Abstract
Ewing Sarcoma (ES) is a rare form of bone cancer that most commonly affects children and adolescents. Chromosomal translocations are fundamental to the development of Ewing Sarcoma, linked to the changes in gene expression affecting transcription factors. Histone acetyl transferases (HATs) and histone deacetylases (HDACs) regulate transcription by modifying acetylation of both histones and transcription factors. Despite the use of multimodal therapeutic approaches current therapies are associated with significant short and long-term side effects. Hence, new therapeutic approaches are needed. In this study, we show that ERG/EWS-ERG, inhibits transcriptional activation properties of RXRα. These results suggest that ERG/EWS-ERG/EWS-Fli-1 may target transcriptional co-activators and transcriptional repressors and thereby regulate RXRα transcriptional activity. To understand the molecular mechanism of action, how the fusion protein targets nuclear receptor function, and to provide a clue for the cancer health disparity seen in Ewing Sarcoma, we hypothesized that the aberrant fusion protein, EWS-ERG/EWS-Fli-1 regulates HDACs-mediated repressor complex and inhibits the binding of transcriptional activator complex causing transcriptional repression of RXRα activity. Since it is known that HDACs regulate nuclear receptors, we proposed that HDAC inhibitor, valproic acid (VPA), an anti-epileptic drug, may reverse the inhibitory properties of EWS-ERG/EWS-Fli-1 oncoprotein on RXRα transcriptional activity and might therefore be used as therapeutic agent in ES. We demonstrate that VPA reverses the inhibitory effect of EWSERG/EWS-Fli-1 on RXRα transcriptional activity and also inhibits the cell growth. Furthermore, VPA induces apoptosis and restored the expression of RXRα target genes RARβ, CRABPII and p21 activity and repressed the expression of aberrant fusion proteins, EWS-ERG and EWS-Fli-1 in Ewing Sarcoma cells. Thus, therapeutic regulation of transcriptional repressor properties of EWS-ERG/EWS-Fli-1 with an anti-epileptic drug with a promising new potential might have a profound impact on prevention, management and treatment of Ewing Sarcoma. Therapeutic use of VPA in minority patients may help reduce the health disparity.
Keywords: Ewing Sarcoma, Valproic Acid, Histone Deacetylase, EWS-ERG, EWS-Fli-1, Retinoid X Receptor α
INTRODUCTION
Ewing Sarcoma is highly aggressive and metastatic tumor that arises in bone or soft tissues. It is the second most common malignant bone or soft tissue tumor in children and adolescents (Ramakrishnan, 2004). It usually occurs in children between the age of 10 and 20 and about 250 new cases are diagnosed every year in US. Based on ethnic and racial differences in patients with Ewing Sarcoma, there is a clear health disparity seen in Black Non-Hispanic and White Hispanic population compared to the White Non-Hispanic population. The survival rates for Black Non-Hispanic and White Hispanic patients are significantly worse compared to White Non-Hispanic patients (Worch, 2010).
Ewing Sarcoma is characterized by chromosomal translocations involving the EWS gene and one of the ETS (Erythroblastosis virus E26 transformation–specific) families of transcription factors (ERG, FLI1, ETV1, ETV4, E1AF and FEV). These chromosomal translocations include t(11;22) of the EWS gene on chromosome 22 juxtaposed with Fli-1 on chromosome 11 (Delattre, 1992) and t(21;22), EWS gene on chromosome 22 juxtaposed with the erg gene on chromosome 21 (Kaneko, 1997, Zucman, 1993). Molecular analysis has shown that the fusion of RNA binding proteins (EWS, TLS/FUS) with the DNA binding proteins (ERG, Fli-1) is responsible for Ewing Sarcoma. ERG and aberrant fusion proteins EWS-ERG/EWS-Fli-1 codes for sequence specific transcriptional activators (Ohno, 1994, Ohno, 1993, Rao, 1993). These chromosomal translocations account for over 95% of Ewing Sarcoma (Ramakrishnan, 2004). The fusion proteins have altered DNA binding and transcriptional activation properties compared to normal Fli-1 and ERG proteins (Ohno, 1994, Prasad, 1994, Yi, 1997). Nuclear receptors were shown to recruit HAT and HDAC associated transcriptional cofactors based on the activation status (Glass, 2000). HDACs are part of co-repressor complex, and are found to be deregulated in various cancers. Over-expression of aberrant oncoproteins and down-regulation of nuclear receptor RXRα transactivation is commonly seen in Ewing Sarcoma (Ramakrishnan, 2004). Consequently, understanding the molecular mechanism of how EWS-ERG/EWS-Fli-1 possesses transforming activity and elucidating the functional role of nuclear receptor RXR and its relation with HDACs may lead to identification of targeted therapeutic agents.
Nuclear receptors (NR) are important regulators of transcription and are involved in a wide variety of physiological processes such as cellular differentiation and homeostasis. They belong to large family of transcription factors that are ligand dependent DNA binding activator proteins (Mangelsdorf, 1995, Zhao, 2007). They are DNA bound transcription factors that act directly by association with specific DNA sequences known as hormone response elements (HREs) (Beato, 1991, Evans, 1988). They initiate transcription by interacting with the transcription initiation apparatus which consists of RNA polymerase II and general transcription factors, TATA box binding proteins (TBP) and TBP associated factors (Roeder, 1996, Tjian, 1994). Retinoid X receptors (RXRs) are important members and they regulate various physiological processes. RXRs either homodimerize or heterodimerize and bind to the hormone response elements and control gene transcription and expression (Zhao, 2007). The transcriptional function of nuclear receptors are regulated by coactivators or corepressors (Perissi, 1999).
There are several studies providing evidences of aberrant patterns of histone modifications with alterations in HAT and HDAC activity in cancer. HAT activity is shown to be modulated by amplification, over-expression, mutation or translocation in various cancers (Pandolfi, 2001, Timmermann, 2001). HDACs have been found to be associated with aberrant transcription factors and are known to mediate the function of the oncogenic translocation products in lymphoma (Dhordain, 1998). In leukemia chromosomal translocations such as PML/RARα, PLZF/RARα and AML1/ETO are known to induce abnormal HAT and HDAC activity (Grignani, 1998, Lin, 1998, Wang, 1998). In acute promyelocytic leukemia cells, aberrant recruitment of HDAC activity has been reported (Grignani, 1998, Lin, 1998). EWS-Fli-1 has shown modulated HAT and HDAC activity (Sakimura, 2005). Additionally, we and others have shown that EWS-Fli-1 and normal Fli-1 targets CBP/p300 and represses its transcriptional co-factor activity (Nakatani, 2003, Ramakrishnan, 2004). We have also shown that in malignant melanoma of soft tissues, EWSATF-1 represses p53 transcriptional activation through binding to CBP (Fujimura, 2001).
Histone acetyl transferases (HAT) and histone deacetylases (HDAC) are key elements in gene regulation, and they determine the acetylation status of histones that play an important role in transcriptional regulation and epigenetic control (Fortson, 2011, Rocchi, 2005). Hypoacetylation is associated with the repression of gene transcription, whereas acetylation is associated with activation of gene transcription (Richon, 2002). The imbalance may, thus lead to dysregulation of transcriptional activity of downstream target genes involved in cell cycle progression, tumor suppression and apoptosis. These altered expressions of genes have been linked to tumor development. HDAC inhibitors have been shown to induce differentiation, cause growth arrest and apoptosis in many cancer cells including human breast cancer cells, ovarian cancer cells, prostate cancer cells, colon cancer cells, lung cancer cells, leukemia, lymphoma and AML (Carrillo, 2009, Fortson, 2011). Thus, HDAC inhibitors are considered as candidates for cancer therapy (Kramer, 2003, Marks, 2001) and are being investigated to use in various cancer treatments.
Valproic acid (VPA, 2-propyl pentanoic acid) is a branched short chain fatty acid (Monti, 2009). It is a commonly used FDA approved, anti-epileptic drug (Alvarez-Breckenridge, 2012). VPA is also, a Class I and Class II HDAC inhibitor that inhibits HDAC 1, 2, 3, and 8 which are constitutively located in the nucleus (Gottlicher, 2001). VPA is one of the most commonly used anticonvulsant and is also used as a mood stabilizer in bipolar disorder and in migraine treatment (Johannessen, 2000, Nalivaeva, 2009).
VPA has been clinically used for over two decades and is currently being investigated in various cancer treatments. VPA is known to induce differentiation, apoptosis and inhibit cellular proliferation in carcinoma cells (Kramer, 2003). It has also been shown to transform leukemic blasts and hematopoietic progenitor cells from acute myeloid leukemia patients (Kramer, 2003). In acute myeloid leukemia cells, VPA represses gene transcription by inhibiting HDAC I and HDAC II. Tumor growth and metastasis were significantly reduced in animal experiments (Gottlicher, 2001). VPA was found to induce apoptosis in human melanoma cells (Facchetti, 2004) and to inhibit cellular proliferation and induce differentiation in human neuroblastoma cells (Rocchi, 2005). VPA was shown to be an effective inhibitor of angiogenesis and vasculogenesis (Michaelis, 2004). Treatment with VPA of cervical cancer induced cell cycle arrest, suppressed cell growth and increased tumor suppressor genes (Tsai, 2013). VPA is under clinical investigation as an anti-cancer drug for treatment of gliomas in children (Blaheta, 2005).
Despite advances in the understanding the molecular basis of this disease, these are still insufficient for the development of new targeted therapeutic approaches. We have demonstrated the inhibition of RXRα transcriptional activity by EWS-Fli-1 suggesting that the aberrant fusion protein EWS-Fli-1 functions as repressor of RXRα transcriptional activity (Ramakrishnan, 2004). We have observed that ERG oncoprotein also inhibits RXRα transcriptional activity, like Fli-1, which is an ERG related protein. Here, we propose that the aberrant fusion protein EWS-ERG/EWS-Fli-1 may regulate RXRα transcriptional activity by forming complex with the transcriptional repressors, HDACs. The fusion onco-protein activates HDACs and other repressors leading to inhibition of normal transcriptional activation properties of RXRα and its target genes. The aim of this study is to elucidate the molecular mechanism as to, how the fusion protein targets nuclear receptor function through regulation of HDACs and the effect of the HDAC inhibitor, an anti-epileptic drug, VPA on EWS-ERG and EWS-Fli-1-positive Ewing Sarcoma cells. In this study, we identified that VPA relieved retinoid X receptor α inhibitory effect mediated by ERG/Fli-1 fusion proteins, induced cell death by apoptosis, restored RXRα target gene activity and down regulate the expression of aberrant fusion proteins in EWSERG and EWS-Fli-1-positive Ewing Sarcoma cells.
MATERIALS AND METHODS
Cell Culture
African green monkey kidney cells, Cos-1 was obtained from American Type Culture Collection (ATCC) Rockville, MD. Cos-1 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) and human Ewing sarcoma cells, 5838 (EWS-ERG translocation) and TC135 (EWS-Fli-1 translocation) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin and 50 μg/ml streptomycin and were maintained at 37 °C in a humidified atmosphere at 5% CO2.
Chemicals and Reagents
Valproic acid (VPA), trichostatin-A (TSA) and 9-cisretinoic acid (9-cis-RA) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Stock solutions of VPA were prepared by dissolving in distilled water, TSA and 9-cis-RA in dimethylsulfoxide (DMSO). All stock solutions were stored at − 80 °C before use. Protein concentrations were determined by Bradford's reagent (Bio-Rad Laboratories Hercules, CA) using bovine serum albumin (BSA) as standard protein. Antibodies Erg1/2/3 (C-20) rabbit polyclonal antibody, Fli-1(C-19) rabbit polyclonal antibody, RARβ (C-19) rabbit polyclonal antibody, CRABPI/II (H-105) rabbit polyclonal antibody and β-actin (C-4) mouse monoclonal antibody were purchased from SantaCruz Biotechnology. Horseradish peroxidase (HRP)-conjugated anti-mouse, anti-rabbit secondary antibodies and enhanced chemiluminescence (ECL) reagents, Amer-sham ECL Western Blotting Analysis System were purchased from GE Healthcare.
Luciferase Assays
Cos-1 cells were co-transfected with pSG5/mRXR expression plasmid, pCRBPII-RARE-Luc reporter plasmid and pRL-SV40 as an internal control with increasing amount of pSG5/ERG or pSG5/ EWS-ERG using FUGENE 6 transfection reagent (Roche Applied Science). Transfected cells were seeded at 5000 cells/well onto 96-well plates (Corning Costar, Sigma-Aldrich) and were incubated for 24 hours. Cells were treated with 9-cis-Retinoic acid or vehicle control for 24 hours or medium was replaced with fresh medium containing VPA (0.1 mM, 1 mM and 3 mM) or TSA (0.02 μM, 0.2 μM and 2 μM) or vehicle control for 24 hours. Luciferase activity was measured using Dual–Glo luciferase assay system following manufacturer's protocol (Promega). The luminescent signal was measured using Fluorskan Ascent FL and data were analyzed with Ascent software version 2.6 (Thermo Electron Corp).
Cell Viability Assay
Ewing sarcoma cells, 5838 or TC135 cells were seeded at 5000 cells/well in 96-well plates (Corning Costar) and were incubated for 24 hours. Medium was then replaced with fresh medium containing VPA at 0.1 mM, 1 mM and 3 mM concentrations or TSA at 0.02 μM, 0.2 μM and 2 μM concentrations or vehicle control for 24 or 48 or 72 hours. The cell viability was measured using CellTiter-Glo reagent according to manufacturers protocol (Promega). The luminescent signal was measured using Fluorskan Ascent FL and data were analyzed with Ascent software version 2.6 (Thermo Electron Corp). Inhibitory Concentration50 (IC50) values of VPA treatments on the Ewing sarcoma cells were calculated using Hill slope model.
Apoptosis Study
Caspase-Glo 3/7 Assay
Caspase 3/7 activity was measured by seeding Ewing Sarcoma cells, 5838 as described above and allowed to attach for 24 hours. Cells were then incubated with various concentrations of VPA (0.1 mM, 1 mM and 3 mM) or TSA (0.02 μM , 0.2 μM and 2 μM ) or vehicle control for 24 hours. Caspase 3/7 activity was measured using Caspase-Glo 3/7 reagent (Promega) according to manufacturer's protocol.
In Situ Cell Death Detection Assay/Terminal De-Oxynucleotidyl Transferase dUTP Nick End Labeling TUNEL Assay
Ewing Sarcoma cells, 5838 and TC135, cells were seeded at 100,000 cells/chamber onto 4 well chamber slides and were incubated overnight. The cells were treated with various concentrations of VPA (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. Cells were washed twice with 1X PBS and allowed to air dry. The cells were fixed using freshly prepared 4% formaldehyde in 1X PBS and incubated for 1 hour at room temperature. Cells were washed with 1X PBS and then incubated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 minutes on ice. These cells were further washed two times with 1XPBS and DNA fragmentation was detected using In Situ Cell Death Detection kit, Fluorescein (Roche Applied Bioscience) according to manufactures procedure. Cells were counter stained with DAPI (Santa Cruz). Olympus IX-71 fluorescence microscope was used to take micro-graphs of fluorescein labeled DNA and DAPI stained cells.
Fluorescence Activated Cell Sorting (FACS) Analysis
Ewing Sarcoma cells, 5838 and TC135 cells were seeded at 50,000 cells/well onto 6 well plates (Corning Costar) and were incubated overnight. The cells were treated with various concentrations of VPA (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. After 24 hours of treatment all the cells were collected and washed with 1× PBS. The cell density was adjusted to 2 × 105 cells/ml and 100 μl of each sample was stained with equal amount of Guava PCA-96 Nexin reagent. Results were acquired on Guava easyCyte HT System using Guava soft software. Acquired data are displayed in a dot-plot with quadrant markers with Annexin V-PE and 7-AAD parameters.
Western Blot Analysis
Ewing Sarcoma cells, 5838 or TC135 cells, were seeded at 5 × 104 cells/ml onto six well plate (Corning Costar). After overnight incubation, cells were treated with VPA at 0.1 mM, 1 mM and 3 mM or vehicle control for 24 hours. Total cell extracts were obtained using ice-cold lysis buffer (20 mM Hepes, 100 mM KCl, 0.4 mM EDTA, 0.2% Igepal CA-630, 1 mM PMSF, 10 mM β-mercaptoethanol and protease inhibitor). Total cell lysates were passed through a 27–1/2 guage needle. After 30 minutes incubation of cell extracts on ice, they were centrifuged for 15 min at 12500 × g and the supernatant were collected. Proteins were quantified using Bradford assay (Bio-Rad). Lysates containing equal amount of total protein were separated on gradient 4–20% by SDS-PAGE and transferred onto nitrocellulose membrane (Hybond ECL, Amer-sham) with overnight incubation at 4 °C. The membranes were incubated for 1 hour in blocking solution containing 5% non-fat dry milk to inhibit non-specific binding. The membranes were further incubated with primary antibodies RARβ (C-19), CRABPI/II (H-105), ERG1/2/3 (C-20), Fli-1(C-19) and β-actin (C-4) in a 1:200 dilution with 1× PBS-T for 1 hour at room temperature. The membranes were washed two times with 1 × PBS-T then incubated with 1:5000 dilution of horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hour at room temperature. After incubation, membrane blots were washed three times with 1XPBS-T and proteins were detected with ECL chemiluminescent kit using LAS3000 imager.
Statistical Analysis
Three independent experiments were carried out. Data were presented as the mean ± standard deviations (SD). The mean values were calculated from triplicate data of each independent experiment. Student t-test was applied to determine the significant values among the samples wherever applicable.
RESULTS
Inhibition of RXRα Mediated Transcriptional Activity by ERG and EWS-ERG
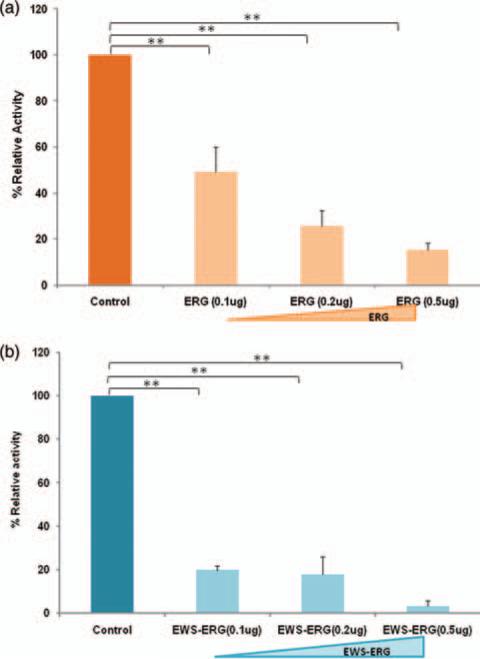
To study the effect of ERG and EWS-ERG on RXR mediated transcriptional activity, Cos-1 cells were co-transfected with expression plasmid pSG5/mRXRα, RXRα reporter plasmid pCRBPII-RARE-Luc and reference plasmid pRLSV-40 with various concentrations 0.1 μg, 0.2 μg and 0.5 μg of pSG5/ERG or pSG5/EWSERG expression plasmid. RXRα mediated transcriptional activity was measured using luciferase assays. Our results demonstrate induction of RXRα transcriptional activation upon addition of 9-cis-retinoic acid and dose dependent inhibition of RXRα mediated transcriptional activity in the presence of ERG and EWS-ERG. ERG inhibited RXRα transcriptional activity approximately by 50% at 0.1 μg, 75% at 0.2 μg and 85% at 0.5 μg (Fig. 1(a)) and aberrant fusion protein EWS-ERG inhibited RXR transcriptional activity approximately by 80% at 0.1 μg, 82% at 0.2 μg and 98% at 0.5 μg (Fig. 1(b)). Taken together these results suggest that RXRα transcriptional activity was inhibited by ERG and EWS-ERG. Based on these results, we planned to investigate whether HDAC inhibitors TSA and VPA could restore the inhibition of aberrant fusion proteins EWS-ERG/ EWS-Fli-1 on RXRα transcriptional activity.
Figure 1.
Dose dependent inhibition of RXRα- mediated transcriptional activity by ERG/ EWS-ERG. (a) Cos-1 cells were cotransfected with pSG5/mRXRα, pCRBPII RARE–Luc, pRLSV-40 and increasing amount of pSG5/ERG (b) Cos-1 cells were cotransfected with pSG5/mRXRα, pCRBPII RARE–Luc, pRLSV-40 and increasing amount of pSG5/EWS-ERG. At 24 hours of transfection 500nm 9-cis-retinoic acid was added and further incubated for 24 hours. The luciferase activity obtained was set at 100%. The values represent the average of three independent experiments with standard deviation. **p < 0.01 compared with controls.
The Effect of TSA/VPA on EWS-ERG/EWS-FLI-1 Inhibition of RXR Transcriptional Activity
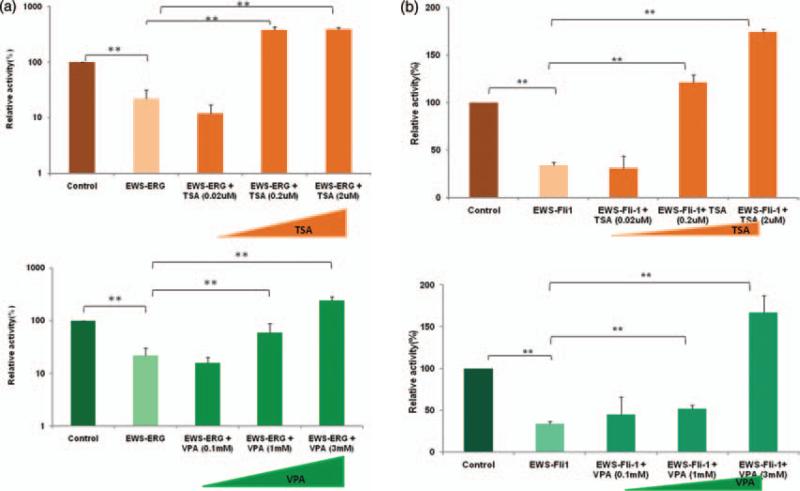
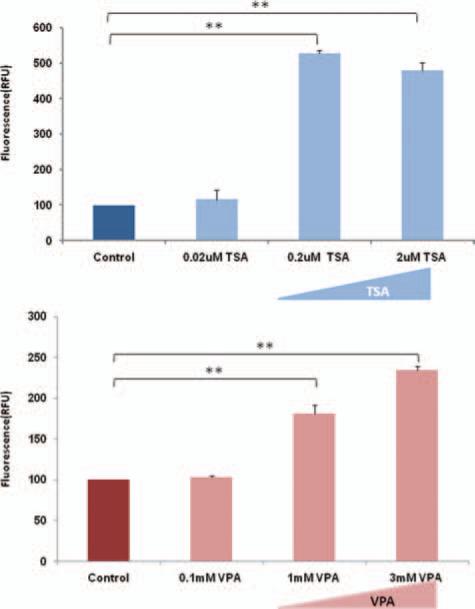
We investigated the effect of TSA and VPA on aberrant fusion protein EWS-ERG/EWS-FLI-1 inhibition of RXRα transcriptional activity. In vitro studies were conducted by cotransfecting 0.2 μg of expression plasmid pSG5/EWSERG (Fig. 2(a)) or pSG5/EWS-Fli-1 (Fig. 2(b)) along with pSG5/mRXR , reporter plasmid pCRBPII-RARE-Luc and reference plasmid pRLSV-40 in Cos-1 cells. After 24 hours of transfection, the cells were treated with various concentrations of TSA (0.02 μM , 0.2 μM and 2 μM ) or VPA (0.1 mM, 1 mM and 3 mM) and vehicle control. The cells were further incubated for 24 hours and RXRα mediated transcriptional activity was measured using luciferase assays. Our results demonstrate about 80% inhibition of RXRα transcriptional activity in the presence of aberrant fusion proteins EWS-ERG (Fig. 2(a)) and dose dependent relief of RXRα transcriptional inhibitory activity with increasing concentration of TSA (0.02 μM , 0.2 μM and 2 μM ) and VPA (0.1 mM, 1 mM and 3 mM). Similar results were observed in the presence of aberrant fusion protein EWS-Fli-1 (Fig. 2(b)) and restoration of the RXRα transcriptional inhibitory activity with increasing concentration of TSA at 0.02 μM , 0.2 μM and 2 μM and VPA at 0.1 mM, 1 mM and 3 mM. The significant relief of transcriptional repression by HDAC inhibitors TSA and VPA suggests that fusion onco-proteins regulate HDACs and thereby effect the transcriptional activities of nuclear receptors.
Figure 2.
Histone deacetylase (HDAC) inhibitors Trichostatin A (TSA) and Valproic acid (VPA) restores EWS-ERG/EWS-Fli-1inhibition of RXRα transcriptional activity. (a) Cos-1 cells were co-transfected with pCRBPII-RARE-Luc, pSG5/mRXRα, pRL-SV40 and pSG5/EWS-ERG. (b) Cos-1 cells were co-transfected with pCRBPII-RARE-Luc, pSG5/mRXRα, pRL-SV40 and pSG5/EWS-Fli-1. After 24 hours of transfection the cells received various concentration of TSA (0.02 μM, 0.2 μM and 2 μM)/VPA (0.1 mM, 1 mM and 3 mM) and were further incubated for 24 hours. The value represents the average of three independent experiments. The error bars indicate the standard deviations. **p < 0 01 between experimental groups.
The Effect of TSA/VPA on the Viability of Ewing Sarcoma Cells, 5838 (EWS-ERG-Positive Ewing Sarcoma) and TC135 Cells (EWS-Fli-1-Positive Ewing Sarcoma)
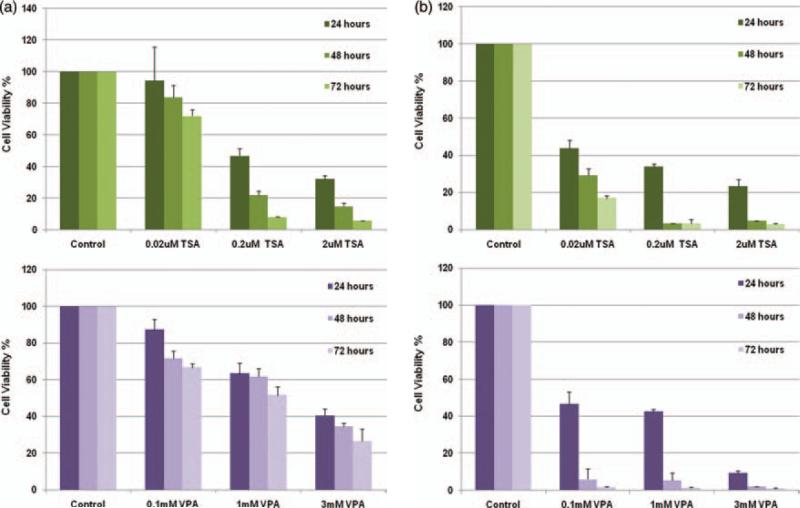
Studies were conducted to evaluate the effect of TSA and VPA on the growth of Ewing sarcoma cells. 5838 cells expressing aberrant fusion protein EWS-ERG and TC135 cells expressing EWS-Fli-1 were treated with various concentrations of TSA (0.02 μM , 0.2 μM and 2 μM ) or VPA (0.1 mM, 1 mM and 3 mM) and vehicle control for 24, 48 and 72 hours. Cell growth was measured using cell titer-Glo assay. Results were calculated as percent relative activity compared to control. The experiments were performed in triplicates with bars indicating standard deviation. Our results show that in 5838 cells (Fig. 3(a)) TSA at 2 μM induced more than 90% cell death at 72 hours and VPA induced more than 60% cell death at 3 mM concentration. Similarly, in TC135 cells (Fig. 3(b)) TSA induced more than 90% cell death at 2 μM concentration and VPA induced more than 90% cell death at 3 mM concentration at 72 hours. These results indicate that TSA and VPA inhibit Ewing sarcoma, 5838 (Fig. 3(a)) and TC135 (Fig. 3(b)) cell growth in a dose and time dependent manner.
Figure 3.
Histone deacetylase (HDAC) inhibitors Trichostatin A (TSA) and Valproic acid (VPA) induce cell death in Ewing Sarcoma cells. (a) Ewing sarcoma (5838) cells were treated with variable amounts of Trichostatin-A (0.02 μM, 0.2 μM and 2 μM) or VPA (0.1 mM, 1 mM and 3 mM ) and vehicle control for 24, 48 and 72 hours. (b) Ewing Sarcoma (TC135) cells were treated with variable amounts of Trichostatin-A (0.02 μM, 0.2 μM and 2 μM) or VPA (0.1 mM, 1 mM and 3 mM) and vehicle control for 24, 48 and 72 hours. Results are shown as percentage of control of three independent experiments with standard deviation.
Inhibitory Concentration 50 (IC50) Values of VPA on Ewing Sarcoma Cells, 5838 (EWS-ERG Translocation) and TC135 Cells (EWS-Fli-1 Translocation)
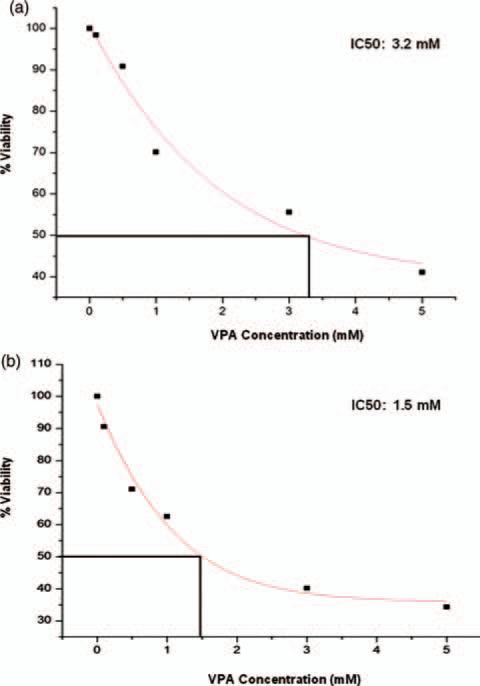
Inhibitory Concentration 50 (IC50) values of VPA on Ewing Sarcoma cells, 5838 and TC135 were evaluated by treating the cells with VPA for 48 hours at various concentrations, 0.1 mM, 0.5 mM, 1.0 mM, 3.0 mM and 5.0 mM. The cell viability was determined by cell-titerGlo assay. The percent viabilities are shown for each concentration of VPA as mean percentage of triplicate experiments. IC50 values were calculated by using Original 6.0 software. IC50 values of VPA in 5838 cells is 3.2 mM (Fig. 4(a)) and TC135 cells is 1.5 mM (Fig. 4(b)).
Figure 4.
Inhibitory Concentration 50 (IC50) values of VPA on Ewing Sarcoma cells. Ewing sarcoma cells (a) 5838 and (b) TC135 cells were treated with Valproic acid (VPA) for 48 hours at various concentrations 0.1 mM, 0.5 mM, 1.0 mM, 3.0 mM and 5.0 mM. The cell viability was determined by cell-titerGlo assay. The percent viabilities are shown for each concentration of VPA. IC50 values are calculated by Original 6.0 software. A. 5838 cells, IC50 value of VPA is 3.2 mM. B. TC-135 cells, IC50 value of VPA is 1.5 mM.
Caspase 3/7 Activity of Ewing Sarcoma, 5838 (EWS-ERG Positive) Cells Treated with VPA and TSA
Induction of apoptosis by TSA and VPA on Ewing sarcoma cells, 5838 was evaluated by caspase 3/7 activity. 5838 cells were treated with variable amounts of Trichostatin-A (0.02 μM , 0.2 μM and 2 μM )/Valproic acid (0.1 mM, 1 mM and 3 mM) and vehicle control for 24 hours. Caspase 3/7 activities were measured using Caspase-Glo 3/7assay reagent. As shown in Figure 5, caspase 3/7 activity in 5838 cells increased in a dose dependent manner. These results suggest that both TSA and VPA induce apoptosis in EWS-ERG positive Ewing Sarcoma cells.
Figure 5.
Histone deacetylase (HDAC) inhibitors Trichostatin A (TSA) and Valproic acid (VPA) increases caspase 3/7 activity. Ewing Sarcoma (5838) cells were treated with variable amounts of VPA (0.1 mM, 1 mM and 3 mM)/TSA (0.02 μM, 0.2 μM and 2 μM) and DMSO as control for 24 hours. Caspase 3/7 activities were measured using Caspase-Glo 3/7assay reagent. Results shown are the mean of percentage of control in three independent experiments with standard deviation. **p < 0 01 compared with untreated controls.
In Situ Cell Death Detection Assay/Terminal De-Oxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) Assay Analysis of Ewing Sarcoma Cells, 5838 (EWS-ERG Positive Ewing Sarcoma) and TC135 (EWS-Fli-1 Positive Ewing Sarcoma) Treated with VPA
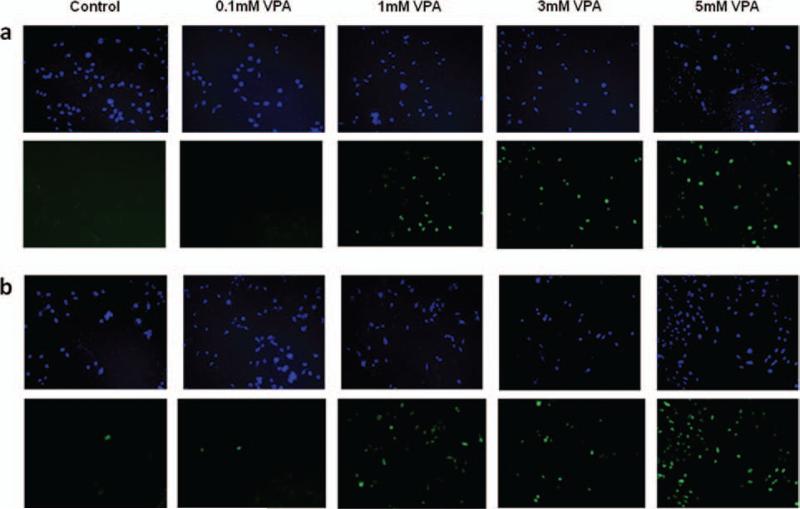
Induction of apoptosis by TSA and VPA on Ewing sarcoma cells, 5838 and TC135 were further evaluated by terminal de-oxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. 5838 and TC135 cells were treated with variable amounts of valproic acid (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. As shown in 5838 cells (Fig. 6(a)) and TC135 cells (Fig. 6(b)), the number of apoptotic cells labeled with fluorescein-dUTP (bottom panel) increased in a dose dependent manner in both 5838 and TC135 cells while the vehicle treated cells showed no significant sign of apoptosis. The cells were counter stained with DAPI (Figs. 6(a) and (b), top panel). These results suggest that VPA induces apoptosis in Ewing sarcoma cells, 5838 and TC135.
Figure 6.
VPA induces apoptosis in Ewing Sarcoma cells. Apoptosis of Ewing Sarcoma cells were analyzed using TUNEL reaction. (a) 5838 cells were treated with variable amounts of VPA (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. (b) TC135 cells were treated with variable amounts of VPA (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. The cells were subjected to TUNEL assay and were counter stained with DAPI (top panel). Fluorescent signals with fluorescein-dUTP (bottom panel).
Flow Cytometry Analysis of Ewing Sarcoma Cells, 5838 (EWS-ERG Positive Ewing Sarcoma) and TC-135 (EWS-Fli-1 Positive Ewing Sarcoma) Treated with VPA
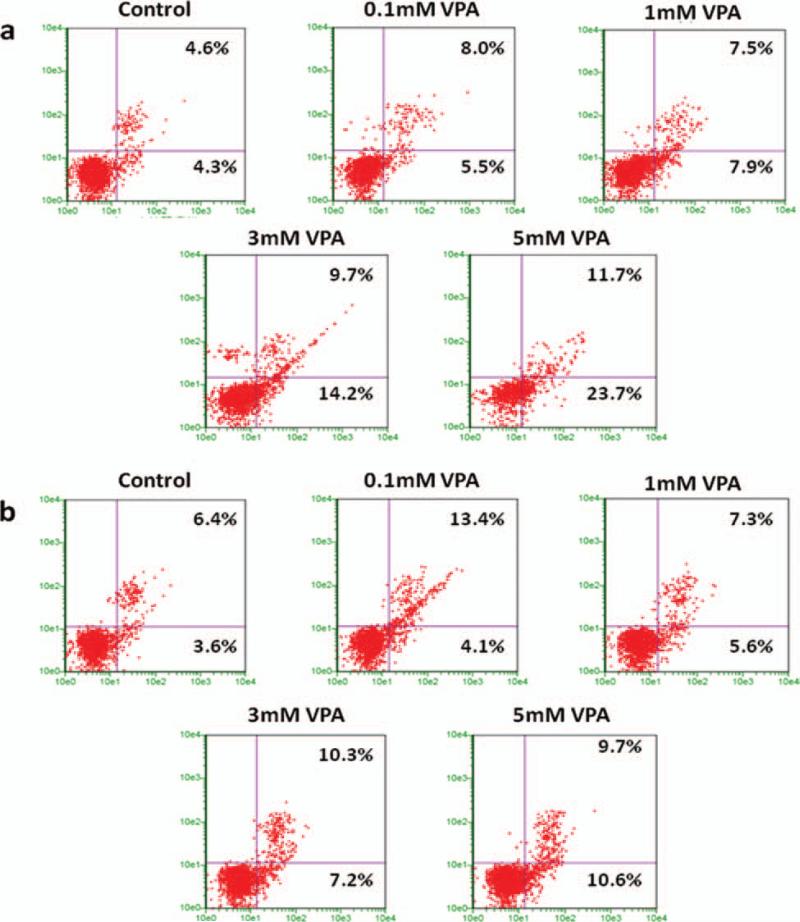
To gain further insight into the alteration in the cell cycle, we analyzed response to VPA treatment in Ewing Sarcoma cells by flow cytometry analysis. Ewing Sarcoma cells, 5838 and TC135 were treated with variable amounts of valproic acid (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. In Figures 7(a) and (b), the lower left quadrant shows normal cells, lower right quadrant shows early stage apoptotic cells, upper right quadrant shows the advanced stage apoptotic cells and upper left quadrant shows necrotic cells. The results indicate the percent of early apoptotic cells on lower right quadrant and percent late apoptotic cells on upper right quadrant increased simultaneously with the increase in the concentration of VPA. In 5838 cells (Fig. 7(a)) the percentage of early apoptotic cells are 5.5%, 7.9%, 14.2% and 23.7% at 0.1 mM, 1 mM, 3 mM and 5 mM respectively compared to control at 4.3%. The percentage of late apoptotic cells are 8.0%, 7.5%, 9.7% and 11.7% at 0.1 mM, 1 mM, 3 mM and 5 mM respectively compared to control at 4.6%. Similarly in TC135 cells (Fig. 7(b)) the percentage of early apoptotic cells are 4.1%, 5.6%, 7.2% and 10.6% at 0.1 mM, 1 mM, 3 mM and 5 mM respectively compared to control at 3.6%. The percentage of late apoptotic cells are 13.4%, 7.3%, 10.3% and 9.7% at 0.1 mM, 1 mM, 3 mM and 5 mM respectively compared to control at 6.4%. These findings further indicate that VPA induces apoptosis in Ewing Sarcoma cells.
Figure 7.
VPA induces apoptosis in Ewing Sarcoma cells. Apoptosis of Ewing Sarcoma cells were analyzed by flow cytometer. (a) 5838 cells were treated with variable amounts of VPA (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. (b) TC135 cells were treated with variable amounts of VPA (0.1 mM, 1 mM, 3 mM and 5 mM) or vehicle control for 24 hours. The cells were stained with Guava Nexin Reagent and acquired on PCA-96 system. The results indicate % early apoptotic cells on lower right quadrant and % late apoptotic cells on upper right quadrant.
Effect of VPA on RXRα Target Genes RARβ, CRABPII and p21 (Tumor Suppressors) in Ewing Sarcoma Cells
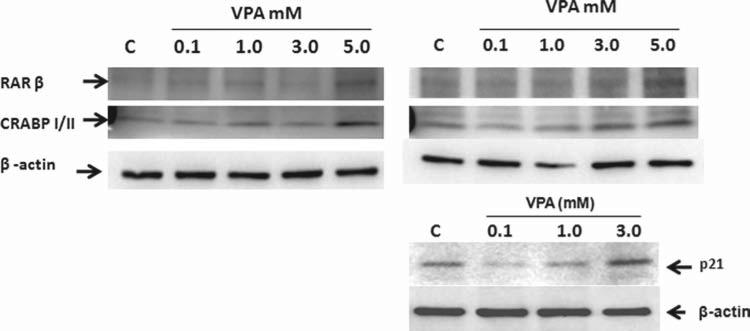
Since VPA relieves EWS-Fli-1 or EWS-ERG inhibition of RXRα transcriptional activity, we tested the effect of VPA on RXRα target genes RARβ and CRABPII and p21 in Ewing sarcoma cells. 5838 cells (Fig. 8, left panel) and TC135 cells (Fig. 8, right panel) were treated with VPA at various concentrations 0.1 mM, 1 mM, 3 mM and 5 mM and vehicle control. Western blot revealed induction of expression of RXRα target genes RARβ and CRABPII at 5 mM VPA at 24 hours (Fig. 8, left panel) in 5838 cells. Similar induction was observed at 5 mM VPA in TC-135 cells (Fig. 8, right top panel); also, RXRα target gene p21 induction was observed at 3 mM VPA in TC-135 cells (Fig. 8, right bottom panel). These results suggest that VPA induces RXRα target genes RARβ, CRABPII and p21 in Ewing sarcoma cells.
Figure 8.
Effect of Valproic acid (VPA) on RXRα target genes RARβ, CRABPII and p21 in Ewing sarcoma cells. 5838 cells (left panel) and TC-135 cells (right panel) were treated with VPA at various concentrations 0.1 mM, 1 mM, 3 mM and 5 mM and vehicle control. Total cell extracts were prepared at 24 hours after treatment and were subjected to SDS-PAGE followed by western blot analysis. RARβ, CRABPI/II-1, p21 and β-actin are indicated by arrows.
Effect of VPA on Ewing Sarcoma Cells, 5838 (EWS-ERG Positive Ewing Sarcoma) and TC135 (EWS-Fli-1 Positive Ewing Sarcoma)
We further analyzed the effect of VPA on EWS-ERG/EWS-FLI-1 fusion protein in Ewing sarcoma cells. 5838 and TC135 cells were treated with VPA at various concentrations 0.1 mM, 1 mM, 3 mM and 5 mM and vehicle control. Western blot analysis showed a dose dependent inhibition in the expression of EWS-ERG (Fig. 9, left panel)/ EWS-Fli-1 (Fig. 9, right panel) in the presence of VPA at 0.1 mM, 1 mM, 3 mM and 5 mM concentrations. These results suggest that VPA inhibits the expression of fusion protein EWS-ERG in 5838 cells and EWS-Fli-1 in TC-135 cells.
Figure 9.

Effect of VPA on EWS-ERG/EWS-FLI-1 fusion protein in Ewing sarcoma cells. Ewing sarcoma cells, 5838 (left panel) and TC135 (right panel) were treated with VPA at various concentrations 0.1 mM, 1 mM , 3 mM and 5 mM and vehicle control. Total cell extracts were prepared at 24 hours after treatment and were subjected to SDS-PAGE followed by western blot analysis. EWS-ERG and β-actin (left panel) and EWS-Fli-1 and β-Actin (right panel) are indicated by arrows.
DISCUSSION
In Ewing Sarcoma, EWS-ERG/EWS-Fli-1 translocation accounts for more than 95% of the cases. They are highly aggressive tumors with incidence of local recurrence and distant metastasis. About 30–40% patients with localized tumor and 80% patients with metastasis die through disease progression (Arpaci, 2013). Although advances have been made in understanding the biology of these tumors and despite the use of multimodal therapies the survival rates have remained stagnant. This underscores the need for targeted therapies to treat the tumors.
Deregulation of HDAC activities have been reported in several cancers. We have shown earlier that normal ERG and EWS-Fli-1 share the target sequences (Ohno, 1994, Reddy, 1991, Siddique, 1993). It was shown that EWS-Fli-1 oncogenic activity is not absolutely dependent on DNA binding domain, but also could be mediated by protein–protein interactions (Jaishankar, 1999). Several studies have demonstrated the association between ERG and HDACs (Matsui, 2003, Yang, 2003). In ERG-positive prostate cancers, ERG contributes directly or indirectly to the hallmarks of cancer. HDAC inhibitors tested in prostate cancer animal models have shown promising antitumor activity (Li, 2005). Therefore, Ewing Sarcoma associated with EWS-ERG and closely related EWS-Fli-1 could also benefit from HDAC inhibitors.
In this study, we found that VPA and TSA reversed the inhibitory properties of RXRα transcriptional activity caused by aberrant EWS-ERG/EWS-Fli-1 oncoproteins and also induced cell death in Ewing Sarcoma cells. Previously, we have shown that Fli-1 and aberrant fusion protein EWS-Fli-1 inhibit RXRα transcriptional activity. We also have shown that dominant negative form of CBP induced apoptosis and it is known that aberrant fusion protein EWS-ERG and EWS-Fli-1 function as anti-apoptotic factors in Ewing Sarcoma cells (Ramakrishnan, 2004). EWS-Fli-1 induces activation or suppression of target genes resulting in uncontrolled proliferation (Hahm, 1999, Nishimori, 2002, Tirado, 2006). Down-regulation of the expression of EWS-Fli-1 has increased susceptibility to apoptosis (Ramakrishnan, 2004, Tanaka, 1997). In EWS-Fli-1 oncogenic fusion protein the p21 expression is down-regulated by inhibition of p300 acetyl transferase activity, and this down-regulation was relieved by over-expression of p300 or by treatment with sodium butyrate (Nakatani, 2003). Knockdown of EWS-Fli-1 expression by siRNA induced p21 expression and introduction of EWSFli-1 resulted in reduction of p21 expression (Li, 2010, Matsumoto, 2001). VPA treatment has shown reduced levels of cyclin A and D1 and caused accumulation of p21 (Takai, 2004). In myeloma cells, a dose dependent inhibition of proliferation was observed with the VPA treatment (Kaiser, 2006). Growth of endometrial cells were inhibited by treatment with VPA. Long-term treatment of VPA inhibited prostate cancer cell growth both in vitro and in vivo. It also showed, an increase in caspase 2 and caspase 3 activation and reduction in tumor xenograft growth in vivo (Xia, 2006). In this study, we have shown that VPA treatment induces apoptosis by activation of caspase 3/7 activity in EWS-ERG associated Ewing Sarcoma and induction of apoptosis by TUNEL and flow cytometry analysis in both EWS-ERG and EWSFli-1 associated Ewing Sarcoma. Furthermore, we have shown loss of expression of the oncogenic fusion protein EWS-ERG/EWS-Fli-1 with the administration of VPA and have demonstrated induction of RXRα downstream target genes RARβ, CRABPII and p21 in Ewing Sarcoma cells. Therefore it might be possible that downstream targets of RXRα is modulated in the presence of oncogenic fusion protein EWS-ERG/EWS-Fli-1 and administration of VPA could relieve the inhibition of RXRα activity and thereby lead to expression of the downstream target genes.
Taken together, in the present study we showed the role of HDAC inhibitor, VPA, in restoring the RXRα transcriptional activity, inducing cell death of Ewing Sarcoma cells by apoptosis, reactivating tumor suppressor genes RARβ, CRABPII and p21 and downregulation of expression of the oncogenic fusion protein EWSERG/EWS-Fli-1. Our observation elucidates the molecular mechanism, how the fusion protein targets nuclear receptor function and the effect of HDAC inhibitor, VPA and its effect on EWS-ERG/EWS-Fli-1 oncoproteins. In contrast to other drugs that are undergoing clinical evaluation, VPA is a well tolerated drug and a well established anti-epileptic drug clinically and pharmacologically. Therefore, HDAC inhibitor, VPA, may be used as a targeted therapy in Ewing Sarcoma associated with EWS-ERG/EWS-Fli-1 translocation.
CONCLUSION
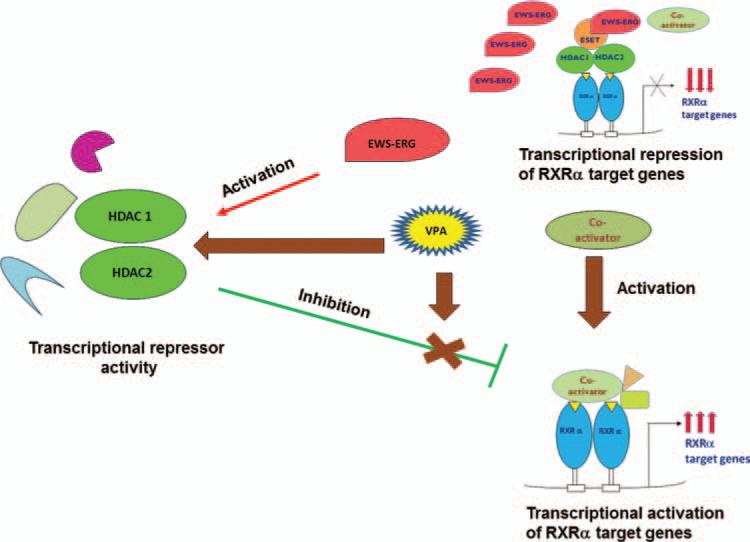
In Ewing Sarcoma, HDACs forms complex with the fusion proteins EWS-ERG/EWS-Fli-1 and interacts more strongly with the nuclear receptor RXRα leading to the repression of RXRα transcriptional activity. The fusion oncoproteins also activates HDACs and other repressors leading to inhibition of normal transcriptional activation of RXRα and its target genes. By addition of anti-epileptic drug valproic acid, an HDAC inhibitor, we could restore the RXRα transcriptional activity (Fig. 10).
Figure 10.
Antiepileptic drug Valproic acid (VPA) reverses the EWS-ERG/EWS-Fli-1 effect of RXRα transcriptional activity and activates target genes in Ewing Sarcoma cells.
We demonstrated that anti-epileptic drug valproic acid (VPA), an HDAC inhibitor reverse the inhibitory effect of RXRα transcriptional activity. VPA targets Ewing sarcoma cells with EWS-ERG and EWS-Fli-1 translocation by inducing apoptosis. VPA also induces the expression of RXRα target genes RARβ, CRABPII and p21 which function as tumor suppressors. These data indicate that VPA may be used as a novel targeted therapeutic agent on Ewing Sarcoma cells based on its relief of RXRα inhibitory activities, induction of apoptosis and its ability to induce RXRα target genes. Therapeutic use of VPA on minority patents can reduce the cancer health disparity. Thus, a promising drug with a new potential will have profound impact on prevention, management and treatment of many types of cancers.
Acknowledgments
We thank all the members of Reddy and Rao laboratories. This study was funded in part by the U.S. Army Medical Research and Materiel Command under W81XWH-08-1-0628, W81XWH-09-1-0236, W81XWH-10-1-0418 (Reddy, ESP) and the Georgia Cancer Coalition Distinguished Cancer Scholar award (Reddy, ESP and Rao, VN), NIH 2U54CA118948, 3U54CA118638-05S1, U54/56 Morehouse School of Medicine/University of Alabama at Birmingham/Tuskegee University Partnership Grant (U54 CA118638), the Research Centers in Minority Institutions (G-12-RR003034) and MBRS/RISE program support to Shubhalaxmi Kayarthodi. We thank Dr. Triche for Ewing Sarcoma cells. We thank K. Ayasola for help with the manuscript.
LIST OF ABBREVIATIONS
- ES
Ewing Sarcoma
- ETS
Erythroblastosis virus E26 transformation– specific
- ERG
ETS Related Gene
- FLI-1
Friend leukemia virus integration 1
- VPA
Valproic acid
- TSA
Trichostatin-A
- RXRα
Retinoid X Receptor α
- RARβ
Retinoic acid receptor β
- CRABP
Cellular retinoic-acid binding protein
- TUNEL
Tri-deoxy UdTP Nick end labeling
Footnotes
Conflict of Interest
We acknowledge that we have no conflict of interest or any financial interest that could influence our work.
REFERENCES
- Alvarez-Breckenridge CA, Yu J, Price R, Wei M, Wang Y, Nowicki MO, Ha YP, Bergin S, Hwang C, Fernandez SA, Kaur B, Caligiuri MA, Chiocca, E. A. The histone deacetylase inhibitor valproic acid lessens NK cell action against oncolytic virus-infected glioblastoma cells by inhibition of STAT5/T-BET signaling and generation of gamma interferon. J. Virol. 2012;86:4566. doi: 10.1128/JVI.05545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaci E, Yetisyigit T, Seker M, Uncu D, Uyeturk U, Oksuzoglu B, Demirci U, Coskun U, Kucukoner M, Isikdogan A, Inanc M, Alkis N, Ozkan M. Prognostic factors and clinical outcome of patients with Ewing's sarcoma family of tumors in adults: Multicentric study of the Anatolian Society of Medical Oncology. Med. Oncol. 2013;30:469. doi: 10.1007/s12032-013-0469-z. [DOI] [PubMed] [Google Scholar]
- Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991;5:2044. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr Evolving anticancer drug valproic acid: Insights into the mechanism and clinical studies. Med. Res. Rev. 2005;25:383. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- Carrillo J, Agra N, Fernandez N, Pestana A, Alonso J. Devazepide, a nonpeptide antagonist of CCK receptors, induces apoptosis and inhibits ewing tumor growth. Anticancer Drugs. 2009;20:527. doi: 10.1097/CAD.0b013e32832c3a4f. [DOI] [PubMed] [Google Scholar]
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Dhordain P, Lin RJ, Quief S, Lantoine D, Kerckaert JP, Evans RM, Albagli O. The LAZ3(BCL-6) oncoprotein recruits a SMRT/mSIN3A/histone deacetylase containing complex to mediate transcriptional repression. Nucleic Acids Res. 1998;26:4645. doi: 10.1093/nar/26.20.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor super-family. Science. 1988;240:889. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchetti F, Previdi S, Ballarini M, Minucci S, Perego P, Porta LCA. Modulation of pro- and anti-apoptotic factors in human melanoma cells exposed to histone deacetylase inhibitors. Apoptosis. 2004;9:573. doi: 10.1023/B:APPT.0000038036.31271.50. [DOI] [PubMed] [Google Scholar]
- Fortson WS, Kayarthodi S, Fujimura Y, Xu H, Matthews R, Grizzle WE, Rao VN, Bhat GK, Reddy ES. Histone deacetylase inhibitors, valproic acid and trichostatin-A induce apoptosis and affect acetylation status of p53 in ERG-positive prostate cancer cells. Int. J. Oncol. 2011;39:111. doi: 10.3892/ijo.2011.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y, Siddique H, Lee L, Rao VN, Reddy ES. EWS-ATF-1 chimeric protein in soft tissue clear cell sarcoma associates with CREB-binding protein and interferes with p53-mediated trans-activation function. Oncogene. 2001;20:6653. doi: 10.1038/sj.onc.1204684. [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121. [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara FF, Zamir I, Seiser C, Lazar MA, Minucci S, Pelicci PG. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature. 1998;391:815. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- Hahm KB. Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat. Genet. 1999;23:481. doi: 10.1038/70611. [DOI] [PubMed] [Google Scholar]
- Jaishankar S, Zhang J, Roussel MF, Baker SJ. Transforming activity of EWS/FLI is not strictly dependent upon DNA-binding activity. Oncogene. 1999;18:5592. doi: 10.1038/sj.onc.1202940. [DOI] [PubMed] [Google Scholar]
- Johannessen CU. Mechanisms of action of valproate: A commentatory. Neurochem. Int. 2000;37:103. doi: 10.1016/s0197-0186(00)00013-9. [DOI] [PubMed] [Google Scholar]
- Kaiser M, Zavrski I, Sterz J, Jakob C, Fleissner C, Kloetzel PM, Sezer O, Heider U. The effects of the histone deacetylase inhibitor valproic acid on cell cycle, growth suppression and apoptosis in multiple myeloma. Haematologica. 2006;91:248. [PubMed] [Google Scholar]
- Kaneko Y, Kobayashi H, Handa M, Satake N, Maseki N. EWS-ERG fusion transcript produced by chromosomal insertion in a Ewing sarcoma. Genes Chromosomes Cancer. 1997;18:228. [PubMed] [Google Scholar]
- Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, Gottlicher M. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003;22:3411. doi: 10.1093/emboj/cdg315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J. Natl. Cancer Inst. 2005;97:103. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- Li Y, Tanaka K, Fan X, Nakatani F, Li X, Nakamura T, Takasaki M, Yamamoto S, Iwamoto Y. Inhibition of the transcriptional function of p53 by EWS-Fli1 chimeric protein in ewing family tumors. Cancer Lett. 2010;294:57. doi: 10.1016/j.canlet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Lin RJ, Nagy L, Inoue S, Shao W, Miller WH, Jr., Evans RM. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr. Opin. Oncol. 2001;13:477. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Chansky HA, Barahmand-Pour F, Zielinska-Kwiatkowska A, Tsumaki N, Myoui A, Yoshikawa H, Yang L, Eyre DR. COL11A2 collagen gene transcription is differentially regulated by EWS/ERG sarcoma fusion protein and wild-type ERG. J. Biol. Chem. 2003;278:11369. doi: 10.1074/jbc.M300164200. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Tanaka K, Nakatani F, Matsunobu T, Matsuda S, Iwamoto Y. Downregulation and forced expression of EWS-Fli1 fusion gene results in changes in the expression of G(1)regulatory genes. Br. J. Cancer. 2001;84:768. doi: 10.1054/bjoc.2000.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Michaelis UR, Fleming I, Suhan T, Cinatl J, Blaheta RA, Hoffmann K, Kotchetkov R, Busse R, Nau H, Cinatl J., Jr Valproic acid inhibits angiogenesis in vitro and in vivo. Mol. Pharmacol. 2004;65:520. doi: 10.1124/mol.65.3.520. [DOI] [PubMed] [Google Scholar]
- Monti B, Polazzi E, Contestabile A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr. Mol. Pharmacol. 2009;2:95. doi: 10.2174/1874467210902010095. [DOI] [PubMed] [Google Scholar]
- Nakatani F, Tanaka K, Sakimura R, Matsumoto Y, Matsunobu T, Li X, Hanada M, Okada T, Iwamoto Y. Identification of p21WAF1/CIP1 as a direct target of EWS-Fli1 oncogenic fusion protein. J. Biol. Chem. 2003;278:15105. doi: 10.1074/jbc.M211470200. [DOI] [PubMed] [Google Scholar]
- Nakatani F, Tanaka K, Sakimura R, Matsumoto Y, Matsunobu T, Li X, Hanada M, Okada T, Iwamoto Y. Identification of p21WAF1/CIP1 as a Direct Target of EWS-Fli1 Oncogenic Fusion Protein. J Biol Chem. 2003;278:15105. doi: 10.1074/jbc.M211470200. [DOI] [PubMed] [Google Scholar]
- Nalivaeva NN, Belyaev ND, Turner AJ. Sodium valproate: an old drug with new roles. Trends Pharmacol. Sci. 2009;30:509. doi: 10.1016/j.tips.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Nishimori H, Sasaki Y, Yoshida K, Irifune H, Zembutsu H, Tanaka T, Aoyama T, Hosaka T, Kawaguchi S, Wada T, Hata J, Toguchida J, Nakamura Y, Tokino T. The Id2 gene is a novel target of transcriptional activation by EWS-ETS fusion proteins in Ewing family tumors. Oncogene. 2002;21:8302. doi: 10.1038/sj.onc.1206025. [DOI] [PubMed] [Google Scholar]
- Ohno T, Ouchida M, Lee L, Gatalica Z, Rao VN, Reddy ES. The EWS gene, involved in Ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene. 1994;9:3087. [PubMed] [Google Scholar]
- Ohno T, Rao VN, Reddy ES. EWS/Fli-1 chimeric protein is a transcriptional activator. Cancer Res. 1993;53:5859. [PubMed] [Google Scholar]
- Pandolfi PP. Transcription therapy for cancer. Oncogene. 2001;20:3116. doi: 10.1038/sj.onc.1204299. [DOI] [PubMed] [Google Scholar]
- Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, Lambert MH, Milburn MV, Glass CK, Rosenfeld MG. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad DD, Ouchida M, Lee L, Rao VN, Reddy ES. TLS/FUS fusion domain of TLS/FUS-erg chimeric protein resulting from the t(16;21) chromosomal translocation in human myeloid leukemia functions as a transcriptional activation domain. Oncogene. 1994;9:3717. [PubMed] [Google Scholar]
- Ramakrishnan R, Fujimura Y, Zou JP, Liu F, Lee L, Rao VN, Reddy ES. Role of protein-protein interactions in the anti-apoptotic function of EWS-Fli-1. Oncogene. 2004;23:7087. doi: 10.1038/sj.onc.1207927. [DOI] [PubMed] [Google Scholar]
- Rao VN, Ohno T, Prasad DD, Bhattacharya G, Reddy ES. Analysis of the DNA-binding and transcriptional activation functions of human Fli-1 protein. Oncogene. 1993;8:2167. [PubMed] [Google Scholar]
- Reddy ES, Rao VN. erg, an ets-related gene, codes for sequence-specific transcriptional activators. Oncogene. 1991;6:2285. [PubMed] [Google Scholar]
- Richon VM, O'Brien JP. Histone deacetylase inhibitors: a new class of potential therapeutic agents for cancer treatment. Clin. Cancer Res. 2002;8:662. [PubMed] [Google Scholar]
- Rocchi P, Tonelli R, Camerin C, Purgato S, Fronza R, Bianucci F, Guerra F, Pession A, Ferreri AM. p21Waf1/Cip1 is a common target induced by short-chain fatty acid HDAC inhibitors (valproic acid, tributyrin and sodium butyrate) in neuroblastoma cells. Oncol. Rep. 2005;13:1139. [PubMed] [Google Scholar]
- Roeder RG. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 1996;21:327. [PubMed] [Google Scholar]
- Sakimura R, Tanaka K, Nakatani F, Matsunobu T, Li X, Hanada M, Okada T, Nakamura T, Matsumoto Y, Iwamoto Y. Antitumor effects of histone deacetylase inhibitor on Ewing's family tumors. Int. J. Cancer. 2005;116:784. doi: 10.1002/ijc.21069. [DOI] [PubMed] [Google Scholar]
- Siddique HR, Rao VN, Lee L, Reddy ES. Characterization of the DNA binding and transcriptional activation domains of the erg protein. Oncogene. 1993;8:1751. [PubMed] [Google Scholar]
- Takai N, Desmond JC, Kumagai T, Gui D, Said JW, Whittaker S, Miyakawa I, Koeffler HP. Histone deacetylase inhibitors have a profound antigrowth activity in endometrial cancer cells. Clin. Cancer Res. 2004;10:1141. doi: 10.1158/1078-0432.ccr-03-0100. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Iwakuma T, Harimaya K, Sato H, Iwamoto Y. EWS-Fli1 antisense oligodeoxynucleotide inhibits proliferation of human Ewing's sarcoma and primitive neuroectodermal tumor cells. J. Clin. Invest. 1997;99:239. doi: 10.1172/JCI119152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann S, Lehrmann H, Polesskaya A, Harel-Bellan A. Histone acetylation and disease. Cell Mol. Life Sci. 2001;58:728. doi: 10.1007/PL00000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado OM, Mateo-Lozano S, Villar J, Dettin LE, Llort A, Gallego S, Ban J, Kovar H, Notario V. Caveolin-1 (CAV1) is a target of EWS/FLI-1 and a key determinant of the oncogenic phenotype and tumorigenicity of Ewing's sarcoma cells. Cancer Res. 2006;66:9937. doi: 10.1158/0008-5472.CAN-06-0927. [DOI] [PubMed] [Google Scholar]
- Tjian R, Maniatis T. Transcriptional activation: A complex puzzle with few easy pieces. Cell. 1994;77:5. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Tsai C, Leslie JS, Franko-Tobin LG, Prasnal MC, Yang T, Vienna Mackey L, Fuselier JA, Coy DH, Liu M, Yu C, Sun L. Valproic acid suppresses cervical cancer tumor progression possibly via activating Notch1 signaling and enhances receptor-targeted cancer chemotherapeutic via activating somatostatin receptor type II. Arch. Gynecol. Obstet. 2013 doi: 10.1007/s00404-013-2762-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Hoshino T, Redner RL, Kajigaya S, Liu JM. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc. Natl. Acad. Sci. USA. 1998;95:10860. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Ethnic and racial differences in patients with Ewing sarcoma. Cancer. 2010;116:983. doi: 10.1002/cncr.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Sung J, Chowdhury W, Chen CL, Hoti N, Shabbeer S, Carducci M, Rodriguez R. Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res. 2006;66:7237. doi: 10.1158/0008-5472.CAN-05-0487. [DOI] [PubMed] [Google Scholar]
- Yang L, Mei Q, Zielinska-Kwiatkowska A, Matsui Y, Blackburn ML, Benedetti D, Krumm AA, Taborsky GJ, Jr, Chansky HA. An ERG (ets-related gene)-associated histone methyltransferase interacts with histone deacetylases 1/2 and transcription corepressors mSin3A/B. Biochem. J. 2003;369:651. doi: 10.1042/BJ20020854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Fujimura Y, Ouchida M, Prasad DD, Rao VN, Reddy ES. Inhibition of apoptosis by normal and aberrant Fli-1 and erg proteins involved in human solid tumors and leukemias. Oncogene. 1997;14:1259. doi: 10.1038/sj.onc.1201099. [DOI] [PubMed] [Google Scholar]
- Zhao WX, Tian M, Zhao BX, Li GD, Liu B, Zhan YY, Chen HZ, Wu Q. Orphan receptor TR3 attenuates the p300-induced acetylation of retinoid X receptor-alpha. Mol. Endocrinol. 2007;21:2877. doi: 10.1210/me.2007-0107. [DOI] [PubMed] [Google Scholar]
- Zucman J, Melot T, Desmaze C, Ghysdael J, Plougastel B, Peter M, Zucker JM, Triche TJ, Sheer D, Turc-Carel C, et al. Combinatorial generation of variable fusion proteins in the Ewing family of tumours. EMBO J. 1993;12:4481. doi: 10.1002/j.1460-2075.1993.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]