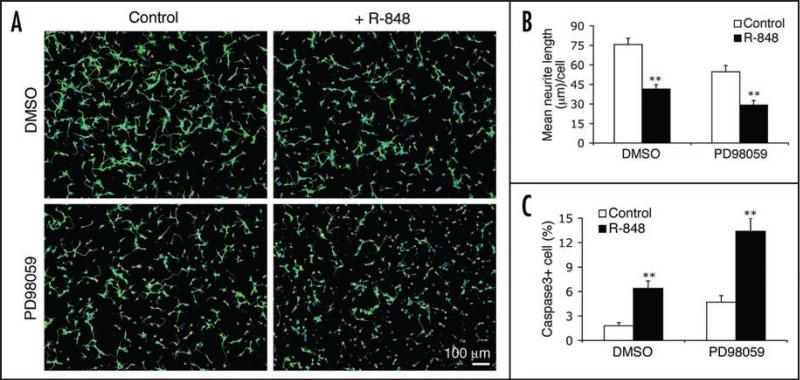
R-848 effects on neurons do not require MAP kinase activity. (A) Representative micrographs of cortical neurons that were untreated (control, left column) or treated with 100 μM R-848 for 24 hours (right column), in the presence of vehicle control (DMSO, upper row) or a MAPKK inhibitor (50 μM PD98059, lower row). (B and C) Quantitative analyses of neurite length (B) and percent of the cleaved caspase3-positive cells (C) show that blocking MAPKK did not prevent the R-848-induced neurite outgrowth suppression and cell apoptosis. Data are presented as the mean ± SEM (n = 12 fields). **p < 0.01, Student's t-Test.
Figure 6.
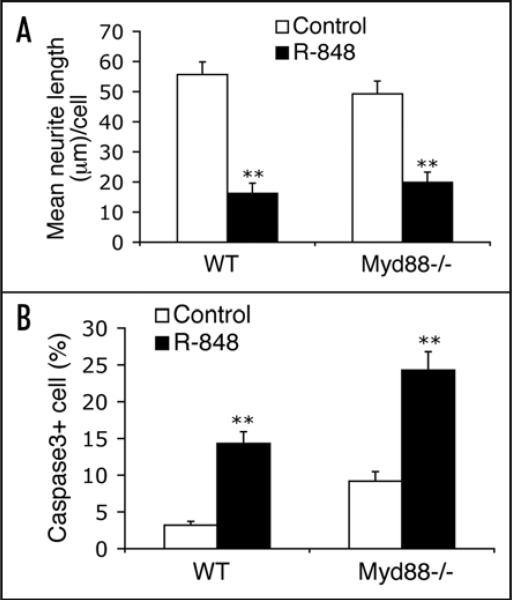
R-848 effects on neurons are independent of MyD88. Cortical neurons cultured from E17 Myd88−/− and wild-type littermate (WT) embryos were treated with PBS (control) or 100 μM R-848 for 24 hours. Quantitative analyses reveal that deficiency of MyD88 does not confer resistance to the R-848-induced inhibition of neurite outgrowth (A) and cell apoptosis (B). Data are presented as the mean ± SEM (n = 12 fields). **p < 0.01, Student's t-Test.
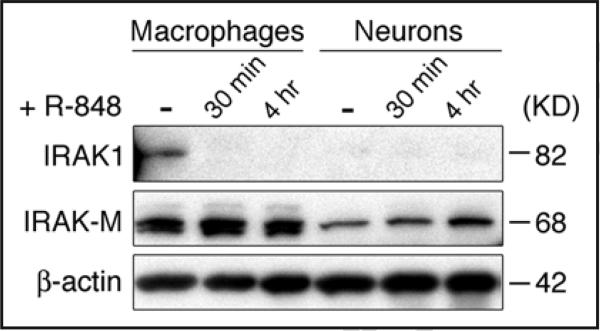
A variety of stimuli, including β-amyloid, TNF-α, transforming growth factor (TGF-β), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and Ca2+/Calmodulin-dependent protein kinase II (CaMKII) can induce NFκB activation in neurons.64 However, it is also believed that the NFκB cascade is tightly regulated in this cell type, since many NFκB activators known to be potent in other cell types are unable to activate NFκB in neurons.65 The hyporesponsiveness of neurons to NFκB induction might represent a specialized adaptation to provide an immunoprivileged status to neurons, and thus contribute to neuroprotection in the brain.65 Hypotheses interpreting this phenomenon include the one that the signaling machinery responsible for NFκB activation may be uniquely regulated in neurons.65,66 In this context, we found that IRAK1, a critical component in the TLR signaling cascade, is not expressed in cortical neurons either under basal conditions or when stimulated with R-848, whereas IRAK1 is constitutively expressed in macrophages and rapidly degraded upon ligand stimulation (Fig. 7). By contrast, IRAK4, another IRAK family member, which lies upstream of IRAK1 in the TLR signaling cascade, is expressed in neurons but becomes degraded slowly upon R-848 stimulation.44 These data suggest the lack of IRAK1 expression by neurons may contribute to their recalcitrance of NFκB activation during TLR signaling. In support, recent studies revealed IRAK1c, a splice variant form of IRAK1 that lacks the intrinsic kinase activity and can potentially suppresses NFκB-inducing signals, exists as the predominant form of IRAK1 in the brain.67,68 Other mechanisms negatively regulating TLR signaling in neurons may also exist. We found IRAK-M, a negative regulator of TLR signaling,69 is also expressed in neurons and is slightly upregulated by R-848 (Fig. 7). Though further analysis is required to clarify whether introducing IRAK1 in neurons would restore responsiveness to NFκB-inducing signals, or whether inhibition by IRAK-M is sufficient to account for the neuronal lack of TLR-induced NFκB activation, the absence of IRAK1 in neurons strongly suggests that TLR8 must induce neuronal responses through a different signaling pathway.
Figure 7.
Cortical neurons lack IRAK1 expression. Neurons cultured from E17 mouse and Raw264.7 macrophages were treated with 100 μM R-848 for the indicated times. Equal amounts of protein lysates were subjected to Western blotting using anti-IRAK1 and anti-IRAK-M polyclonal antibodies. β-actin serves as the loading control.
How, then, are TLR8 signals transduced in neurons? Two components of the canonical TLR signaling pathway found to be degraded in response to neuronal stimulation by R-848 are IκBα and IRAK4.44 The timing of their degradation coincides with the onset of morphological changes in neurons, suggesting a possible link between these events.44 As no degradation of other TLR signaling machinery was observed, the downregulation of IκBα and IRAK4 likely resulted from a specific process rather than from some overall degradation caused by caspase activation. In fact, caspase family inhibitors were incapable of blocking the prolonged TLR stimulation-induced degradation of IRAK4 in macrophages,70 and should be tested also in neurons.
Of particular note, TLR8 stimulation in neurons causes phosphor-ylation (Ser32)-independent IκBα degradation that does not lead to NFκB transactivation.44 Intriguingly, a similar phenomenon was recently noted in a report that LPS stimulation of TLR4-expressing neuroblastoma NB-1 cells induced a transient, partial degradation of IκBα, while NFκB activation was not detected.40 These findings invite the speculation that IκBα in neurons might be uniquely regulated in the context of TLR signaling. Emerging evidence indicates that IκBα may have broader roles other than inhibiting NFκB activation by blocking its nuclear translocation.71 IκBα can shuttle between cytoplasm and nucleus, and recruit through physical interactions nuclear corepressors (N-CoRs) or histone acetyltransferases and deacetylases (HDACs) to the NFκB-dependent or -independent promoters of target genes.72,73 Hence, IκBα is able on its own to activate or repress gene transcription, or crosstalk with other signaling cascades such as the Notch pathway.72 Such IκBα-mediated gene regulation may be relevant to NFκB-independent TLR signaling. Application of the HDAC inhibitors increased the acetylation status at the IL-12p40 gene promoter and thus strongly inhibited TLR-induced expression of this cytokine.74 Also, TLR4 signaling was shown to control IL12p40 gene expression by inducing chromatin remodeling of its promoter, in a manner independent of NFκB induction.75 Based on these observations, it might be plausible that TLR8 signaling in neurons causes morphological responses through an NFκB-independent, IκBα-mediated transcriptional activation/ repression of as-yet unknown target genes. Future experiments using approaches such as chromatin immunoprecipitation (ChIP) to identify IκBα-regulated genes during TLR8 stimulation might provide insights on how TLR8 signaling translates into neuronal responses.
AN AXONAL VIEW
TLR activation has been implicated in pathological processes associated with axonal injury. Spinal nerve axotomy triggered TLR-mediated glia cell activation and subsequently led to pain hyper-sensitivity, and further analysis with TLR2- and TLR4-knockout mice confirmed that both receptors are critically involved in this neuropathic pain.76,77 In another model of axonal injury, stereotactic transection of axons in the entorhinal cortex led to upregulation of TLR2 by microglia in the denervated zones of the hippocampus.78 The damaged axons apparently induced cytokine production and inflammation by releasing undefined factors that caused TLR activation in microglia or astrocytes. The intensity and duration of such inflammatory responses correlated well with the extent of the axon injury, and in addition, TLR activation may cause injury of the axon itself. Microbial lipopeptides trigger Schwann cell apoptosis in vitro via TLR2, and expression of TLR2 in vivo was found to correlate with Schwann cell apoptosis in human leprosy lesions.79 These observations suggest that TLR activation and axonal injury may be closely linked, a role for which TLR8 appears well suited due to its distinct localization in axons.
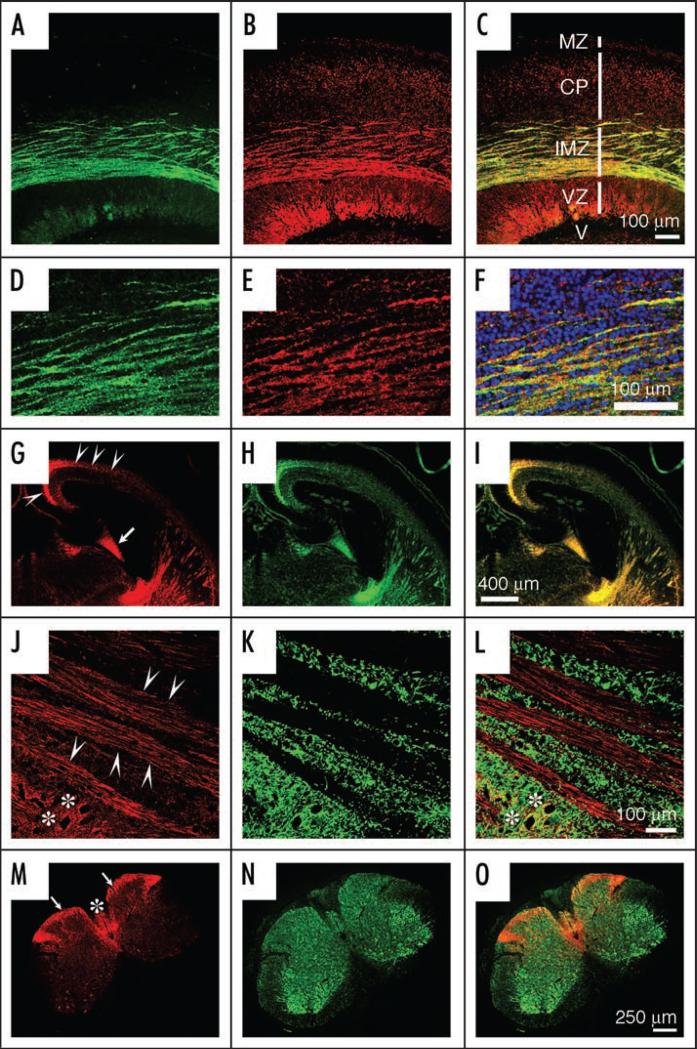
In cultured cortical neurons, TLR8 is highly enriched in growth cones, and is also distributed as punctuate structures throughout the soma and cell processes (Fig. 1). In the embryonic mouse brain TLR8 expression is restricted largely to the axonal fiber tracts, and is absent in dendrites (Fig. 8A–I).44 In the adult CNS TLR8 expression becomes diffuse and diminished,44 but such an axon-specific expression pattern remains characteristic in some areas, including the striatal bundles in the forebrain and tracts in the dorsal spinal cord (Fig. 8J–O). This predilection for localization to axons might indicate that TLR8 is a regulator of axonal dynamics during CNS development, and in pathological processes. With regard to the latter, TLR8 expression was distinctively upregulated in axons of cerebellar granule cell neurons in a mouse model of parasitic neurocysticercosis.53 Of note, the TLR8-positive axons appeared in close contact with CD11c-positive dendritic/microglial cells, suggesting that TLR8 may mediate interactions between axons and immune cells in a CNS inflammatory process.53 It would be particularly interesting to examine whether TLR8 activation is involved in pathological processes featuring both axonal injury and inflammation, such as multiple sclerosis.80
Figure 8.
TLR8 is expressed in developing and adult axons. (A–F) Dual-immunolabeling of TLR8 (green) and the neuronal/axonal marker βIII-tubulin (red) on sagittal sections of E18 mouse cortex. Note that TLR8 is specifically expressed in the cortical intermediate zone (IMZ) where thalamocortical and corticofugal axons are enriched. Additional zones shown in (C) include marginal zone (MZ), cortical plate (CP), ventricular zone (VZ), and lateral ventricle (V). (D–F) Corresponding higher magnification views of the IMZ regions of (A-C). (G-I) Dual-immunolabeling of TLR8 (red) and the axonal marker growth-associated protein 43 (GAP43, green) on coronal sections of E18 mouse brain. Arrowheads indicate the IMZ and arrows point to the fimbra (an axonal bundle) of the hippocampus. (J-L) Dual-immunolabeling of TLR8 (red) and the neuronal dendrite marker microtubule-associated protein 2 (MAP2, green) on sagittal sections of three-month-old mouse brain caudae/putamen, showing striatal axonal bundles, with arrowheads pointing to individual axons. Asterisks indicate neuronal somas co-stained with TLR8 and MAP2. (M-O) Dual-immunolabeling of TLR8 (red) and βIII-tubulin (green) on three-month-old mouse spinal cord sections. Note that TLR8 is highly expressed in the axons in the dorsal funiculus (arrows) and the dorsal column (asterisk).
ISSUES AND CHALLENGES
Our data uncovered novel functions for TLR8 in neurons, but also raise new questions. Might TLR8 have a neurodevelopmental role? TLR8 is expressed in the CNS prominently in embryonic axons, is developmentally regulated, and is capable upon activation of inhibiting neurite outgrowth and trigger neuronal apoptosis. As an innate immune receptor, might TLR8 participate in CNS inflammatory processes? Since TLR8 expression in the adult brain is sharply diminished but not absent, we speculate that a role switch from development to inflammation might occur as mice mature. The latent expression of TLR8 in the adult CNS suggests that an innate immunity function of TLR8 may be quiescent under normal conditions, in keeping with the immune-privileged status of the brain, but that TLR8 might spring into action by directly mediating neuronal or axonal responses to endogenous stimuli such as traumatic and hypoxic tissue injury, or exogenous stimuli such as viral infection. This idea is considered further below.
What are the mediators and effectors of TLR8 signaling in neurons? How does TLR8 activation in neurons translate into two independent processes-neurite outgrowth suppression and apoptosis? Although the TLR8 signaling cascade in neurons remains largely undefined, some clues are emerging. Our data indicate that both IκBα and IRAK4 probably mediate TLR8 signaling in neurons, even though it remains to be solved how R-848 triggers IκBα- and IRAK4-downregulation. IRAK4 is a central mediator of the canonical TLR signaling pathway,81 and it has a role in cell apoptosis, at least with respect to human cancer cells.82 Also, mice deficient in IRAK4 were protected against hyperoxia-induced neuronal death.83 Thus, it is plausible that IRAK4 is involved in the process leading to neuronal apoptosis. However, other mediators might exist, among which Bim, a Bcl-2 homology domain 3-only protein, is an attractive candidate. Bim is a known key mediator of neuronal apoptosis whose expression in neurons is increased in certain neurodegenerative disease.84,85 Intriguingly, TLR activation can upregulate the expression of Bim in macrophages, and is required for Bim-triggered cell apoptosis.86 It remains to be examined whether TLR8 activation induces apoptosis in neurons by upregulating their expression of Bim.
TLR activation in neurons also induces suppression of neurite outgrowth, probably through cytoskeletal mechanisms. It was previously demonstrated that TLR activation induced actin reorganization in dendritic cells and macrophages.87,88 A particularly relevant candidate might be the small GTPase Rab7, for a proteome analysis revealed that R-848 stimulation significantly increased the expression of Rab7 in macrophages.89 Rab7 in neurons mediates endosomal trafficking of TrkA,90 a process crucial to NGF-induced neurite outgrowth.91 It is tempting to speculate that TLR8 signaling leads to the upregulation of Rab7 expression in neurons, which in turn would disrupt TrkA trafficking and thus suppress NGF-mediated neurite outgrowth. A further issue would be whether Rab7 is one of the target genes regulated by IκBα in the context of TLR8 activation in neurons.
Does TLR8 have an endogenous ligand in mice? A variety of host-derived molecules, usually released from damaged cells or degraded upon tissue injury, have been proposed as endogenous ligands for various TLRs, including fibronectin, hyaluronan, biglycan, uric acid, heat-shock proteins HSP60, HSP70, and GP96, high mobility group box1 (HMGB1), eosinophil-derived neurotoxin (EDN), mammalian DNA and various RNA species, and small nuclear ribonucleoprotein particles (snRNPs).92,93 What does murine TLR8 recognize? We initially pursued this question with an expression cloning approach, a classical methodology to identify ligands.94 We constructed two different versions of recombinant protein encoding the ectodomain or the entire non-TIR region of murine TLR8 fused in their C-terminals with the reporter gene alkaline phosphatase. However, these recombinant proteins, though correctly expressed, lacked enzymatic activity and thus did not serve our goal. Still, we are persuaded to continue seeking an endogenous ligand for TLR8.
The idea that TLR8 might have an endogenous ligand in mice stems from the observation that murine TLR8 differs from its human homologue in its ability to activate NFκB in response to R-848 or ssRNA.46,47 Because the intracellular TIR domain in both murine and human TLR8 possesses intact signaling capacity,59 the difference might be attributed to structural variations in the leucine-rich repeats (LRRs) of their ectodomains. The LRR domains of TLRs form a horseshoe structure thought to be directly involved in recognition of specific PAMPs.63 Murine and human TLR8 have substantial variations in LRR organization, which likely impacts the efficiency or even the specificity of ligand recognition.95 Thus, it might explain why R-848 can effectively induce NFκB activation through human but not murine TLR8. The evolutionary variations in TLR8 ectodo-main structure might represent a response to a changing microbial environment that allowed human TLR8 better to recognize PAMPs, and in turn to eliminate more efficiently invading pathogens through a robust induction of NFκB-dependent immune responses. Yet, the inefficiency of murine TLR8 in inducing NFκB activation begs the question why TLR8 is still expressed and tightly controlled in a variety of mouse cell types including neurons.18,20,32,33,48-53 From the CNS perspective, the speculation raised in the opening paragraph of this section is pertinent, that TLR8 in adult mouse brain, albeit expressed, remains quiescent under normal conditions due to its limited efficiency in inducing NFκB-controlled inflammatory responses. TLR8, however, might retain the potential to engage in CNS innate immunity when appropriate stimuli are present. The observation favoring this idea is that R-848 combined with polyT oligodeoxynucleotides can induce robust NFκB activation via murine TLR8.61 An alternative hypothesis is that an endogenous ligand able to activate TLR8 in a biological process not involving NFκB might exist in neurons of developing brains where TLR8 is abundantly expressed. Adding to the plausibility of this idea is our evidence that TLR8 functions in neurite outgrowth and neuronal apoptosis though an NFκB-independent mechanism.44 The putative endogenous ligand, likely a protein, would not necessarily bind to the same site in the TLR8 ectodomain as R-848. The LRR motif of the TLR ectodomain provides a particularly suitable molecular framework for protein interactions,96 and has been found in a large number of mammalian proteins with diverse functions, including neuronal differentiation, migration and axonal guidance.97 In this context, studies from the fruit fly give the clue that a proteolytically-processed polypeptide named Spätzle directly binds to the Toll ectodomain, which in turn induces Toll homodimerization and subsequent activation.98 Spätzle, however, has no mammalian counterpart, and was shown not to bind to the immobilized human TLR2 ectodomain in a Biacore sensor chip experiment.15 Nonetheless, the C-terminal domain of Spätzle is structurally similar to a cystine-knot cytokine superfamily in mammals, which includes NGF, platelet-derived growth factor (PDGF) and TGF-β.99 It appears reasonable to ask whether a mammalian cystine-knot cytokine-like protein might activate TLR8.
In sum, while many questions remain unanswered, particularly how TLR8 is activated in neurons and how the intracellular signaling transduced, our finding suggests an emerging role for TLR8 in bridging development, inflammation, and axonal injury in the murine CNS.
ACKNOWLEDGEMENTS
We thank colleagues I. Chiu, J. Lu, and J. Sloane for critical reading of the manuscript. This work was supported by grants from the US National Institutes of Health and the National Multiple Sclerosis Society.
ABBREVIATIONS
- IκBα
nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor α
- IRAK
interleukin-1 receptor-associated kinase
- LPS
lipopolysaccharide
- MyD88
myeloid differentiation factor 88
- NFκB
nuclear factor-κB
- PAMPs
pathogen-associated molecular patterns
- TLR
Toll-like receptor
References
- 1.Lemaitre B, Nicolas E, Michaut L, Reichart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 3.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–93. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–74. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Belvin MP, Anderson KV. A conserved signaling pathway: The Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong NJ, Steinbeisser H, Prothmann C, DeLotto R, Rupp RA. Conserved Spatzle/ Toll signaling in dorsoventral patterning of Xenopus embryos. Mech Dev. 1998;71:99–105. doi: 10.1016/s0925-4773(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 8.Rose D, Zhu X, Kose H, Hoang B, Cho J, Chiba A. Toll, a muscle cell surface molecule, locally inhibits synaptic initiation of the RP3 motoneuron growth cone in Drosophila. Development. 1997;124:1561–71. doi: 10.1242/dev.124.8.1561. [DOI] [PubMed] [Google Scholar]
- 9.Ritzenthaler S, Chiba A. Myopodia (postsynaptic filopodia) participate in synaptic target recognition. J Neurobiol. 2003;55:31–40. doi: 10.1002/neu.10180. [DOI] [PubMed] [Google Scholar]
- 10.Halfon MS, Keshishian H. The Toll pathway is required in the epidermis for muscle development in the Drosophila embryo. Dev Biol. 1998;199:164–74. doi: 10.1006/dbio.1998.8915. [DOI] [PubMed] [Google Scholar]
- 11.Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–20. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- 12.Chuang CF, Bargmann CI. A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes Dev. 2005;19:270–81. doi: 10.1101/gad.1276505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 14.Beutler B, Poltorak A. Toll we meet again. Nat Immunol. 2001;2:9–10. doi: 10.1038/83222. [DOI] [PubMed] [Google Scholar]
- 15.Gangloff M, Weber AN, Gibbard RJ, Gay NJ. Evolutionary relationships, but functional differences, between the Drosophila and human Toll-like receptor families. Biochem Soc Trans. 2003;31:659–63. doi: 10.1042/bst0310659. [DOI] [PubMed] [Google Scholar]
- 16.Hwa Cho H, Bae YC, Jung JS. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24:2744–52. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- 17.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–32. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 19.Ni L, Acevedo G, Muralidharan B, Padala N, To J, Jonakait GM. Toll-like receptor ligands and CD154 stimulate microglia to produce a factor(s) that promotes excess cholinergic differentiation in the developing rat basal forebrain: Implications for neurodevelopmental disorders. Pediatr Res. 2007;61:15–20. doi: 10.1203/01.pdr.0000249981.70618.18. [DOI] [PubMed] [Google Scholar]
- 20.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–8. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 21.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–9. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–30. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konat GW, Kielian T, Marriott I. The role of Toll-like receptors in CNS response to microbial challenge. J Neurochem. 2006;99:1–12. doi: 10.1111/j.1471-4159.2006.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crack PJ, Bray PJ. Toll-like receptors in the brain and their potential roles in neuropathology. Immunol Cell Biol. 2007;85:476–80. doi: 10.1038/sj.icb.7100103. [DOI] [PubMed] [Google Scholar]
- 25.van Noort JM. Toll-like receptors as targets for inflammation, development and repair in the central nervous system. Curr Opin Investig Drugs. 2007;8:60–5. [PubMed] [Google Scholar]
- 26.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–86. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–9. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, Bechmann I, Nitsch R, Weber JR, Golenbock DT, Vartanian T. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177:583–92. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM, Wang JM. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem. 2006;281:3651–9. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- 30.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–19. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Touil T, Fitzgerald D, Zhang GX, Rostami A, Gran B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol. 2006;177:7505–9. doi: 10.4049/jimmunol.177.11.7505. [DOI] [PubMed] [Google Scholar]
- 32.Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ, Rochford CD, Bruck W, Becher B. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–64. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–804. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–9. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- 35.Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: Human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29:185–94. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 36.Nessa BN, Tanaka T, Kamino K, Sadik G, Ansar AB, Kimura R, Tanii H, Okochi M, Morihara T, Tagami S, Kudo T, Takeda M. Toll-like receptor 3 mediated hyperphosphorylation of tau in human SH-SY5Y neuroblastoma cells. Psychiatry Clin Neurosci. 2006;60(Suppl 1):S27–33. [Google Scholar]
- 37.Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: A mechanism for pain due to infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J Neurosci. 2004;24:9623–31. doi: 10.1523/JNEUROSCI.2392-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassan F, Islam S, Tumurkhuu G, Naiki Y, Koide N, Mori I, Yoshida T, Yokochi T. Intracellular expression of toll-like receptor 4 in neuroblastoma cells and their unresponsiveness to lipopolysaccharide. BMC Cancer. 2006;6:281. doi: 10.1186/1471-2407-6-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis CN, Mann E, Behrens MM, Gaidarova S, Rebek M, Rebek J, Jr, Bartfai T. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc Natl Acad Sci USA. 2006;103:2953–8. doi: 10.1073/pnas.0510802103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson AC, Rossiter JP, Lafon M. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol. 2006;12:229–34. doi: 10.1080/13550280600848399. [DOI] [PubMed] [Google Scholar]
- 43.Werts C, Girardin SE, Philpott DJ. TIR, CARD and PYRIN: Three domains for an antimicrobial triad. Cell Death Differ. 2006;13:798–815. doi: 10.1038/sj.cdd.4401890. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–15. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: Gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362–71. [PubMed] [Google Scholar]
- 46.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 47.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 48.Kurt-Jones EA, Sandor F, Ortiz Y, Bowen GN, Counter SL, Wang TC, Finberg RW. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J Endotoxin Res. 2004;10:419–24. doi: 10.1179/096805104225006516. [DOI] [PubMed] [Google Scholar]
- 49.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: Lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–33. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 50.Yrlid U, Cerovic V, Milling S, Jenkins CD, Klavinskis LS, MacPherson GG. A distinct subset of intestinal dendritic cells responds selectively to oral TLR7/8 stimulation. Eur J Immunol. 2006;36:2639–48. doi: 10.1002/eji.200636426. [DOI] [PubMed] [Google Scholar]
- 51.McKimmie CS, Johnson N, Fooks AR, Fazakerley JK. Viruses selectively upregulate Toll-like receptors in the central nervous system. Biochem Biophys Res Commun. 2005;336:925–33. doi: 10.1016/j.bbrc.2005.08.209. [DOI] [PubMed] [Google Scholar]
- 52.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 53.Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 55.Rehli M. Of mice and men: Species variations of Toll-like receptor expression. Trends Immunol. 2002;23:375–8. doi: 10.1016/s1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 56.Grabiec A, Meng G, Fichte S, Bessler W, Wagner H, Kirschning CJ. Human but not murine toll-like receptor 2 discriminates between tri-palmitoylated and tri-lauroylated peptides. J Biol Chem. 2004;279:48004–12. doi: 10.1074/jbc.M405311200. [DOI] [PubMed] [Google Scholar]
- 57.Philbin VJ, Iqbal M, Boyd Y, Goodchild MJ, Beal RK, Bumstead N, Young J, Smith AL. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology. 2005;114:507–21. doi: 10.1111/j.1365-2567.2005.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanghavi SK, Shankarappa R, Reinhart TA. Genetic analysis of Toll/Interleukin-1 Receptor (TIR) domain sequences from rhesus macaque Toll-like receptors (TLRs) 1-10 reveals high homology to human TLR/TIR sequences. Immunogenetics. 2004;56:667–74. doi: 10.1007/s00251-004-0734-6. [DOI] [PubMed] [Google Scholar]
- 59.Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J Biol Chem. 2004;279:19008–17. doi: 10.1074/jbc.M311618200. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Shao Y, Bennett TA, Shankar RA, Wightman PD, Reddy LG. The functional effects of physical interactions among Toll-like receptors 7, 8, and 9. J Biol Chem. 2006;281:37427–34. doi: 10.1074/jbc.M605311200. [DOI] [PubMed] [Google Scholar]
- 61.Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: Activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol. 2006;177:6584–7. doi: 10.4049/jimmunol.177.10.6584. [DOI] [PubMed] [Google Scholar]
- 62.Lundberg AM, Drexler SK, Monaco C, Williams LM, Sacre SM, Feldmann M, Foxwell BM. Key differences in TLR3/POLY I:C signaling and cytokine induction by human pri-mary cells: A phenomenon absent from murine cell systems. Blood. 2007;110:3245–52. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- 63.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 64.Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Jarosinski KW, Whitney LW, Massa PT. Specific deficiency in nuclear factor-kappaB activa- tion in neurons of the central nervous system. Lab Invest. 2001;81:1275–88. doi: 10.1038/labinvest.3780341. [DOI] [PubMed] [Google Scholar]
- 66.Massa PT, Aleyasin H, Park DS, Mao X, Barger SW. NFkappaB in neurons? The uncer- tainty principle in neurobiology. J Neurochem. 2006;97:607–18. doi: 10.1111/j.1471-4159.2006.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao N, Nguyen S, Ngo K, Fung-Leung WP. A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in Toll/IL-1R-induced inflammatory signaling. Mol Cell Biol. 2005;25:6521–32. doi: 10.1128/MCB.25.15.6521-6532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su J, Richter K, Zhang C, Gu Q, Li L. Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol. 2007;44:900–5. doi: 10.1016/j.molimm.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 70.Hatao F, Muroi M, Hiki N, Ogawa T, Mimura Y, Kaminishi M, Tanamoto K. Prolonged Toll-like receptor stimulation leads to down-regulation of IRAK-4 protein. J Leukoc Biol. 2004;76:904–8. doi: 10.1189/jlb.0504277. [DOI] [PubMed] [Google Scholar]
- 71.Bates PW, Miyamoto S. Expanded nuclear roles for IkappaBs. Sci STKE. 2004;2004:pe48. doi: 10.1126/stke.2542004pe48. [DOI] [PubMed] [Google Scholar]
- 72.Espinosa L, Ingles-Esteve J, Robert-Moreno A, Bigas A. IkappaBalpha and p65 regulate the cytoplasmic shuttling of nuclear corepressors: Cross-talk between Notch and NFkappaB pathways. Mol Biol Cell. 2003;14:491–502. doi: 10.1091/mbc.E02-07-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Viatour P, Legrand-Poels S, van Lint C, Warnier M, Merville MP, Gielen J, Piette J, Bours V, Chariot A. Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J Biol Chem. 2003;278:46541–8. doi: 10.1074/jbc.M306381200. [DOI] [PubMed] [Google Scholar]
- 74.Bode KA, Schroder K, Hume DA, Ravasi T, Heeg K, Sweet MJ, Dalpke AH. Histone deacetylase inhibitors decrease Toll - like receptor - mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology. 2007;122:596–606. doi: 10.1111/j.1365-2567.2007.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weinmann AS, Mitchell DM, Sanjabi S, Bradley MN, Hoffmann A, Liou HC, Smale ST. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat Immunol. 2001;2:51–7. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- 76.Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–83. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 77.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–61. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B, Owens T. Toll-like receptor 2 signaling in response to brain injury: An innate bridge to neuroinflammation. J Neurosci. 2006;26:12826–37. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oliveira RB, Ochoa MT, Sieling PA, Rea TH, Rambukkana A, Sarno EN, Modlin RL. Expression of Toll-like receptor 2 on human Schwann cells: A mechanism of nerve damage in leprosy. Infect Immun. 2003;71:1427–33. doi: 10.1128/IAI.71.3.1427-1433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis: Mechanisms and functional consequences. Curr Opin Neurol. 2001;14:271–8. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki N, Suzuki S, Yeh WC. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 2002;23:503–6. doi: 10.1016/s1471-4906(02)02298-6. [DOI] [PubMed] [Google Scholar]
- 82.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 83.Felderhoff-Mueser U, Sifringer M, Polley O, Dzietko M, Leineweber B, Mahler L, Baier M, Bittigau P, Obladen M, Ikonomidou C, Buhrer C. Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. Ann Neurol. 2005;57:50–9. doi: 10.1002/ana.20322. [DOI] [PubMed] [Google Scholar]
- 84.Biswas SC, Shi Y, Vonsattel JP, Leung CL, Troy CM, Greene LA. Bim is elevated in Alzheimer's disease neurons and is required for beta-amyloid-induced neuronal apoptosis. J Neurosci. 2007;27:893–900. doi: 10.1523/JNEUROSCI.3524-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ham J, Towers E, Gilley J, Terzano S, Randall R. BH3-only proteins: Key regulators of neuronal apoptosis. Cell Death Differ. 2005;12:1015–20. doi: 10.1038/sj.cdd.4401689. [DOI] [PubMed] [Google Scholar]
- 86.Kirschnek S, Ying S, Fischer SF, Hacker H, Villunger A, Hochrein H, Hacker G. Phagocytosis-induced apoptosis in macrophages is mediated by up-regulation and activation of the Bcl-2 homology domain 3-only protein Bim. J Immunol. 2005;174:671–9. doi: 10.4049/jimmunol.174.2.671. [DOI] [PubMed] [Google Scholar]
- 87.Hazeki K, Masuda N, Funami K, Sukenobu N, Matsumoto M, Akira S, Takeda K, Seya T, Hazeki O. Toll-like receptor-mediated tyrosine phosphorylation of paxillin via MyD88-dependent and -independent pathways. Eur J Immunol. 2003;33:740–7. doi: 10.1002/eji.200323375. [DOI] [PubMed] [Google Scholar]
- 88.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 89.Rakkola R, Matikainen S, Nyman TA. Proteome analysis of human macrophages reveals the upregulation of manganese-containing superoxide dismutase after toll-like receptor activation. Proteomics. 2007;7:378–84. doi: 10.1002/pmic.200600582. [DOI] [PubMed] [Google Scholar]
- 90.Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005;25:10930–40. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002;12:268–74. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 92.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 93.Wagner H. Endogenous TLR ligands and autoimmunity. Adv Immunol. 2006;91:159–73. doi: 10.1016/S0065-2776(06)91004-9. [DOI] [PubMed] [Google Scholar]
- 94.Flanagan JG, Cheng HJ. Alkaline phosphatase fusion proteins for molecular characterization and cloning of receptors and their ligands. Methods Enzymol. 2000;327:198–210. doi: 10.1016/s0076-6879(00)27277-7. [DOI] [PubMed] [Google Scholar]
- 95.Cargill EJ, Womack JE. Detection of polymorphisms in bovine toll-like receptors 3, 7, 8, and 9. Genomics. 2007;89:745–55. doi: 10.1016/j.ygeno.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–32. doi: 10.1016/s0959-440x(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 97.Carim-Todd L, Escarceller M, Estivill X, Sumoy L. LRRN6A/LERN1 (leucine-rich repeat neuronal protein 1), a novel gene with enriched expression in limbic system and neocortex. Eur J Neurosci. 2003;18:3167–82. doi: 10.1111/j.1460-9568.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- 98.Weber AN, Tauszig-Delamasure S, Hoffmann JA, Lelievre E, Gascan H, Ray KP, Morse MA, Imler JL, Gay NJ. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 99.DeLotto Y, DeLotto R. Proteolytic processing of the Drosophila Spatzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech Dev. 1998;72:141–8. doi: 10.1016/s0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]