Abstract
Previous studies have demonstrated the influence of calcium phosphate (CaP) mineral coating characteristics on cell attachment, proliferation, and differentiation. However, the wide range of mineral properties that can potentially influence cell behavior calls for an efficient platform to screen for the effects of specific mineral properties. To address this need, we have developed an efficient well-plate format to probe for the effects of mineral coating properties on stem cell behavior. Specifically, here we systematically controlled mineral coating morphology by modulating ion concentrations in modified simulated body fluids (mSBF) during mineral nucleation and growth. We found that mineral micro-morphology could be gradually changed from spherulitic, to plate-like, to net-like depending on [Ca2+] and [PO43−] in mSBF solutions, while other mineral properties (Ca/P ratio, crystallinity, dissolution rate) remained constant. Differences in mineral morphology resulted in significant differences in stem cell attachment and expansion in vitro. These findings suggest that an enhanced throughput mineral coating format may be useful to identify mineral coating properties for optimal stem cell attachment and expansion, which may ultimately permit efficient intraoperative seeding of patient derived stem cells.
1. Introduction
Calcium phosphate (CaP)-based mineral coatings have been widely used in bone tissue engineering applications as osteoconductive materials that can promote bone formation by interacting with bone-forming cells.1–3 Previous studies have demonstrated the importance of mineral coating characteristics on cell attachment, proliferation, and differentiation.4–6 Among various mineral deposition methods, “biomimetic” approaches have been widely used to deposit CaP minerals on various materials because the mineral deposition process occurs in near physiological conditions, and the resultant mineral coatings are similar to human bone structure and composition.7–10 In addition, several studies highlight the potential of biomimetic mineral coating approaches as a tool to modulate mineral properties and thereby understand mineral-cell interactions.5,8,11 The properties of mineral coatings formed from simulated body fluids (SBF), which mimic inorganic components of human blood plasma, can be modified by varying ion concentrations, ion contents, pH, and temperature.12–15 Specifically, changes in SBF characteristics have resulted in differences in mineral morphology,16 phase,17 crystallinity,18 and dissolution.19 However, although the relationship between some mineral forming conditions and resultant mineral properties have been identified, the wide range of mineral properties that can potentially influence cell behavior calls for an efficient platform to screen for the effects of mineral properties.
Screening of cell-biomaterials interactions in a high throughput manner have been developed to generate diverse libraries of biomaterials and thereafter to characterize biomaterial properties that can influence cell behaviors. For example, a large library of polylactide-based polymers was synthesized on a platform that enables combinational approach, allowing for screening polymers effects on human mesenchymal stem cell (hMSC) behaviors.20 We hypothesized that this concept used to characterize cell behaviors on biodegradable polymers could also be expanded on inorganic materials through formation of mineral coatings via a biomimetic process that enables systematic change of mineral morphology.
It is well known that cell behavior on minerals is regulated by differences in surface nano- and micro-morphology.13,21–23 For example, mineral coatings formed in different SBF conditions can have different surface topography and surface chemistry, leading to significant differences in cell viability, spreading, proliferation, and differentiation.5 Although prior studies have demonstrated a correlation between mineral surface morphology and cell response, it is difficult to systematically vary surface morphology while keeping other parameters that influence cell response constant. For example, variations in mineral dissolution rates are often dependent on mineral morphology, composition, crystallinity, and porosity, and the products of mineral dissolution can affect cell behavior.24 Taken together, these previous results suggest a need to develop efficient ways to manipulate specific mineral properties, such as mineral morphology, and study cell-mineral interactions.
In view of the complex array of mineral properties that can influence cell behavior, here we describe an enhanced throughput approach to form mineral coatings with diverse properties and probe for their effects on hMSC behavior. We particularly focus on the effect of mineral coating morphology on hMSC attachment and expansion, as each of these outputs are of direct relevance to stem cell-based tissue engineering applications. Mineral coatings were formed on poly (lactide-co-glycolide) (PLGA), which is a bioresorbable polymer commonly used in design of orthopedic devices (e.g. screws, sutures, plates) and has also been used in scaffolds for tissue engineering applications.19, 5–28 We regulated mineral morphology by modifying ion concentrations in modified simulated body fluid (mSBF) solutions, and particularly explored conditions in which other mineral characteristics (phase, crystallinity, dissolution kinetics) remained consistent. Our results indicate that mineral coating morphology significantly influences stem cell attachment and expansion. This enhanced throughput mineral coating approach may have potential applications for screening and optimizing mineral properties for stem cell-based tissue engineering applications.
2. Experimental
2.1. Formation of mineral coatings
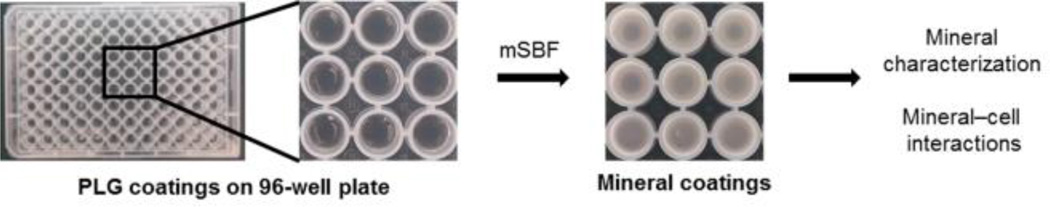
Poly (lactide-co-glycolide) (PLGA, lactide:glycolide = 85:15, average MW = 50,000–70,000 kDa) pellets were dissolved in acetone and solvent casted on polypropylene 96-well plates. For mineral formation on PLGA-coated plates, first, each well was incubated in 0.5 M NaOH solution to hydrolyze PLGA surface and reveal carboxylate and alcohol groups on the surface. Hydrolyzed surfaces were then incubated in mSBF solutions containing 2 times more Ca2+ and PO43− ions than that in human blood plasma with Ca2+/PO43− ratio of 2.5 (this condition is referred to as a “2 × − 2.5”) for 3 d at 37 °C at pH 6.8 for formation of precursor mineral coatings. mSBF solutions with different [Ca2+] and [PO43−] and Ca2+/PO43− ratios were prepared and pH of each solution was adjusted to maintain super-saturated conditions. The detailed composition of mSBF solutions is shown in Table 1. (Note that mSBF solutions are named as A × − B (A: [Ca2+]/[Ca2+]in blood plasma; B: Ca2+/PO43−). Plates were then incubated in various mSBF solutions for 4 d at 37 °C with mSBF refreshed daily. Resulting mineral coatings were rinsed in DI water (18 MΩ cm) and immediately used for cell culture or dried for characterizing properties of mineral coatings. The experimental procedure is illustrated in Scheme 1.
Table 1.
Ion concentrations of the mSBF solutions (unit: mM).
| [Ca2+]/[Ca2+]in blood plasma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 × | 3.5 × | 5 × | ||||||||||
| Ca2+/PO43− | 2.5 | 2 | 1.5 | 1 | 2.5 | 2 | 1.5 | 1 | 2.5 | 2 | 1.5 | 1 |
| Ca2+ | 5 | 5 | 5 | 5 | 8.8 | 8.8 | 8.8 | 8.8 | 12.5 | 12.5 | 12.5 | 12.5 |
| PO43− | 2 | 2.5 | 3.3 | 5 | 3.5 | 4.4 | 5.8 | 8.8 | 5 | 6.3 | 8.3 | 12.5 |
| CO32− | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| pH | 6.8 | 6.6 | 6.5 | 6.2 | 6.1 | 5.9 | 5.8 | 5.7 | 5.8 | 5.7 | 5.5 | 5.3 |
Scheme 1.
Schematic representation of processes to produce mineral coatings for probing mineral-cell interactions.
2.2. Characterization of mineral coatings
Morphology and composition of mineral coatings were first analyzed by scanning electron microscopy (SEM) (Carl Zeiss SMT, model LEO-1530) in conjunction with energy dispersive X-ray spectroscopy (EDS). Compositional analysis of mineral coatings was further examined by Fourier-transformed infrared (FT-IR) spectroscopy (Bruker, model EQUINOX 55) after pellet formation with potassium bromide (KBR). X-ray diffractometry (XRD) (Bruker AXS, model HI-STAR) was used to measure the phase of mineral coatings under Cu Kα radiation. The pore size and porosity of mineral coatings were estimated from SEM images taken at 6 different locations of each sample from 4 different samples (n = 24) by ImageJ software (National Institutes of Health, Bethesda, MD). The roughness of mineral coating was measured using an optical surface profiler (Zygo Corporation, middlefield, CT).
2.3. Dissolution of mineral coatings
The dissolution of mineral coatings was examined by measuring the change of Ca2+ amount in Dulbecco’s Modified Eagle Medium (DMEM) (Mediatech, Manassas, VA) cell culture solution. Mineral coatings were incubated in DMEM at 37 °C. At specific time points, the incubating solution was collected and mixed with working solutions of colorimetric assay (0.4 mM Arsenazo III, MP Biomedicals, Solon, OH). Light adsorption at 650 nm was converted to Ca2+ amount using standard curves relating absorbance intensity to Ca2+ amount.
2.4. Multipotent stem cell attachment and expansion on mineral coatings
Human mesenchymal stem cells (hMSC) were grown in α-Minimum Essential Medium (MEM) (Mediatech, Manassas, VA) cell culture solution supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 37 °C and 5% CO2. C3H10T1/2 cells were grown in DMEM solution supplemented with 10% cosmic calf serum (CCS) and 1% P/S. At the time of seeding, cells were seeded onto the mineral coatings in aforementioned media at a density of 2.4 × 104 cells/cm2. Cell culture media were refreshed every 4 d. At specific time points, cells grown on mineral coatings were lysed by buffer containing 0.2% Triton X–100. At specified time points, total DNA amounts from cell lysate were measured using a cell proliferation kit (Invitrogen, Carlsbad, CA). In order to take images of cells on mineral coatings, hMSCs were exposed to 2 µM of CellTracker Green (Invitogen, Carlsbad, CA) for 30 min and then were fixed with 3.7% formaldehyde in phosphate buffer saline (PBS). Cells were rinsed with PBS and were observed through an inverted epifluorescence microscope equipped with FITC filter (Eclipse Ti, Nikon).
3. Results and Discussion
3.1. Modulating mineral morphology
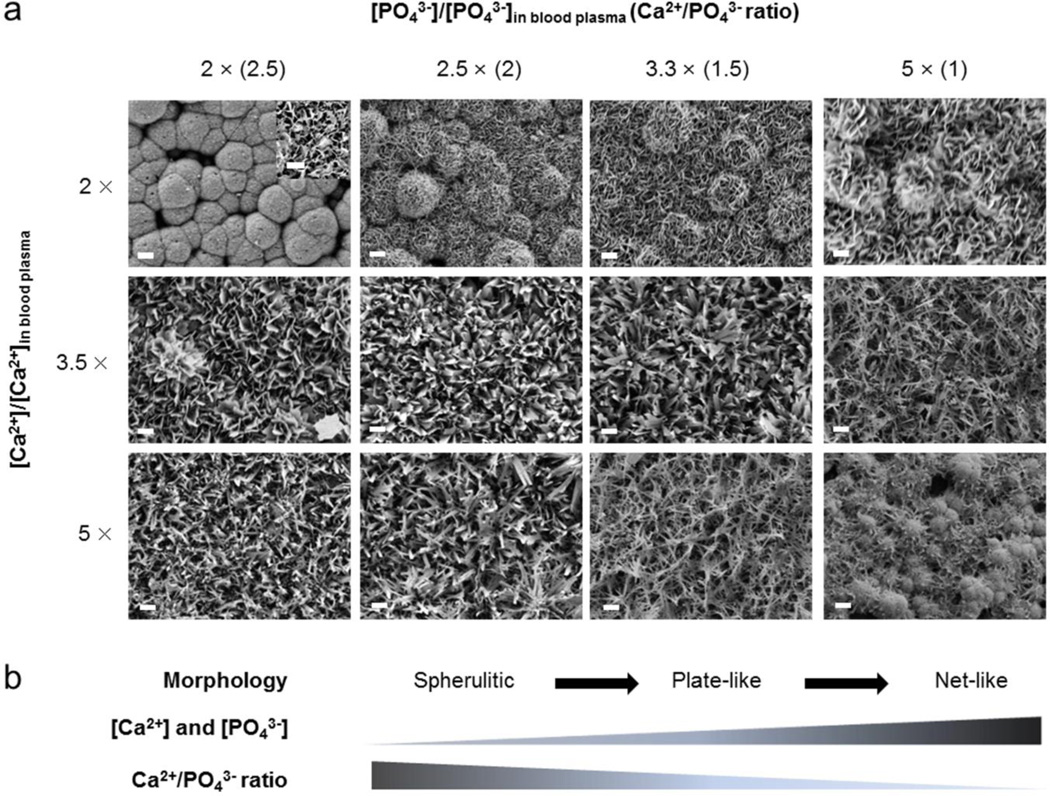
SEM images demonstrated that the Ca2+/PO43− ratio and [Ca2+] and [PO43−] in mSBF regulated mineral micro-morphology after a 4 day incubation (Fig. 1a). All mineral coatings formed on PLG surfaces were continuous layers, with differences in micro-scale morphology. Mineral coatings formed in a solution containing 5 mM of Ca2+ and 2 mM of PO43−, which constitutes 2 times the [Ca2+] and [PO43−] in blood plasma and a Ca2+/PO43− ratio of 2.5 (here noted as “2 × − 2.5”) exhibited spherulitic micro-morphology composed of plate-like nano-morphology (inset of 2 × − 2.5 condition in Fig. 1a). Spherulitic micro-morphology was transformed into plate-like micro-morphology with increasing [PO43−] (and correspondingly decreased Ca2+/PO43− ratio), and the nano-scale plate-like crystal size also increased with [PO43−].
Fig. 1.
(a) SEM images of the surface of mineral coatings. Mineral coatings were formed on hydrolyzed poly (lactide-co-glycolide) surface after 7 days of incubation in modified simulated body fluids (mSBF). mSBF solutions have different Ca2+ and PO43− ion concentrations with different Ca2+/PO43− ratios. Scale bar = 10 µm. Inset shows SEM image of mineral coating formed in 2 × − 2.5 condition at high magnification. Scale bar = 1 µm. (b) Schematic representation of effect of Ca2+ and PO43− ion concentrations and Ca2+/PO43− ratios on morphology of mineral coatings.
We also evaluated the influence of [Ca2+] and [PO43−] on mineral morphology by increasing [Ca2+] and [PO43−] up to 5 times more than that in blood plasma (5 ×). Previous studies have used increased ion concentrations to accelerate mineral formation and demonstrated an interdependent relationship between mineral properties and solution conditions.15, 17 Theoretical analysis reported by Lu et al. indicated that high [Ca2+] and [PO43−] is favorable to the formation of several mineral phases that can be difficult to form at lower concentrations.25 In this study, increasing [Ca2+] and [PO43−] systematically altered the morphology of mineral coatings. With increasing [Ca2+] and [PO43−], mineral coatings formed in 2 × − 2.5 and 2 × − 2 conditions changed from spherulitic micro-morphology to plate-like micro-morphology and mineral coatings formed in 2 × − 1.5 and 2 × − 1 conditions changed from plate-like micro-morphology into net-like micro-morphology. Plate-like mineral coatings formed in 3.5 × − 2.5 and 5 × − 2.5 conditions gradually changed to net-like micro-morphology with decreasing Ca2+/PO43− ratio. Thus, mineral morphology tended to change from spherulitic, to plate-like, to net-like micro-morphology with increasing [Ca2+] and [PO43−] and decreasing Ca2+/PO43− ratio. These differences in mineral morphology result from not only ion concentrations and Ca2+/PO43− ratio but also changes in pH. As shown in Table 1, each mSBF solution requires a distinct pH to maintain super-saturation conditions and avoid homogeneous mineral precipitation. Previous studies demonstrated that the initial pH can result in distinctive morphology changes.14
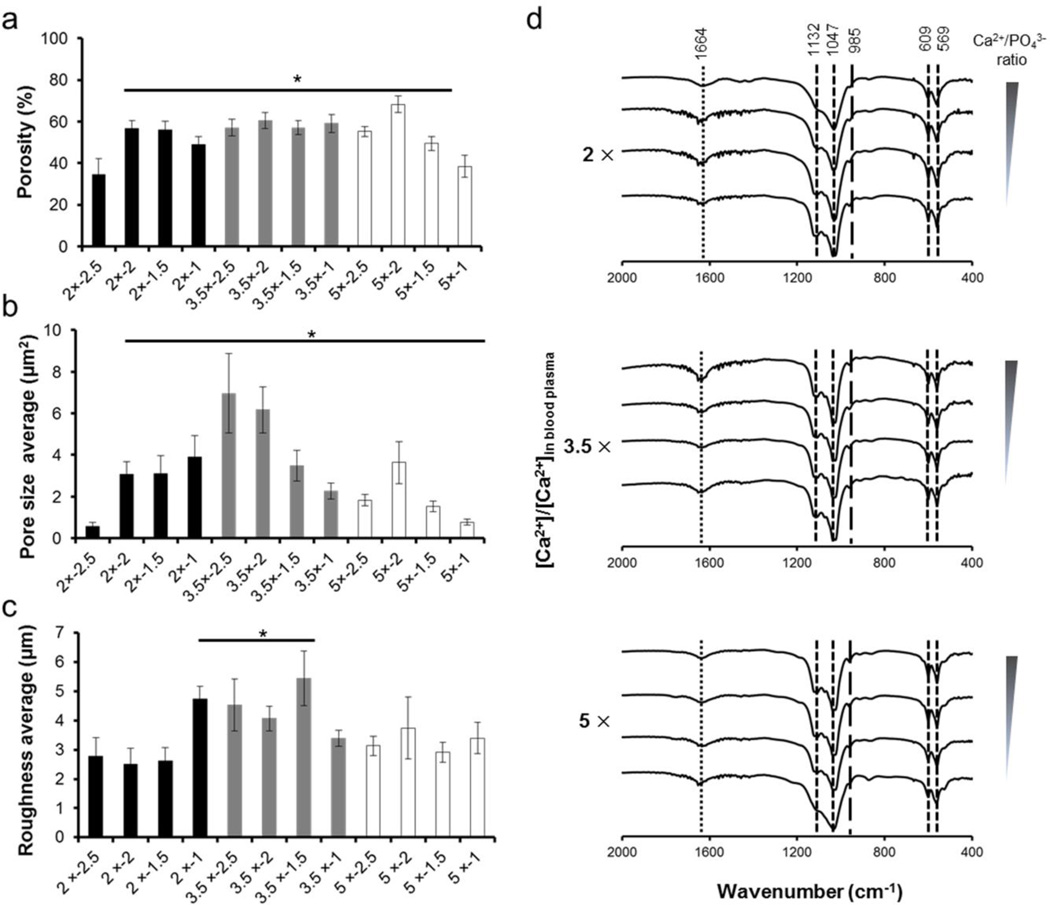
Morphological differences in mineral coatings also provide diversity in pore size, porosity and surface roughness. At the interface of mineral coatings, micro-porosity of minerals plays important role to regulate cell response.29–31 To evaluate micro-porosity of mineral coatings, we measured porosity and pore size from SEM images (Fig. 1a). The porosity of mineral coating formed in 2 × − 2.5 conditions is less than other mineral coatings (Fig. 2a). This mineral coating has spherulitic micro-morphology composed of small pore size area (less than 0.6 ± 0.19 µm2), while large pore size area (3.1 ± 0.61 µm2 – 7.0 ± 1.91 µm2) from plate-like micro-morphology produced greater porosity (Fig. 2b). In addition to pore size variation, surface roughness also was varied by changing mSBF characteristics. The surface roughness of the mineral coatings with plate-like micro-morphology was higher than other mineral coatings, as the plate-like micro-morphology is composed of crystal structure that grew vertically when compared with mineral coatings with spherulitic and net-like micro-morphology (Fig. 2c). This result is potentially important, as variation in surface roughness has been reported to influence cell attachment and subsequent cellular response.32
Fig. 2.
Characterization of mineral coatings. (a) Porosity, (b) Pore size area, (c) Roughness, and (d) FTIR spectra of mineral coatings. Porosity and pore size area were measured from imageJ software using SEM images of mineral coatings. Roughness was measured using an optical surface profiler. * indicate significant difference when compared with 2 × − 2.5 condition. Each peak from (d) indicates PO43− (- -), HPO42− (—) and CO32− (⋯) groups.
Taken together, these results indicate that modulating [Ca2+] and [PO43−], Ca2+/PO43− ratio, and pH can systematically change the micro-morphology, porosity, pore size, and roughness of mineral coatings in an enhanced throughput format (Fig. 1b).
3.2. Mineral structure and composition
FTIR analysis of all mineral coatings showed two main peak areas associated with phosphate, between 1047 and 1132 cm−1 and between 569 and 609 cm−1 (Fig. 2d). Mineral coatings formed in 2 × − 2.5 conditions exhibited a broad PO43− peak between 1047 and 1132 cm−1, while the peak was more distinct with increasing [Ca2+] and [PO43−]. In addition, the peak at 985 cm−1, which can be assigned to protonated phosphate ions (HPO42−), was more strongly detected in mineral coatings formed in more acidic mSBF conditions when compared with mineral coatings in 2 × − 2.5 conditions (pH = 6.8). This observation can be attributed to changes in the pH of mSBF solutions, which was adjusted to prevent solution precipitation, and was reduced from 6.8 to 5.3 with increasing [Ca2+] and [PO43−] in mSBF solutions, and more acidic environments are known to lead to emergence of hydrogen phosphate (HPO42−) and dihydrogen phosphate (H2PO4–). Overall, the results of FTIR analysis suggest that changes in mSBF solution conditions, such as ion concentrations and pH, can lead to protonated phosphate incorporation into the mineral structure.
To determine relative crystallinity we used FTIR data to calculate a “crystallinity index”, as described in previous studies,33,34 which reflects the combination of relative sizes of the crystals and the degree of crystal order in the lattice (Supplementary Fig. 1). Relative crystallinity gradually increased with decreasing Ca2+/PO43− ratio in 2 × and 3.5 × conditions, while the crystallinity decreased with decreasing Ca2+/PO43− ratio in 5 × conditions. This result correlated with the changes in crystal size observed from SEM images (Fig. 1a), indicating that crystallinity index directly correlated with crystal size. Calcium/Phosphorous (Ca/P) ratio and XRD patterns from resultant mineral coatings indicated that mineral coatings each have hydroxyapatite (HA)-like phase, with no significant differences in the measured Ca/P ratio (Supplementary Figs. 2 and 3). Taken together, mineral coatings showed systematic changes in morphology, porosity, pore size, and roughness, while chemical structure, Ca/P ratio, and mineral phase of mineral coatings were consistent among the experimental conditions.
3.3. Mineral dissolution in cell culture solution
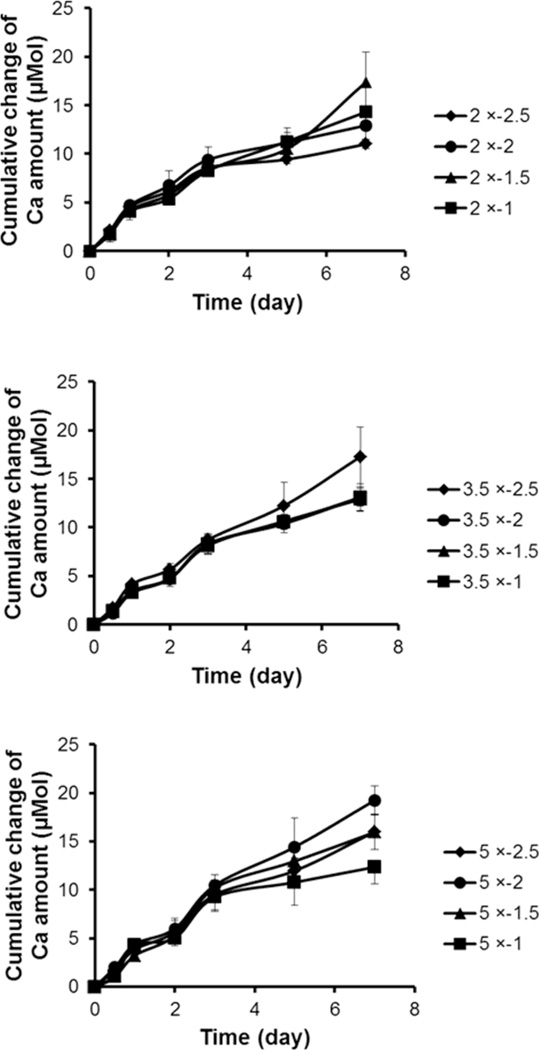
Dissolution rates of mineral coatings were similar across all coating conditions initially, and the rates changed slightly after a 5 day incubation in buffered DMEM cell culture solution (Fig. 3). Previous studies indicated that dissolved CaP minerals can cause local environmental changes by releasing ions, and the dissolution rates result from a combination of mineral properties (e.g. crystallinity, porosity, morphology) and environmental conditions (e.g. presence of serum, pH, ionic strength). Even though various physiological conditions exist in body fluids, we have characterized mineral dissolution from mineral coatings in cell culture medium buffered with carbonate and HEPES to gain insights into mineral properties that influence mineral coating dissolution in cell culture. The initially similar dissolution characteristics were surprising, as mineral coatings had unique physical properties (morphology, crystal size, roughness, and porosity) depending on the conditions of the mSBF solutions (Figs. 1 and 2), and these properties have previously been shown to influence dissolution rate in cell culture conditions.4,8,11 The surface area that medium solution can contact within the mineral coatings likely is an important factor that regulates mineral dissolution. For example, mineral coatings formed in 2 × − 2.5 condition have less porosity than other mineral coatings, and other coatings with plate-like micro-morphology and higher porosity might be expected to have higher reactivity with DMEM solutions. However, the small pore size of mineral coatings formed in the 2 × − 2.5 condition can increase the surface area of mineral coatings. Therefore, the balance between porosity and pore size may result in similar dissolution rates of mineral coatings across different conditions in this study.
Fig. 3.
Cumulative Ca2+ ions release of different mineral coatings in DMEM cell culture media. Mineral coatings formed in 2 × (top), 3.5 × (middle), and 5 × (bottom) mSBF solutions.
The slight dissolution changes observed between days 5 and 7 may be attributed to gradual phase transformation of mineral coatings to more stable forms of apatite. It has been reported that several precursor phases such as dicalcium phosphate dihydrate (DCPD), and octacalcium phosphate (OCP) can convert to hydroxyapatite (HA) in physiological conditions, as HA phase is the most stable mineral phase in physiological conditions.35 Studies have indicated that OCP transformation to carbonated apatite resulted from an interfacial dissolution-reprecipitation mechanism in the presence of Ca2+, PO43−, and CO32− ions in MEM cell culture medium. For example, Barrère et al. demonstrated that OCP coatings on Ti surfaces transformed to a carbonated apatite phase after a 2 week incubation in MEM medium.36 Mineral coatings used in this study, which have relatively similar mineral structure, and Ca/P ratio, may be transformed to a more stable apatite phase in cell culture conditions, and the slightly different dissolution rates of mineral coatings between days 5 and 7 of incubation may be due to different transformation rates.
3.4. Morphology effects on cell attachment and expansion on mineral coatings
Cell attachment on mineral coatings is correlated with changes in mineral micro-morphology. Mineral coatings with spherulitic micro-morphology exhibited higher attached cell number relative to that from mineral coatings composed of plate-like and net-like micro-morphology (Fig. 4). Several studies have indicated that mineral-coated surfaces can adsorb serum proteins and support cell adhesion.37,38 For example, Sawyer et al. have shown that cell attachment and spreading significantly increased on minerals coated with FBS. In addition, the adsorption of RGD peptides along with FBS synergistically enhanced cell attachment and spreading.38 Another study demonstrated that micro-porosity of HA influenced protein adsorption.39 In this study, mineral coatings that provided the highest cell attachment had the smallest pore size. This effect may be attributed to increased surface area of coatings with small pore size, and related increases in protein adsorption. In contrast, mineral coatings composed of plate-like micro-morphology have larger pore size due to the increased space between plate-like crystals, which leads to reduced surface area and may explain lower cell attachment.
Fig. 4.
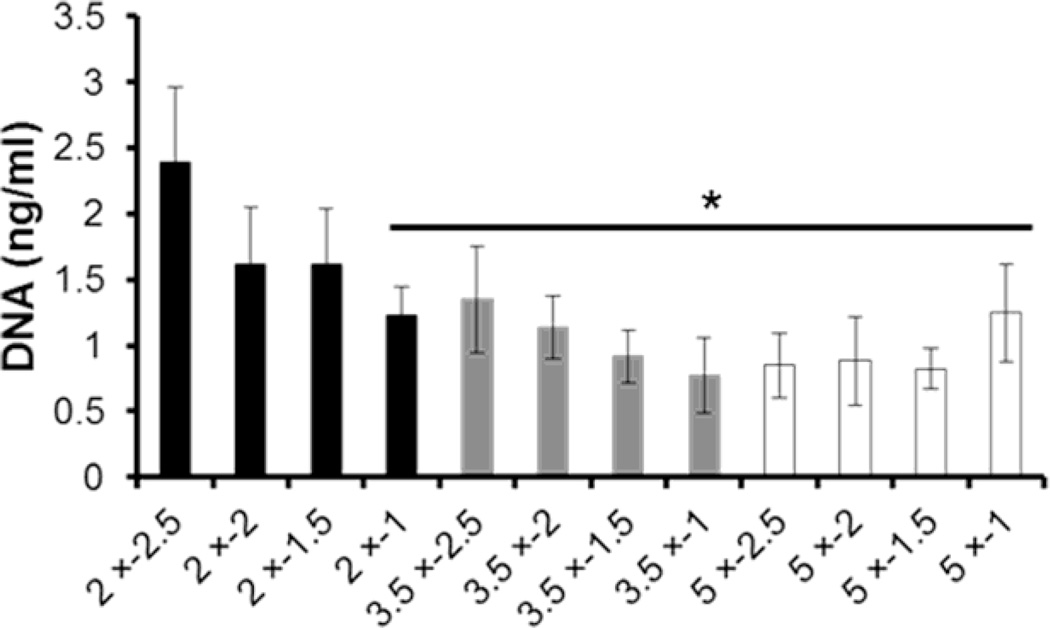
hMSC attachment on mineral coatings. Total DNA amount (a measure of cell attachment) after 12 h incubation on mineral coatings. * indicate significant difference when compared with 2 × − 2.5 condition.
Mineral crystal size also influenced surface roughness, which is known to contribute to cell attachment.4 Mineral coatings that provided high cell attachment had spherulitic micro-morphology with relatively smooth surface roughness when compared with plate-like micro-morphology. Net-like micro-morphology, which has relatively low roughness, cannot mediate high cell attachment. For efficient cell attachment, the formation of focal contacts on mineral surface is required. However, the increased crystal size from both plate-like and net-like micro-morphology might reduce focal contact area regardless of surface roughness. Besides the physical factors derived from morphology, other factors should be considered to regulate cell attachment. In this study, we demonstrated that crystallinity, Ca/P ratio, mineral phase (Supplementary Figs. 1‒3), and dissolution (Fig. 3) were not significantly affected by mineral forming conditions. Therefore, this result indicated that mineral morphology can influence cell attachment on mineral coatings. Such an effect of mineral morphology on cell attachment suggests that orthopedic devices that require efficient hMSC attachment can be modulated by controlling mineral coating conditions.
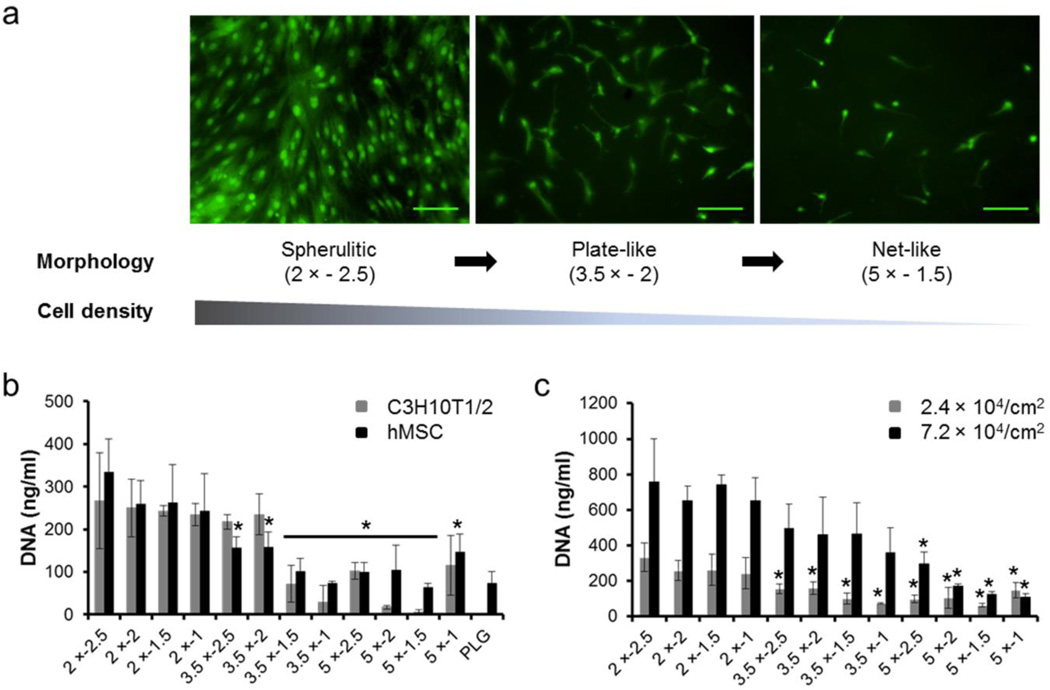
Differences in mineral morphology also influence expansion of multipotent stem cells that adhere on mineral coatings (Fig. 5). Fluorescence images of hMSCs after 4 days in culture on mineral coatings formed in 2 × − 2.5 conditions (spherulitic morphology in Fig. 1) show well-spread cell morphology with a high density of cells when compared with cells on mineral coatings formed in 3.5 × − 2 conditions (plate-like morphology in Fig. 1). In comparison, fewer cells with rounded morphology were found on mineral coatings formed in 5 × − 1.5 conditions (net-like morphology in Fig. 1) (Fig. 5a). The cell expansion dependence on mineral morphology was more obvious after 8 days of cell culture on mineral coatings (Fig. 5b). Cell density on mineral coatings composed of spherulitic micro-morphology was higher than that on mineral coatings composed of plate-like and net-like micro-morphology. This difference in cell density on mineral coatings correlates with the cell attachment trend we observed (Fig. 4), as expected. In addition, cell density on mineral coatings that have spherulitic micro-morphology is higher than that on the un-coated PLGA surface, indicating that mineral morphology change can improve cell proliferation. Overall, these results indicated that cell populations can expand on mineral coatings and the degree of cell expansion is dependent on micro-morphology of mineral coatings.
Fig. 5.
Multipotent stem cell expansion on mineral coatings. (a) Fluorescence images of hMSC after a 4 day incubation on 2 × − 2.5 (left), 3.5 × − 2 (middle) and 5 × − 1.5 (right) mineral coatings. Scale bar = 100 µm. (b) hMSC and C3H10T1/2 cells expansion on mineral coatings and PLGA surface. Total DNA amount (a measure of cell number) of cells after 8 day incubations. (c) Seeding density effect on hMSC expansion after 8 day incubations. * indicate significant difference when compared with 2 × − 2.5 conditions respectively.
We also preliminarily investigated the cell seeding density effect on hMSC expansion (Fig. 5c), as cell-cell contact, paracrine signaling, and cell spreading are each involved in cell proliferation and differentiation.40–44 For example, Peng et al. have shown that the extent of differentiation of MSC derived from rats was regulated by cell density on micropatterned surfaces with various cell sizes and cell-cell contacts.43 In this study, we increased cell density by seeding 3 times more hMSCs than that used for previous experiments to investigate hMSC expansion on mineral coatings. After 8 days of incubation on mineral coatings, the cell number with high seeding density was 2.6 times higher than that with low seeding density on mineral coatings formed in 2 × conditions. In addition, cell numbers on mineral coatings with high seeding density showed similar dependence on mineral coating morphology observed on previous results (Fig. 5a,b). These results further suggest that cell expansion is affected by differences in morphology of mineral coatings.
4. Conclusions
The results in this study indicate that enhanced throughput mineral coatings are an efficient platform to characterize mineral-stem cell interactions. Specifically, we used a well-plate format to efficiently control morphology of mineral coatings, and screened for the effects of coating properties on multipotent stem cell attachment and expansion. Systematic changes in the ion concentrations and pH in mSBF solutions led to clear changes in mineral coating morphology. The spherulitic micro-morphology at low [Ca2+] and [PO43−] transformed into plate- and net-like micro-morphology with increasing [Ca2+] and [PO43−] and reducing Ca2+/PO43− ratio in mSBF solutions. In contrast, physicochemical mineral characteristics including Ca/P ratio, crystallinity, and dissolution rates of mineral coatings were not significantly different among all experimental conditions. Therefore, our experimental approach identified unique conditions to explore the effects of mineral coating morphology on cell behavior while other confounding variables remained relatively constant. Mineral coating morphology directly influenced multipotent stem cell attachment and expansion. Mineral coatings composed of spherulitic micro-morphology supported more efficient cell attachment than those composed of plate-like or net-like micro-morphology. After attachment on mineral coatings, multipotent stem cells expanded at relatively higher rates on mineral coatings that had spherulitic micro-morphology, indicating cell proliferation can be modulated by morphology changes. Collectively, the results of this study indicate a significant influence of mineral morphology on attachment and expansion of multipotent stem cells on mineral coatings. These findings suggest that enhanced throughput mineral coatings may be useful to identify mineral coating properties for orthopedic devices that permit efficient intraoperative seeding of patient derived hMSCs.
Supplementary Material
Acknowledgements
The authors acknowledge financial support from the AO Research Foundation (Exploratory Research Grant) and the National Institutes of Health (R01AR059916).
Footnotes
Electronic Supplementary Information (ESI) available: Figures with crystallinity index, calcium/phosphorous ratio and XRD patterns of mineral coatings. See DOI: 10.1039/b000000x/
Notes and references
- 1.Weinand C, Pomerantseva I, Neville CM, Gupta R, Weinberg E, Madisch I, Shapiro F, Abukawa H, Troulis MJ, Vacanti JP. Bone. 2006;38:555–563. doi: 10.1016/j.bone.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Weir MD, Xu HHK. J. Biomed. Mater. Res. A. 2010;94A:223–233. doi: 10.1002/jbm.a.32665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dekker RJ, de Bruijn JD, Stigter M, Barrere F, Layrolle P, van Blitterswijk CA. Biomaterials. 2005;26:5231–5239. doi: 10.1016/j.biomaterials.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 4.Chou L, Marek B, Wagner WR. Biomaterials. 1999;20:977–985. doi: 10.1016/s0142-9612(98)00254-3. [DOI] [PubMed] [Google Scholar]
- 5.Chou Y-F, Huang W, Dunn JCY, Miller TA, Wu BM. Biomaterials. 2005;26:285–295. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Murphy WL, Hsiong S, Richardson TP, Simmons CA, Mooney DJ. Biomaterials. 2005;26:303–310. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Sikirić MD, Gergely C, Elkaim R, Wachtel E, Cuisinier FJG, Füredi-Milhofer H. J. Biomed. Mater. Res. A. 2009;89A:759–771. doi: 10.1002/jbm.a.32021. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Layrolle P, Stigter M, de Groot K. Biomaterials. 2004;25:583–592. doi: 10.1016/s0142-9612(03)00559-3. [DOI] [PubMed] [Google Scholar]
- 9.Kretlow JD, Mikos AG. Tissue Eng. 2007;13:927–938. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 10.Kokubo T, Ito S, Huang ZT, Hayashi T, Sakka S, Kitsugi T, Yamamuro T. J. Biomed. Mater. Res. 1990;24:331–343. doi: 10.1002/jbm.820240306. [DOI] [PubMed] [Google Scholar]
- 11.Bertazzo S, Zambuzzi WF, Campos DDP, Ogeda TL, Ferreira CV, Bertran CA. Colloids Surf. B. 2010;78:177–184. doi: 10.1016/j.colsurfb.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Chou Y-F, Chiou W-A, Xu Y, Dunn JCY, Wu BM. Biomaterials. 2004;25:5323–5331. doi: 10.1016/j.biomaterials.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Jalota S, Bhaduri SB, Tas AC. J. Mater. Sci. Mater. Med. 2006;17:697–707. doi: 10.1007/s10856-006-9680-1. [DOI] [PubMed] [Google Scholar]
- 14.Qu H, Wei M. J. Biomed. Mater. Res. B: Appl. Biomater. 2008;87B:204–212. doi: 10.1002/jbm.b.31096. [DOI] [PubMed] [Google Scholar]
- 15.Barrère F, Blitterswijk CA, de Groot K, Layrolle P. Biomaterials. 2002;23:1921–1930. doi: 10.1016/s0142-9612(01)00318-0. [DOI] [PubMed] [Google Scholar]
- 16.Ban S, Maruno S. Biomaterials. 1998;19:1245–1253. doi: 10.1016/s0142-9612(98)00032-5. [DOI] [PubMed] [Google Scholar]
- 17.Barrere F, Layrolle P, van Blitterswijk CA, de Groot K. Bone. 1999;25:107S–111S. doi: 10.1016/s8756-3282(99)00145-3. [DOI] [PubMed] [Google Scholar]
- 18.Müller L, Müller FA. Acta Biomater. 2006;2:181–189. doi: 10.1016/j.actbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Lu Y, Baer GS, Markel MD, Murphy WL. J. Mater. Chem. 2010;20:8894–8903. [Google Scholar]
- 20.Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 21.Tan J, Saltzman WM. Biomaterials. 2004;25:3593–3601. doi: 10.1016/j.biomaterials.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Okada S, Ito H, Nagai A, Komotori J, Imai H. Acta Biomater. 2010;6:591–597. doi: 10.1016/j.actbio.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 23.Okada S, Nagai A, Oaki Y, Komotori J, Imai H. Acta Biomater. 2011;7:1290–1297. doi: 10.1016/j.actbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Jung GY, Park YJ, Han JS. J. Mater. Sci. Mater. Med. 2010;21:1649–1654. doi: 10.1007/s10856-010-4011-y. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Leng Y. Biomaterials. 2005;26:1097–1108. doi: 10.1016/j.biomaterials.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 26.Kim SS, Park MS, Gwak SJ, Choi CY, Kim BS. Tissue Eng. 2006;12:2997–3006. doi: 10.1089/ten.2006.12.2997. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Ding J. Biomaterials. 2004;25:5821–5830. doi: 10.1016/j.biomaterials.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Pan Z, Ding J. Interface Focus. 2012;2:366–377. doi: 10.1098/rsfs.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bignon A, Chouteau J, Chevalier J, Fantozzi G, Carret JP, Chavassieux P, Boivin G, Melin M, Hartmann D. J. Mater. Sci. Mater. Med. 2003;14:1089–1097. doi: 10.1023/b:jmsm.0000004006.90399.b4. [DOI] [PubMed] [Google Scholar]
- 30.Annaz B, Hing KA, Kayser M, Buckland T, Di Silvio L. J. Microsc. 2004;216:97–109. doi: 10.1111/j.0022-2720.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 31.Specchia N, Pagnotta A, Cappella M, Tampieri A, Greco F. J. Mater. Sci. 2002;37:577–584. [Google Scholar]
- 32.Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Biomaterials. 2000;22:87–96. doi: 10.1016/s0142-9612(00)00174-5. [DOI] [PubMed] [Google Scholar]
- 33.Termine JD, Posner AS. Science. 1966;153:1523–1525. doi: 10.1126/science.153.3743.1523. [DOI] [PubMed] [Google Scholar]
- 34.Farlay D, Panczer G, Rey C, Delmas P, Boivin G. J. Bone Miner. Metab. 2010;28:433–445. doi: 10.1007/s00774-009-0146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horváthová R, Müller L, Helebrant A, Greil P, Müller FA. Mater. Sci. Eng. C. 2008;28:1414–1419. [Google Scholar]
- 36.Barrère F, van der Valk CM, Dalmeijer RAJ, van Blitterswijk CA, de Groot K, Layrolle P. J. Biomed. Mater. Res. A. 2003;64A:378–387. doi: 10.1002/jbm.a.10291. [DOI] [PubMed] [Google Scholar]
- 37.dos Santos E, Farina M, Soares G, Anselme K. J. Mater. Sci. Mater. Med. 2008;19:2307–2316. doi: 10.1007/s10856-007-3347-4. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer AA, Hennessy KM, Bellis SL. Biomaterials. 2005;26:1467–1475. doi: 10.1016/j.biomaterials.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Rouahi M, Champion E, Gallet O, Jada A, Anselme K. Colloids Surf. B Biointerfaces. 2006;47:10–19. doi: 10.1016/j.colsurfb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Nelson CM, Chen CS. FEBS Lett. 2002;514:238–242. doi: 10.1016/s0014-5793(02)02370-0. [DOI] [PubMed] [Google Scholar]
- 41.Kim K, Dean D, Mikos AG, Fisher JP. Biomacromolecules. 2009;10:1810–1817. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 43.Peng R, Yao X, Cao B, Tang J, Ding J. Biomaterials. 2012;33:6008–6019. doi: 10.1016/j.biomaterials.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Tang J, Peng R, Ding J. Biomaterials. 2010;31:2470–2476. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.