Abstract
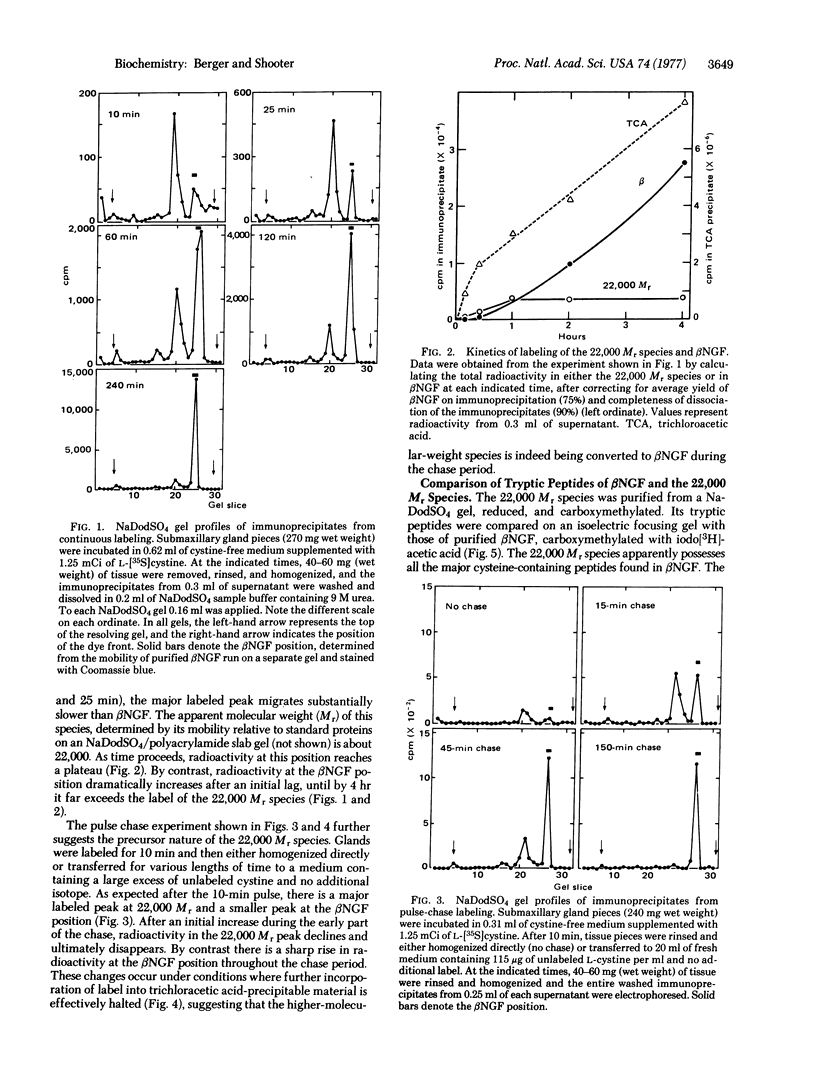
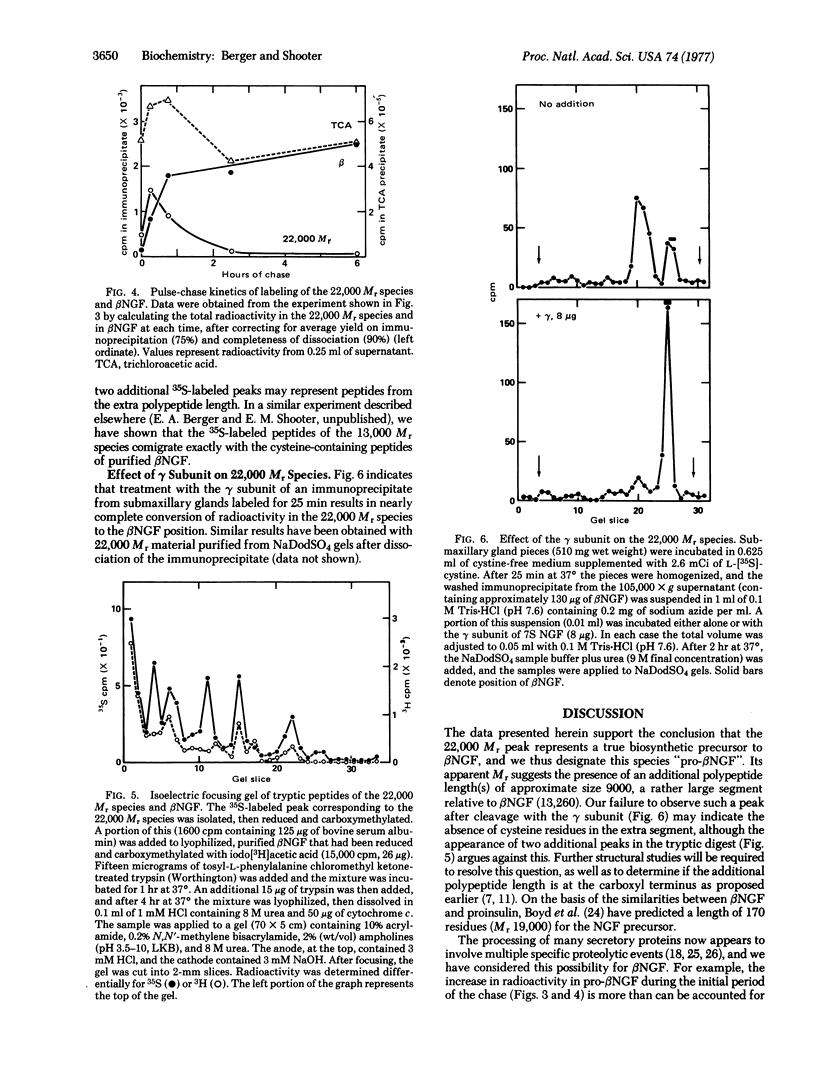
The biosynthesis of β-nerve growth factor (βNGF) was studied in mouse submaxillary glands incubated with L-[35S]cystine. βNGF was isolated from tissue extracts by the addition of antiserum against βNGF and the washed immunoprecipitates were analyzed by sodium dodecyl sulfate gel electrophoresis. With short labeling periods (10 and 25 min) there is a major labeled species with an apparent molecular weight of 22,000 and a smaller peak comigrating with purified βNGF chains (13,260). As time proceeds, the radioactivity in the 22,000 molecular weight peak plateaus, while the label in βNGF continues to increase, until by 4 hr it greatly exceeds the radioactivity of the 22,000 molecular weight species. When glands incubated for 10 min are transferred to medium containing a large excess of unlabeled L-cystine, the 22,000 molecular weight peak gradually declines, and there is a corresponding increase in radioactivity at the βNGF position. The 22,000 molecular weight species isolated from sodium dodecyl sulfate gels possesses all the cystine-containing peptides of βNGF, and possibly two additional ones. When immunoprecipitates from submaxillary glands labeled for 25 min are incubated with the γ subunit (a specific arginyl-esteropeptidase associated with βNGF in the 7S NGF complex), the radioactivity in the 22,000 molecular weight species is converted to the βNGF position. The results suggest that the 22,000 molecular weight species is a biosynthetic precursor to βNGF, and that the γ subunit may function as a specific protease in the processing event.
Keywords: arginyl-esteropeptidase, protein processing, submaxillary gland, gamma subunit, epidermal growth factor
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti R. H., Bradshaw R. A. Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2417–2420. doi: 10.1073/pnas.68.10.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeletti R. H., Bradshaw R. A., Wade R. D. Subunit structure and amino acid composition of mouse submaxillary gland nerve growth factor. Biochemistry. 1971 Feb 2;10(3):463–469. doi: 10.1021/bi00779a018. [DOI] [PubMed] [Google Scholar]
- Attardi D. G., Schlesinger M. J., Schlesinger S. Submaxillary gland of mouse: properties of a purified protein affecting muscle tissue in vitro. Science. 1967 Jun 2;156(3779):1253–1255. doi: 10.1126/science.156.3779.1253. [DOI] [PubMed] [Google Scholar]
- Boyd L. F., Bradshaw R. A., Frazier W. A., Hogue-Angeletti R. A., Jeng I. M., Pulliam M. W., Szutowicz A. Nerve growth factor. Life Sci. 1974 Oct 15;15(8):1381–1391. doi: 10.1016/0024-3205(74)90113-1. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M., Varon S. Enzymatic activities of mouse nerve growth factor and its subunits. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1383–1388. doi: 10.1073/pnas.60.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M., Varon S. Subunit interaction and enzymatic activity of mouse 7S nerve growth factor. Biochemistry. 1969 Sep;8(9):3735–3741. doi: 10.1021/bi00837a037. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta nerve growth factor receptor of avian dorsal root ganglia. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3884–3888. doi: 10.1073/pnas.70.12.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D. N., Shooter E. M. Regulation of nerve growth factor synthesis in mouse submaxillary glands by testosterone. J Neurochem. 1975 Dec;25(6):843–851. doi: 10.1111/j.1471-4159.1975.tb04416.x. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Schenker A., Shooter E. M. Characterization and isolation of proteolytically modified nerve growth factor. Biochemistry. 1976 Dec 14;15(25):5543–5552. doi: 10.1021/bi00670a019. [DOI] [PubMed] [Google Scholar]
- Moore J. B., Jr, Mobley W. C., Shooter E. M. Proteolytic modification of the beta nerve growth factor protein. Biochemistry. 1974 Feb 12;13(4):833–840. doi: 10.1021/bi00701a030. [DOI] [PubMed] [Google Scholar]
- Naughton M. A., Koch J., Hoffman H., Bender V., Hagopian H. Isolation and activity of a thymocyte-trasforming factor from the mouse submaxillary gland. Exp Cell Res. 1969 Sep;57(1):95–103. doi: 10.1016/0014-4827(69)90371-1. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Server A. C., Shooter E. M. Comparison of the arginine esteropeptidases associated with the nerve and epidermal growth factor. J Biol Chem. 1976 Jan 10;251(1):165–173. [PubMed] [Google Scholar]
- Server A. C., Sutter A., Shooter E. M. Modification of the epidermal growth factor affecting the stability of its high molecular weight complex. J Biol Chem. 1976 Feb 25;251(4):1188–1196. [PubMed] [Google Scholar]
- Smith A. P., Varon S., Shooter E. M. Multiple forms of the nerve growth factor protein and its subunits. Biochemistry. 1968 Sep;7(9):3259–3268. doi: 10.1021/bi00849a032. [DOI] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Cohen S., Mitchell W. M. Epidermal growth factor: high and low molecular weight forms. Proc Natl Acad Sci U S A. 1970 Sep;67(1):164–171. doi: 10.1073/pnas.67.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon S., Nomura J., Shooter E. M. The isolation of the mouse nerve growth factor protein in a high molecular weight form. Biochemistry. 1967 Jul;6(7):2202–2209. doi: 10.1021/bi00859a043. [DOI] [PubMed] [Google Scholar]
- Varon S., Normura J., Shooter E. M. Reversible dissociation of the mouse nerve growth factor protein into different subunits. Biochemistry. 1968 Apr;7(4):1296–1303. doi: 10.1021/bi00844a008. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Weimar V. L., Haraguchi K. H. A potent new mesodermal growth factor from mouse submaxillary gland. A quantitative, comparative study with previously described submaxillary gland growth factors. Physiol Chem Phys. 1975;7(1):7–21. [PubMed] [Google Scholar]
- Wlodawer A., Hodgson K. O., Shooter E. M. Crystallization of nerve growth factor from mouse submaxillary glands. Proc Natl Acad Sci U S A. 1975 Mar;72(3):777–779. doi: 10.1073/pnas.72.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]



