Abstract
Background:
Muscle strains are one of the most common injuries treated by physicians. Standard conservative therapy for acute muscle strains usually involves short-term rest, ice, and nonsteroidal anti-inflammatory medications, but there is no clear consensus regarding treatments to accelerate recovery. Recently, clinical use of platelet-rich plasma (PRP) has gained momentum as an option for therapy and is appealing for many reasons, most notably because it provides growth factors in physiological proportions and it is autologous, safe, easily accessible, and potentially beneficial. Local delivery of PRP to injured muscles can hasten recovery of function. However, specific targeting of PRP to sites of tissue damage in vivo is a major challenge that can limit its efficacy.
Hypothesis:
Location of PRP delivery can be monitored and controlled in vivo with noninvasive tools.
Study Design:
Controlled laboratory study.
Methods:
Superparamagnetic iron oxide nanoparticles (SPIONs) can be visualized by both magnetic resonance imaging (MRI) (in vivo) and fluorescence microscopy (after tissue harvesting). PRP was labeled with SPIONs and administered by intramuscular injections of SPION-containing platelets. MRI was used to monitor the ability to manipulate and retain the location of PRP in vivo by placement of an external magnet. Platelets were isolated from whole blood and incubated with SPIONs. Following SPION incubation with PRP, a magnetic field was used to manipulate platelet location in culture dishes. In vivo, the tibialis anterior (TA) muscles of anesthetized Sprague-Dawley rats were injected with SPION-containing platelets, and MRI was used to track platelet position with and without a magnet worn over the TA muscles for 4 days.
Results:
The method used to isolate PRP yielded a high concentration (almost 4-fold increase) of platelets. In vitro experiments showed that the platelets successfully took up SPIONs and then rapidly responded to an applied magnetic field. Platelets without SPIONs did not respond to the magnetic field. In vivo experiments showed that the SPION-containing platelets can be noninvasively maintained at a specific site with the application of a magnetic field.
Conclusion:
PRP may be a useful product in the clinical treatment of muscle injuries, but one problem with using it as a therapeutic tool is retaining PRP at the site of injury. This study proposes a potential solution, with findings that support this method at the cell, whole muscle, and in vivo levels. Controlling the location of PRP will allow the clustering of PRP to enrich the target area with growth factors and will prevent loss of platelets over time at the site of injury.
Keywords: injury, muscle strain, muscle damage, nanoparticles, MRI
Platelets are small, nonnucleated, discoid-shaped fragments released into the circulation from large bone marrow megakaryocytes. A high volume of new platelets (approximately 1 × 1011) is produced daily,14 resulting in turnover of the total number of circulating platelets approximately every 10 days. Platelets contain secretory vesicles called α-granules, which contain a multitude of growth factors7,11,35 that have been associated with improved healing of damaged tissue, such as tendon, muscle, and bone.12 Plasma containing a concentration of platelets that is higher than the concentration in whole blood is referred to as platelet-rich plasma (PRP).34 Using established methods or commercial systems, PRP can be isolated and has up to 8-fold the concentration of platelets found in whole blood.7 The resulting enrichment of growth factors is present in physiological proportions, an appealing benefit compared with using isolated growth factors.
However, maintaining PRP for the lifespan of the platelets (8-10 days) at a specific site of tissue damage in vivo is a major challenge, and the escape of PRP from the area of interest can limit its beneficial effects. Bovine thrombin can be used to induce clotting and activate platelets, allowing them to secrete their growth factors; however, based on enzyme-linked immunosorbent assays (ELISAs), the efficacy of thrombin-activated clots is questionable.38 An alternative method to induce local activation over a prolonged period of time is the use of biosynthetic scaffolds, but this has similar limitations.15 Repeated injections are undesirable both from a convenience and comfort standpoint and are more likely to have unwanted systemic effects.34,39 Furthermore, storing platelets from a single draw becomes cumbersome as one has to be concerned with premature activation of platelets during storage.
Platelet-rich plasma is currently used for a wide variety of musculoskeletal disorders,19 but with weaknesses in efficacy as described above. The overall goal in this study was to describe a novel method to inject a muscle with platelets containing fluorescent superparamagnetic iron oxide nanoparticles (SPIONs), which can be visualized by both magnetic resonance imaging (MRI) (in vivo) and fluorescence microscopy (after harvesting). We tested the hypothesis that we can noninvasively detect the location of PRP in vivo in rats and also control its location with noninvasive tools. To manipulate and retain the location of PRP, we used an external magnet32 to trap the SPION-containing platelets. If successful, this method could allow homing of PRP to a desired site and clustering of PRP to enrich the target area with growth factors and prevent premature loss of the platelets at the site of damaged muscle. Although not tested here, the ultimate goal of this method is to improve muscle healing after injury and enhance recovery.
Materials and Methods
In vitro experiments were performed to confirm SPION uptake by platelets and to confirm that platelets maintained normal morphology. In vivo experiments were performed to test the ability to track intramuscular SPIONS by MRI and to delay their dispersion by use of an external magnet over a period of 4 days. The proposed technology is described in noninjured muscle, but with the intent of future use in injured or diseased muscle.
Animals
All protocols were approved by the University of Maryland Institutional Animal Care and Use Committee (IACUC). Adult male Sprague-Dawley rats (N = 15) weighing 341 ± 21 g (3 months old) were anesthetized with isoflurane (2% with oxygen flow rate of 0.5 L/min) for experimental procedures. After the completion of experiments, tibialis anterior (TA) muscles were harvested at respective time points from the anesthetized rat, snap frozen in liquid nitrogen, and stored at –80°C.
Platelet-Rich Plasma
Whole blood was collected from adult male Sprague-Dawley rats (withdrawn via cardiac puncture; 10-20 mL per draw). PRP was separated from the blood during centrifugation using the ACP Double Syringe System (Arthrex), and PRP from a single rat was used for each injection of 1 to 3 rats. For all injections, only fresh PRP (same-day blood draw) was used. Platelet concentration was determined using an automated hemocytometer (TC20 Automated Cell Counter; Bio-Rad) and confirmed with hemocytometers designed for manual counting (DHC-N01; INCYTO or DRM-700; Millennium).
SPION Labeling of Platelets
For labeling of platelets with SPIONs, we used Molday ION (MION) with fluorescent tags: Molday ION Rhodamine B SPIONs (MIRB) (CL-50Q02-6A-50; BioPAL) or Molday ION EverGreen SPIONs (MIEG) (CL-50Q02-6A-51; BioPAL). MIRB was immediately added to the suspension of PRP at a concentration of 20 μg Fe/mL for 30 minutes to 1 hour unagitated and at room temperature, as recommended. MIRB is a homogeneous, rhodamine fluorescent (MIEG for fluorescein) contrast reagent designed to label cells efficiently and simply without use of added transfection reagents. It is an unmodified, ultrasmall contrast agent similar in size, zeta potential, and magnetic properties to MION, and it can be visualized by both MRI and fluorescence microscopy. MIRB is stable for 4 days at 37°C in phosphate-buffered saline or saline. MIRB has been used previously in live cells and shows excellent retention with no perturbation of cell proliferation, migration, survival, or differentiation.36
Electron Microscopy
Standard methods were used to prepare platelets for electron microscopy,33,40 and care was taken to maintain morphology by controlling temperature, fixative, pH, and buffer type. After incubation with the SPIONs, a 1.5-mL suspension of SPION-containing platelets was centrifuged at 3000 rpm for 10 minutes, and the supernatant was decanted from the pellet. The pellet was resuspended 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH, 7.4) for 2 hours, then stained with heavy metals, dehydrated, and postfixed in 1% osmium tetroxide in 0.5 M acetate buffer for 1 hour. The pellet was rinsed in buffer 2 times for 15 minutes each and dehydrated in a graded series of ethanols (50%, 70%, 90%, and 100%) for 2 minutes each. The pellet was infiltrated with resin and sectioned for electron microscopy at 90 nm, then viewed with a Phillips 201 microscope. Images were taken on Kodak 4489 film and digitally scanned at 600 dpi.
Targeting
We injected 200 μL of SPION-containing platelets into both TA muscles of each rat (n = 3). A “sleeve” was fashioned externally over the skin of the left leg, anterior to the TA; each sleeve held six 8 mm–diameter magnets with remanence magnetization of around 0.15 tesla each (Neodymium, Grade N52; K&J Magnetics Inc) to form a Halbach array over the muscle. A Halbach array is a special arrangement of magnets that augments the magnetic field on one side of the array. For animals, this can be done with a fit neoprene sleeve or tape, or even surgically placed. Here, we used tape covered by superglue to form a solid covering. To further prevent the animals from chewing the magnet loose, we placed them in Elizabethan collars (EC404VS-5; Kent Scientific Corp) so as not to reach the tape while under treatment.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed immediately after intramuscular injections of SPION-containing platelets in both TA muscles. After MRI, the external sleeves were then applied to the left leg only, with the magnets positioned over the TA muscle. MRI was performed again 4 days after application of the magnets to compare TA injected muscles with and without a magnetic sleeve. MRI studies were performed on a Bruker Biospec AVAVCE III 7.0-T 30-cm horizontal bore scanner using Paravision 5.1 software (Bruker Biospin MRI). A Bruker 4-element 1H surface coil array was used as the receiver and a Bruker 72-mm linear-volume coil as the transmitter. The rat was anesthetized in an animal induction chamber with a gas mixture of O2 (1 L/min) and isoflurane (3%). The animal was then placed supine on a custom-made body holder bed, and the radio frequency coil was positioned and fixed with surgical tape in the region of interest on the animal leg. After the animal was moved into the center of the magnet, the isoflurane level was maintained at 1.0% to 1.5% for the remainder of the experiment. An MR-compatible small-animal monitoring and gating system (SA Instruments) was used to monitor animal respiration rates and body temperatures. Rat body temperature was maintained at 36°C to 37°C using a warm-water circulator. Three-slice (axial, midsagittal, and coronal) scout rapid acquisition with fast low-angle shot MRI (FLASH) was used to localize the leg. Multiple 2-dimensional high-resolution T1-weighted MRIs in the cross-sectional view between the knee and the ankle were acquired using rapid acquisition using the FLASH sequence with repetition time/echo time (TR/TE) = 377/3 ms, flap angle = 30°, field of view (FOV) = 45 × 45 mm2, in-plane resolution = 100 × 100 μm2, slice thickness = 1.0 mm without a gap, averages = 8, and number of slices = 40. MRI was simply used to provide a binary positive or negative, based on the presence/absence of platelets. The imaging was not conducted in a blind fashion; however, images of the same image slice relative to the tibial plateau were used, and axial slices above and below were examined.
Statistics
Data (platelet counts) were analyzed using a single-factor analysis of variance (Sigma-Stat). When a significant ratio was found, a Tukey post hoc analysis was performed to determine where significant differences (P < .05) had occurred.
Results
We used a commercial system to obtain PRP. Platelet concentration of the PRP was measured using both automated and manual hemocytometers, which confirmed that the platelet isolation yielded PRP with a 3.7-fold increase in platelets compared with whole blood (Figure 1). Incubation of the PRP with SPIONS resulted in SPION uptake by platelets, as evidenced by colocalization with microscopic imaging (Figure 2).
Figure 1.
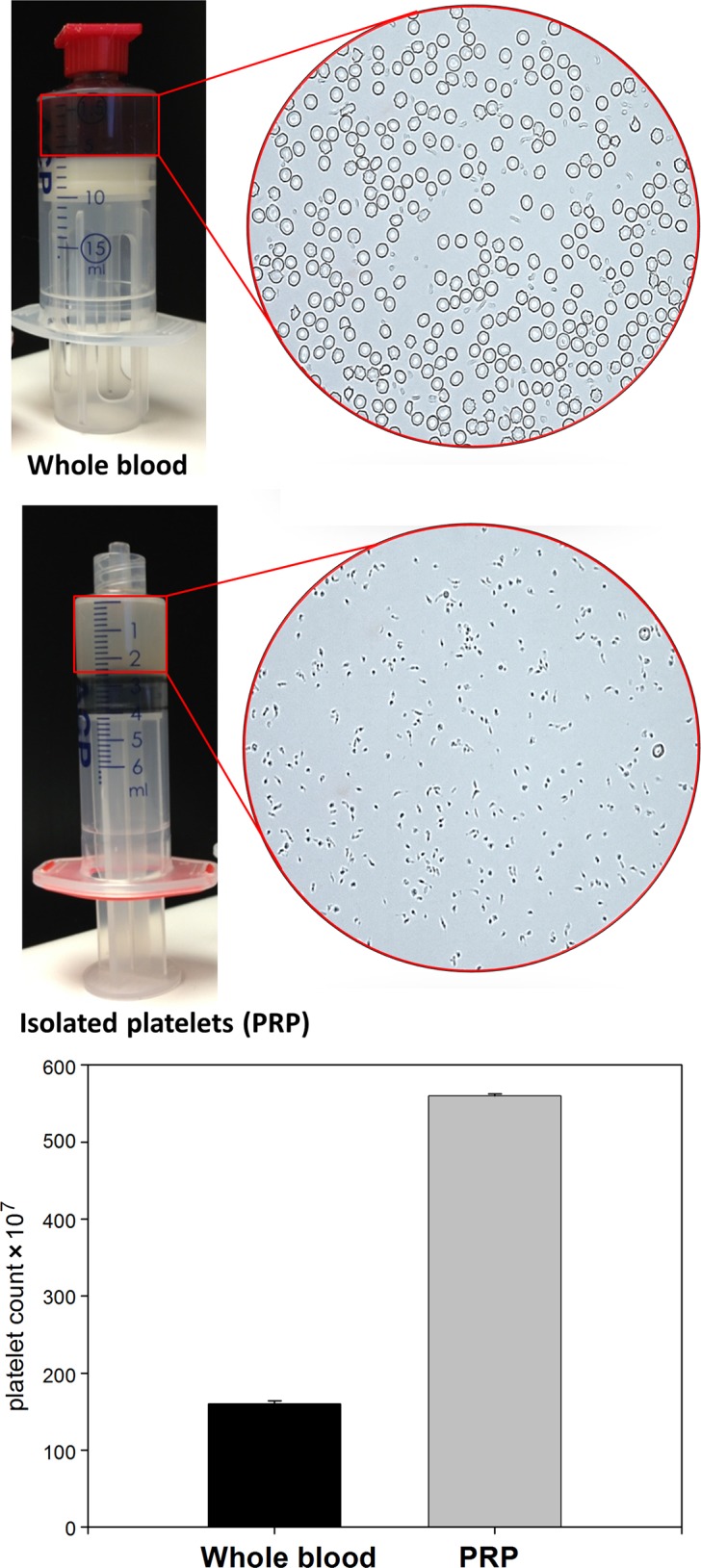
Isolation of platelet-rich plasma (PRP). Platelets were isolated from whole blood using the Arthrex AC Double Syringe System. Photographs show the PRP and whole blood after separation by centrifugation. Micrographs show representative images of the respective products using phase contrast microscopy. The bar graph shows quantification of the almost 4-fold increase in platelet concentration.
Figure 2.
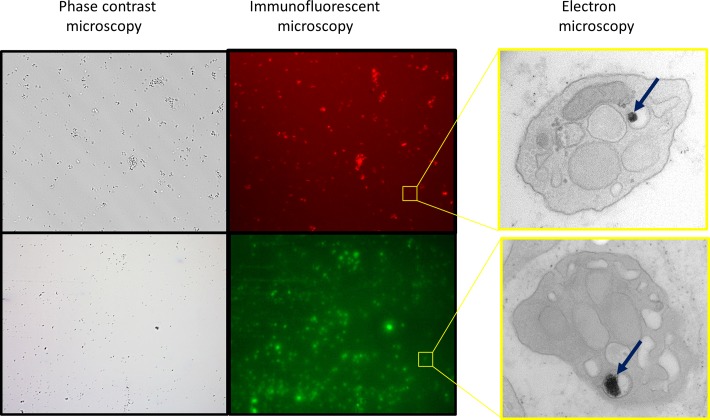
Fluorescent superparamagnetic iron oxide nanoparticles (SPIONs) are taken up by platelets. The micrographs show isolated platelets on a dish (phase contrast microscopy). Using SPIONs conjugated to rhodamine (red) or fluorescein (green), the platelets can also be seen using a standard epifluorescent microscope. Transmission electron microscopy was performed to confirm that the SPIONs are inside the platelets. The SPIONs are typically seen in an endocytotic vesicle. The iron oxide core of the SPIONs (arrow) is present as small dark spheres within the vesicles.
Based on preliminary experiments (data not shown), detectable labeling occurred as rapidly as 30 minutes, and maximal labeling for platelets occurs at 30 to 60 minutes. Following a 1-hour coculture, platelets with SPIONs conjugated to rhodamine (MIRB, red) or fluorescein (MIEG, green) were visible with fluorescent microscopy (Figure 2). Normal platelets (with no SPIONs) imaged under fluorescent microscopy were black (not shown); this negative control ensured that the fluorescence seen from SPION-containing platelets was not a false positive. Transmission electron microscopy was used to further confirm that the platelets could indeed take up the SPIONs (Figure 2). The SPIONs are typically seen in an endocytotic vesicle, with the iron oxide core of the SPIONs present as small dark spheres within the vesicles. Viability of platelets was measured by Trypan blue exclusion following SPION labeling, and no difference in viability between SPION-labeled and unlabeled platelets was detected (not shown).
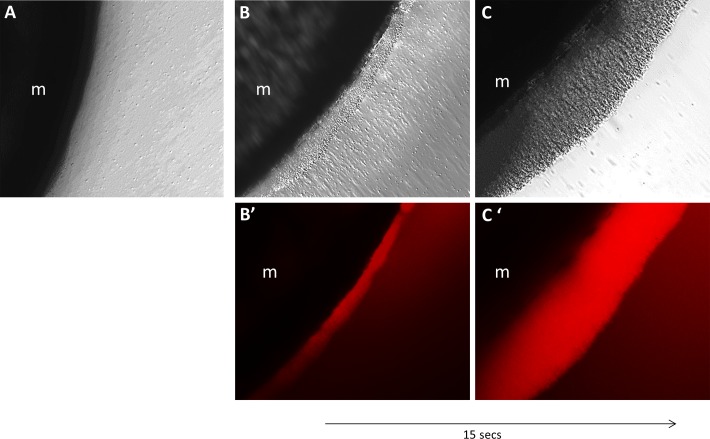
In vitro experiments showed that platelets were easily mobilized to and maintained at a set position. Platelets added to a standard 35-mm culture dish did not show any response to a magnet (Figure 3A), whereas SPION-containing platelets were immediately (<15 seconds) attracted to a magnet when added to the dish (Figure 3, B and C).
Figure 3.
Manipulation of superparamagnetic iron oxide nanoparticle (SPION)–containing platelets in vitro. To test the ability to mobilize SPION-containing platelets in vitro, platelets were cultured in SPION media. Dark field microscopy shows that the SPION-containing platelets were immediately attracted by placement of a magnet (m) underneath the culture dish (B-C). This attraction over the magnet did not occur when platelets were not incubated with SPIONs (A). (B′-C′) Time-dependent increase in the rhodamine signal emitted by the SPIONs inside the platelets.
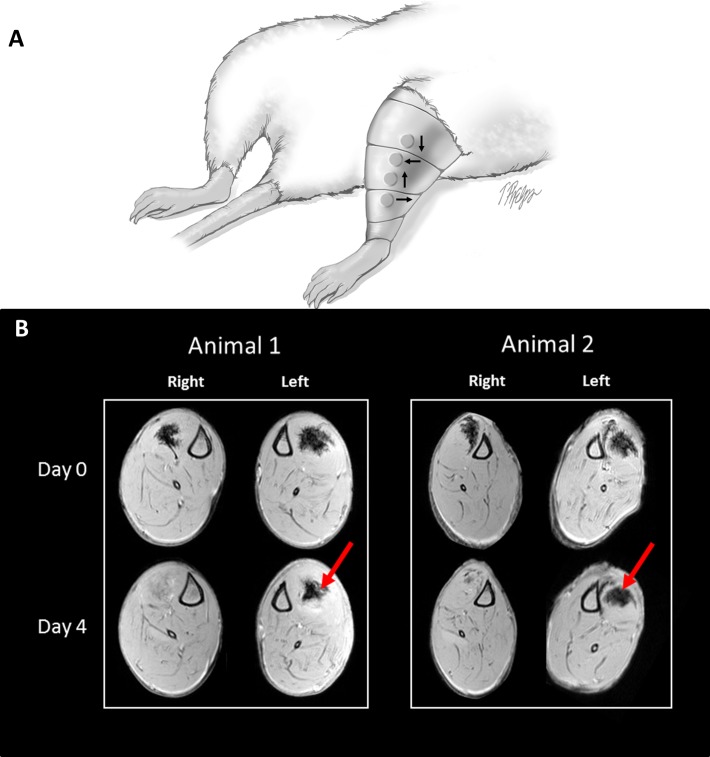
In vivo experiments showed that MRI could track the SPION-containing platelets. Both TA muscles, injected with equal volumes of the SPION-containing platelets, were tracked over time with or without a magnet on the leg. Results show that the magnet was able to keep the platelets at a specific location and over a longer period of time than without the magnet (Figure 4). Without an external magnet (Figure 4, right leg), the SPION-containing platelets dissipated within 1 to 2 days. With an external magnet (Figure 4, left leg), the SPION-containing platelets were visible at least 4 days after injection, but never beyond 8 days.
Figure 4.
In vivo assessment and retention of superparamagnetic iron oxide nanoparticle (SPION)–containing platelets. (A) Immediately after injection of SPION-containing platelets into both tibialis anterior muscles, magnets were arranged into a specialized array (Halbach array) and were positioned over the muscle on the left leg and covered by a customized sleeve; the arrows indicate the rotating pattern of polarization in the Halbach array. The right leg was used as a control (no magnets). An Elizabethan collar was used (not pictured) to keep animals from chewing on or removing the sleeve. (B) Magnetic resonance imaging was used to track the SPION-containing platelets in vivo. Both tibialis anterior muscles were injected with equal volumes of the SPION-containing platelets and tracked over time with or without a magnet on the leg. Representative images show that the application of a magnetic field is able to retain the SPION-containing platelets at a specific location and over a longer period of time (left leg, red arrows indicate same anatomical position on day 4) when compared with injected muscles without the magnetic field (right leg).
Discussion
There are a variety of methods to isolate and concentrate platelets, resulting in different products with variation (3- to 27-fold) in the concentration of growth factors.9,24,31 PRP has been used for a plethora of orthopaedic ailments to facilitate healing in muscle, bone, cartilage, skin, and intervertebral disc.1,23 To date, there has been little evidence of a systemic effect from local PRP injections,23,34 but this notion has been challenged recently.39 PRP is safe, but effectiveness remains to be unquestionably proven. Clinical studies have shown variable results,2,16,18,28,30 and basic science and animal studies range from positive outcomes11,20,25,35 to suggesting little benefit for some types of injury. For example, a recent article by Delos et al8 showed no benefit of using PRP in an animal model of contusion injury. There are many potential causes for the variation, such as the amount of PRP, concentration of PRP, and timing of PRP administration. In any case, PRP is currently used in a variety of tissues, and certainly, care needs to be taken to avoid deleterious consequences.4
Platelet-rich plasma contains up to 8 times the concentration of platelets found in whole blood,7 and these platelets contain α-granules, which can release a multitude of growth factors, such as platelet-derived growth factor (PGF), insulin-like growth factor–1 (IGF-1), transforming growth factor–β (TGF-β), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and hepatocyte growth factor (HGF).7,11,35 The fact that PRP contains several different growth factors, present in physiological proportions, is an appealing benefit compared with using isolated growth factors. Other advantages are that it is relatively simple and easy to obtain PRP from a human blood sample, and there is little risk of developing an immune response from autologous PRP. A platelet’s life span is approximately 8 to 10 days. Once activated by exposure to collagen in the tissues, they secrete growth factors with an initial burst and then throughout their lifespans.19 Whether the regenerative capacity of growth factors is the direct effect of growth factors on the target tissue or some indirect/secondary activation of pathways is unclear.
SPIONs are easily taken up by a variety of cells, reaching levels suitable for tracking, with labeled cells showing no signs of toxicity.36,42 Particles are internalized through spontaneous endocytosis or phagocytosis,22 and cell labeling is simple, chemically safe, and typically requires no more than 1 hour of laboratory contact time. SPIONs are increasingly used to label cells, to track them by imaging, or to quantify them in vivo.10,27,29 The use of SPIONs eliminates the need for complex cellular labeling strategies that rely on the overexpression of iron-binding receptors or the conjugation of iron oxides to specific ligands. Labeled cells can be stored frozen, thawed, and replated with levels of viability identical to untreated cells. These materials, sometimes referred to as USPIOs (ultrasmall superparamagnetic iron oxide contrast agents), have been extraordinarily useful in a broad range of applications.17,26,37 They label cells with high efficiency and are retained within the cell for extended time periods, providing an alternative to cell labeling with MRI contrast agents. A recent study3 has shown that platelets incorporate SPIONs by endocytosis (without linkers or binding agents) and that approximately 98% of platelets were labeled.
Muscle strains are one of the most common complaints treated by physicians13,21 and account for the majority of all sports-related injuries.6,41 Given the common nature of muscle strain injuries, a treatment that can improve recovery time could have a tremendous impact in athletics. Wearing a magnet over the site of injury to keep the SPION-containing platelets localized to an area would be a noninvasive, convenient, and cost-effective way to concentrate growth factors locally. Injection of isolated growth factors, even if effective, could be prohibitively more expensive. To date, studies suggest that SPIONs do not alter cell viability, proliferation, or differentiation properties.27,29,36 Thus, SPION-containing cells are unlikely to have any adverse effects on the cellular, tissue, or systemic levels, although this notion has not been tested via clinical studies.
This study has several limitations. We presented novel methodology in an animal model using a specific species, age, and sex; however, extensive work is still needed to confirm safety and optimize this method. We have shown using MRI that the nanoparticles can be concentrated and therefore persist in the injected area, but more work is needed to determine if platelet structure, activation, or the release of growth factors is altered in vivo or after muscle injury.
Only 1 volume of PRP and SPIONs was tested, and the optimal concentration of SPIONs for a given concentration of platelets and the amount delivered for a specific type of muscle injury are still variables to be studied. Cellular uptake of SPIONs can be affected by the nanoparticle properties, and the larger the cell the higher the number of SPIONs absorbed.22 There can also be a lack of consistency of the PRP based on an individual’s physiological state at the time of the blood draw, as well as the system used to isolate the PRP (and therefore the yield of platelets/volume), and both are likely contributing factors to reported variability in the effectiveness of PRP.24,25,31 A critical dose (threshold) may exist, but which quantities are too little or too much is not clear.
Preclinical animal studies are clearly required for any new therapies, but the lack of standardized methods to isolate and deliver PRP together with the dearth of controlled clinical studies means that the efficacy and optimization of PRP therapy is still not clear. Indeed, platelet concentration can vary depending on the system and methods used to obtain the PRP,5 and care must be taken to avoid excessive aggregates that might impair vascular function in small vessels. Despite the lack of standardization, the use of PRP to facilitate soft tissue healing appears promising and magnetic manipulation in vivo for therapeutic delivery could offer some exciting advances in how we treat muscle injury and even muscle disease.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported by a grant to R.M.L. from the National Institutes of Health (1R01AR059179).
References
- 1. Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9:721–730. [DOI] [PubMed] [Google Scholar]
- 2. Antonello Gde M, Torres do Couto R, Giongo CC, Corrêa MB, Chagas Júnior OL, Lemes CH. Evaluation of the effects of the use of platelet-rich plasma (PRP) on alveolar bone repair following extraction of impacted third molars: prospective study. J Craniomaxillofac Surg. 2013;41:e70–e75. [DOI] [PubMed] [Google Scholar]
- 3. Aurich K, Spoerl MC, Fürll B, et al. Development of a method for magnetic labeling of platelets. Nanomedicine. 2012;8:537–544. [DOI] [PubMed] [Google Scholar]
- 4. Bowman KF, Jr, Muller B, Middleton K, Fink C, Harner CD, Fu FH. Progression of patellar tendinitis following treatment with platelet-rich plasma: case reports. Knee Surg Sports Traumatol Arthrosc. 2013;21:2035–2039. [DOI] [PubMed] [Google Scholar]
- 5. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–271. [DOI] [PubMed] [Google Scholar]
- 6. Chan YS, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33:43–51. [DOI] [PubMed] [Google Scholar]
- 7. Creaney L, Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med. 2008;42:314–320. [DOI] [PubMed] [Google Scholar]
- 8. Delos D, Leineweber MJ, Chaudhury S, Alzoobaee S, Gao Y, Rodeo SA. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am J Sports Med. 2014;42:2067–2074. [DOI] [PubMed] [Google Scholar]
- 9. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27:158–167. [DOI] [PubMed] [Google Scholar]
- 10. El Haj AJ, Glossop JR, Hura HS, et al. An in vitro model of mesenchymal stem cell targeting using magnetic particle labelling [published online December 27, 2012]. J Tissue Eng Regen Med. doi:10.1002/term.1636. [DOI] [PubMed] [Google Scholar]
- 11. El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. [DOI] [PubMed] [Google Scholar]
- 12. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. [DOI] [PubMed] [Google Scholar]
- 13. Garrett WE ., Jr Muscle strain injuries. Am J Sports Med. 1996;24 (suppl):S2–S8. [PubMed] [Google Scholar]
- 14. Geddis AE, Kaushansky K. Immunology. The root of platelet production. Science. 2007;317:1689–1691. [DOI] [PubMed] [Google Scholar]
- 15. Getgood A, Henson F, Brooks R, Fortier LA, Rushton N. Platelet-rich plasma activation in combination with biphasic osteochondral scaffolds—conditions for maximal growth factor production. Knee Surg Sports Traumatol Arthrosc. 2011;19:1942–1947. [DOI] [PubMed] [Google Scholar]
- 16. Gosens T, Peerbooms JC, van Laar W, Den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–1208. [DOI] [PubMed] [Google Scholar]
- 17. Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine (Lond). 2007;2:23–39. [DOI] [PubMed] [Google Scholar]
- 18. Halpern B, Chaudhury S, Rodeo SA, et al. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013;23:238–239. [DOI] [PubMed] [Google Scholar]
- 19. Halpern BC, Chaudhury S, Rodeo SA. The role of platelet-rich plasma in inducing musculoskeletal tissue healing. HSS J. 2012;8:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hammond JW, Hinton RY, Curl LA, Muriel JM, Lovering RM. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirkendall DT, Garrett WE., Jr Clinical perspectives regarding eccentric muscle injury. Clin Orthop Relat Res. 2002;(403 suppl):S81–S89. [DOI] [PubMed] [Google Scholar]
- 22. Kolosnjaj-Tabi J, Wilhelm C, Clément O, Gazeau F. Cell labeling with magnetic nanoparticles: opportunity for magnetic cell imaging and cell manipulation. J Nanobiotechnology. 2013;11 (suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maffulli N, Del Buono A. Platelet plasma rich products in musculoskeletal medicine: any evidence? Surgeon. 2012;10:148–150. [DOI] [PubMed] [Google Scholar]
- 24. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308–316. [DOI] [PubMed] [Google Scholar]
- 25. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40:1742–1749. [DOI] [PubMed] [Google Scholar]
- 26. Moore A, Weissleder R, Bogdanov A., Jr Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J Magn Reson Imaging. 1997;7:1140–1145. [DOI] [PubMed] [Google Scholar]
- 27. Odintsov B, Chun JL, Mulligan JA, Berry SE. 14.1 T whole body MRI for detection of mesoangioblast stem cells in a murine model of Duchenne muscular dystrophy. Magn Reson Med. 2011;66:1704–1714. [DOI] [PubMed] [Google Scholar]
- 28. Peerbooms JC, Colaris JW, Hakkert AA, et al. No positive bone healing after using platelet rich plasma in a skeletal defect. An observational prospective cohort study. Int Orthop. 2012;36:2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riegler J, Liew A, Hynes SO, et al. Superparamagnetic iron oxide nanoparticle targeting of MSCs in vascular injury. Biomaterials. 2013;34:1987–1994. [DOI] [PubMed] [Google Scholar]
- 30. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40:1234–1241. [DOI] [PubMed] [Google Scholar]
- 31. Russell RP, Apostolakos J, Hirose T, Cote MP, Mazzocca AD. Variability of platelet-rich plasma preparations. Sports Med Arthrosc. 2013;21:186–190. [DOI] [PubMed] [Google Scholar]
- 32. Sarwar A, Nemirovski A, Shapiro B. Optimal Halbach permanent magnet designs for maximally pulling and pushing nanoparticles. J Magn Magn Mater. 2012;324:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sawatzke CL, Solomons CC. Fixation and embedding of small volumes of platelets for transmission electron microscopy. J Clin Pathol. 1980;33:600–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schippinger G, Fankhauser F, Oettl K, Spirk S, Hofmann P. Does single intramuscular application of autologous conditioned plasma influence systemic circulating growth factors? J Sports Sci Med. 2012;11:551–556. [PMC free article] [PubMed] [Google Scholar]
- 35. Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. [DOI] [PubMed] [Google Scholar]
- 36. Shen WB, Plachez C, Chan A, et al. Human neural progenitor cells retain viability, phenotype, proliferation, and lineage differentiation when labeled with a novel iron oxide nanoparticle, Molday ION Rhodamine B. Int J Nanomedicine. 2013;8:4593–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tallheden T, Nannmark U, Lorentzon M, et al. In vivo MR imaging of magnetically labeled human embryonic stem cells. Life Sci. 2006;79:999–1006. [DOI] [PubMed] [Google Scholar]
- 38. Textor JA, Tablin F. Activation of equine platelet-rich plasma: comparison of methods and characterization of equine autologous thrombin. Vet Surg. 2012;41:784–794. [DOI] [PubMed] [Google Scholar]
- 39. Wasterlain AS, Braun HJ, Harris AH, Kim HJ, Dragoo JL. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 2013;41:186–193. [DOI] [PubMed] [Google Scholar]
- 40. White JG. Electron microscopy methods for studying platelet structure and function. Methods Mol Biol. 2004;272:47–63. [DOI] [PubMed] [Google Scholar]
- 41. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2:167–182. [PubMed] [Google Scholar]
- 42. Zhang Y, Zhang J. Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J Colloid Interface Sci. 2005;283:352–357. [DOI] [PubMed] [Google Scholar]