Abstract
This review is an example of the use of an animal model to try to understand the immune biology of pregnancy. A well-known model of recurrent spontaneous pregnancy loss is put in clinical, historical and theoretical context, with emphasis on T cell biology.
Introduction
The ‘problem of viviparity’ (Medawar 1954) remains among the most fundamental questions in biology. From an immunological standpoint, this problem stems from the conundrum presented by the fetus as a ‘semi-allograft and the mother's need to both protect and tolerate the fetus. The implications of the relationship(s) between the mother, fetus and immune system reach clinical significance with regard to emerging infectious disease, vaccinations, autoimmunity, specific disease of pregnancy, fetal development and neonatal health. It has been our hypothesis that understanding of critical clinical and basic biological problems can result from the iterative use of good clinical/epidemiological data and well-understood animal models (Bonney 2013). Such an approach should lead to not only significant progress in our understanding of pregnancy, but also in logical and successful clinical intervention.
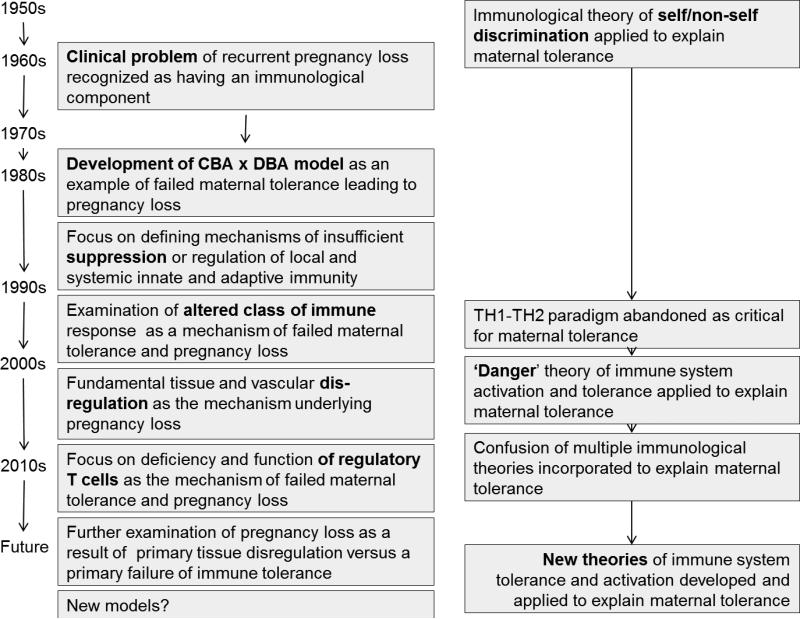
Depending on one's viewpoint it seems that, studies using animal models of pregnancy-related disease, as compared to study of other disease models, have failed to deliver. Why? As an outsider we would posit that due to unrecognized subtle and not so subtle differences in human and animal physiology that the models themselves take some of the blame (Bonney 2013) but not all of it. In addition, we propose that the theoretical framework which elicited the use of and interpretation of the data generated by these models, and further, the experimental direction lead by this interpretation may have impeded the finding of solutions. To support this assertion, what follows is a focused discussion of one animal model wherein female mice of the CBA/J strain mated with male mice of the DBA/2 strain experience a high rate of reproductive failure. We think the theoretical context that drives the use of this model is founded on classical immune theory of how the maternal immune system decides between activation and tolerance, namely the discrimination between self and non-self. We will discuss how this model appears to have driven thinking about the T cell biology of pregnancy, and how thinking about this model might be redirected in the context of an alternative to self/non self determination theory (Bonney 2007, Matzinger 1994) to generate experiments leading to better understanding. Figure 1 illustrates the path of the model and our discussion.
Figure 1.
Immune theory and the path of the CBA x DBA model
A driver: the clinical problem of recurrent spontaneous pregnancy loss
It has long been recognized that there were couples who suffered from recurrent pregnancy loss (see (Branch, et al. 2010) for a recent review). The most concerning were those who never had a successful pregnancy and who at a similar time in each of several pregnancies suffered this loss. While some of this phenomenon was attributed to such factors as chromosomal defects (Kim, et al. 1975) progesterone insufficiency (Shearman and Garrett 1963), uterine anomalies (Branch, et al. 2010), or psychosocial factors (Weil and Tupper 1960) many of these factors were hotly debated and it soon became clear that that there was a subset of couples for whom no cause could be found (Christiansen, et al. 2008). Over many years, several lines of evidence (briefly summarized here) have suggested the involvement of the immune system in this disease. Earlier (for example (McIntyre, et al. 1989, McIntyre and Faulk 1983) and more recent studies have suggested a role for HLA and minor antigens (e.g. H-Y (Christiansen, et al. 2012, Nielsen, et al. 2009) in recurrent pregnancy loss. Moreover, the association between the generation of auto-antibodies, autoimmune disease (Firkin, et al. 1980), disorders of complement regulation (Laitinen, et al. 1991) and recurrent pregnancy loss then and now (Mohlin, et al. 2013, Oku, et al. 2009) support the idea that the immune system plays a role in this disease. In addition, there exists evidence of the important role played by natural killer cells both in normal pregnancy (Bulmer, et al. 1991, Koopman, et al. 2003) and in recurrent pregnancy loss, with the later thought to stem from lack of trophic supportive (e.g. growth, development) activity and/or overactive killer activity (Nakashima, et al. 2012) although this remains controversial (Tang, et al. 2011) and awaits further study (Tang, et al. 2013). Earlier studies of distinct immune-reactive antigens expressed on the human placenta (for example (Faulk, et al. 1978)) suggested the possibility of alternative flavors of immune responses- some protective and some harmful to the fetal-placental unit (McIntyre and Faulk 1979) (Ecker, et al. 1993). Finally, there is evidence supporting an association between altered decidual T cell populations and recurrent pregnancy loss (Inada, et al. 2013, Nakashima, et al. 2012, Sasaki, et al. 2004).
Immune theory, bolstered by years of basic experimental data, suggested that the immune system's primary and activating focus was to respond to that which was non-self. Pregnancy was considered an example of this theory (Medawar 1954), and successful pregnancy was considered critically dependent on suppression of maternal immunity. However, it had been observed that human (McIntyre, et al. 1983) (Faulk and McIntyre 1983) and animal females could make complex immune responses to fetal/placental antigens during normal pregnancy. An early, prominent report suggested that women with recurrent abortion who shared several HLA antigens with their partners could be “immunized” by transfusion with allogeneic leukocytes (Taylor and Faulk 1981) to enhance fetal-protective immunity and therefore helped to have successful pregnancies. Although subsequent randomized trials and meta-analyses have cast some doubt on its use in most couples (Ober, et al. 1999, Porter, et al. 2006), the possibility of an immune-based therapy for recurrent abortion at that time supported the development of animal models to address the issue.
The origins of the ‘CBA x DBA’ model
History of the mouse strains (Table 1)
Table 1.
Genetic and immunogenetic features of the CBA × DBA model
| Strain | Origin | MHC | H2-T23 (Qa1) | H2-T18 | MMTV-7 | Known Features/mutations | Response to the male antigen H-Y |
|---|---|---|---|---|---|---|---|
| CBA/J | Cross between Bagg female and DBA male in 1920; selected for low incidence of mammary tumors | H-2 haplotype k | b | b | a | homozygous for the retinal degeneration allele Pde6brd1; causes blindness High frequency of renal tubulointerstitial lesions |
Non-responder |
| DBA/2 | Oldest of all inbred strains; produced by CC Little in 1909 | H-2 haplotype d | b | c | a | Widely used strain; traits often contrasted with C57BL/6J Hearing loss CD94 deficient (DBA/2) Low incidence of atherosclerosis Eye abnormality used as model for glaucoma |
Non-responder |
| Balb/c | H-2 haplotype d | b | c | b | Well known for susecptibiltiy to autoimmune encephalomyelitis (EAE) Widely used for the production of monoclonal antibodies. |
Non-responder |
CBA/J has been maintained at Jackson labs since 1948. It was originally created in as in inbred strain in 1920 by Strong, by crossing a Bagg albino female with a DBA/2 male (see http://jaxmice.jax.org). CBA/J mice were selected for having a low incidence of mammary tumors. Based on this history, modern CBA/J and DBA/2 are expected to be genetically identical at half of all loci. Like all inbred strains, CBA/J has some specific features. In particular, CBA/J mice are homozygous for Pde6brd1 (phosphodiesterase 6B, cGMP, rod receptor, beta polypeptide) which is a mutation causing early retinal degeneration and blindness by age of weaning. Thus, all CBA/J adult mice are blind. In addition, CBA/J mice are known to have a high incidence of tubulointerstitial renal lesions.
DBA mice are the oldest of all inbred strains, and were originally developed by CC Little in 1909. DBA/1 and DBA/2 were established as sub-strains in 1929-1930. DBA/2 mice are homozygous for a mutation that results in progressive hearing loss. In addition, DBA/2 mice develop progressive glaucoma-like eye abnormalities, the inheritance of which involves at least two genes.
T cell antigens These two strains were among those extensively used to gain understanding of major, minor and ‘super antigens’. CBA/J is (histocompatibility) H-2 haplotype k. The CBA/J H2-T23 (Qa1) and H2-T18 types are both b (Fischer Lindahl 1997). An important minor antigen, presented as a peptide in a histocompatibility molecule, and capable of inducing tissue graft rejection by females, is the male antigen, H-Y (Scott, et al. 1997, Simpson, et al. 1997). CBA/J is a “non responder” to the male antigen H-Y (Gordon, et al. 1975, von Boehmer, et al. 1978), although CBA/J x C57BL/6 F1 female mice do respond to this antigen. Both CBA/J and DBA/2 mice express the mouse mammary tumor virus locus 7 allele originally called Mls-1a. Strains carrying this allele delete T cells expressing specific V beta chains (e.g. V beta 8.1) from their peripheral T cell pool (Kappler, et al. 1988). DBA/2 is H-2 haplotype d, its H2-T23 (Qa1) type is b (Fischer Lindahl 1997) and its H2-T18 type is c. DBA/2 mice have the unique characteristic that they do not express the NK cell surface receptor CD94/NKG2A, due to a deletion within the Klrd2 gene(Vance, et al. 2002). Balb/c mice are H-2 d, their H2-T23 and H2-T18 types are the same as DBA/2, and their Mtv7 type is b. Balb/c mice do not respond to the male antigen H-Y (von Boehmer, et al. 1978).
The pregnancy-loss phenotype
CBA/J mice have been housed and bred in several facilities around the world. The primary model comprises a comparison between CBA/J females mated to DBA/2 males and CBA/J females mated to males of other strains, including C3H, C57BL/6 and the most common, Balb/c. Early studies suggested a high rate of non-pregnancy in DBA/2-mated CBA/J females (Clark, et al. 1980). However, mothers of related CBA/J strains, CBA/Ca and CBA/N do not exhibit this phenomenon (Bobe and Kiger 1989). Investigators have noted that in DBA/2-mated CBA/J females, beginning around day 7 of gestation, there is a loss of cellular contact between decidual cells and cells of the ectoplacental cone (Gendron and Baines 1989). This is accompanied by an infiltration of natural killer and non- natural killer leukocytes, including T cells and B cells (Gendron and Baines 1988, 1989). Later studies also suggest that resorbing tissues have an increased infiltration of mast cells (Zenclussen, et al. 2003). The pathology of these pregnancies progresses by day 8 to comprising increased tissue spaces and abnormal syncytium formation by day 9 (Gendron and Baines 1989). Finally by day 10-12 there is evidence of embryonic loss and pronounced infiltration of polymorphonuclear leukocytes, CD8 positive and negative T cells, and B cells. By day 12 there is a clear difference between “resorbing” fetal placental units and normal units whereby the resorbing units are dark, small, and necrotic, with in-determinant fetal and placental elements while normal fetal placental units are pink and show overt evidence of a developed placenta separate from a fetus within an amniotic sac. From this discussion, it should be obvious that not every fetal-placental unit undergoes resorption. In addition from time to time, fetal placental units resorb in all strains. The importance of specific factors that differentiate resorbing from non-resorbing fetal placental units in either ‘normal’ or ‘abnormal’ strain combinations appears to have been afforded less relevance in the study of this model.
While it has been reported that the number of early implantation sites in this breeding is relatively normal (Chavez, et al. 1987), ultimately, litter size in CBA/J X DBA/2 matings is variable, from 2 to 8 pups per litter (Heine, et al. 1989). Data on litter size is not as prevalent as data on what is called “resorption. Pregnancy loss in the CBA/J x DBA/2 model is usually reported as the number (or percent) of resorptions/total number of implantations (R/T), pooling data from individual mice (Clark, et al. 2008b) and this is deemed preferable to reporting median and range of resorbed units or to comparing data from individual mice. The percentage of resorbing fetal-placental units in this mating are highly variable, with some values as low as 10 % and others upwards of 80% depending on several factors—in particular, the microbial barrier status of husbandry { Hamilton, 1987 #73;Clark, 2003 #168 }. A large range in resorption rate can be seen within the same set of experiments (Clark, et al. 1994). There also appears to be some variability with regard to second pregnancies in this mating, with most reports suggesting that second pregnancies decrease the resorption rate (Chavez, et al. 1987, Clark, et al. 1994) (Ahmed, et al. 2010). However, this has not been observed by all investigators (Baines, et al. 1996). This later discrepancy may be related to the time-lapsed between the first and second pregnancies. As in human populations, this mating combination experiences increased pregnancy loss with increasing maternal age (Chavez, et al. 1987, Ho, et al. 1994).
Evolving views of immune suppression and the ‘CBA/J x DBA’ model
Inherent pregnancy-related suppression?
Clonal selection theory and the related self/non self discrimination model of immune system activation (Burnet 1962) led immunologists to suggest model(s) wherein the maternal immune system was suppressed during normal pregnancy (Medawar 1954). Though some early (e.g. (Clark and McDermott 1978)) studies documented the expansion rather than contraction of maternal lymphoid tissue during allogeneic pregnancy, this theoretical context, and studies in humans (Finn, et al. 1972), and varied animal species (e.g. armadillos (Anderson and Benirschke 1964)) drove the search for immune-suppressive factors. It was thought that expression of such factors in lymphoid and other tissues (e.g. placenta) would affect both systemic and local immunity of pregnant mice and support fetal tolerance. The CBA/J x DBA/2 model first arose in the context of delineating these putative suppressive factors (Clark, et al. 1980) as the model was held up as an example of adverse pregnancy outcome (resorption) in the face of deficiency of such suppression (Clark, et al. 1980). Continued study of the model was also a venue for examining the production of soluble suppressive factors produced in the placenta or decidua. For example, it was observed that a factor in normal fetal-placental units suppressed systemic NK cell activity but that this factor was not in CBA/J x DBA/2 (Gendron, et al. 1990). However these factors were not tested on purified populations of local (decidual) NK cells. Another issue that deserved analysis was, given this suppressor factor, the mechanisms which led to the resorption of some fetal-placental units and not others.
Early investigations suggested that within the uterus there is an early infiltration of a CD8 T cell population with suppressive activity which later gives way to a non-T cell suppressor population (Clark, et al. 1989). However, it was not clear the extent to which the infiltration of these cells is deficient in normal versus resorption-prone matings. Through examination of this model, another population of cells thought to provide site-specific suppressor activity was described as consistent with gamma-delta T cells (Clark, et al. 1997) and while depletion on day 8-9 of gestation increased the resorption rate (Arck, et al. 1999), peri-implantation depletion of this T cell subset had no effect. Although apparently not available in the CBA/J strain background, generally available strains of mice deficient in this particular cell type are not known to have an overt pregnancy failure (see http://jaxmice.jax.org).
Is the model evidence of antigen specific, T cell-mediated resorption?
CBA/J x DBA/2pregnancies are “semi-allogeneic” in that CBA/J are H-2 k and DBA/2 mice are H-2 d. A potential myriad of minor and other antigens might be relevant in the immune response of the CBA/J immune system against DBA/2 cells, yet the specific antigenic focus of the resorption-generating immune response is not known. Nor is it clear which cell is the primary effector. Histologic and other findings suggest NK cells (de Fougerolles and Baines 1987, Gendron and Baines 1988) macrophages (Redecha, et al. 2009), and neutrophils (Gendron and Baines 1989) (Girardi 2011) play an important role in the pregnancy loss observed in this model. Over time however, the drive to understand pregnancy loss in the context of self/non-self theory lead to an examination of T cell biology, and in particular defects in T cell tolerance, as a major mechanism of pathology. Several studies suggested that pregnancy loss in the CBA/J x DBA/2model was related to altered T cell function. For example, low, but not high doses of cyclosporine A (Du, et al. 2007, Zhou, et al. 2008) blockade of T cell costimulation (Jin, et al. 2004, Zhu, et al. 2005), and over expression of the T cell regulatory molecule CTLA-4 (Li, et al. 2009) decreased resorptions.
These studies led to examinations in other mating combinations and suggested a role for T cell mediated resorption (Riella, et al. 2013). One of these important studies in another mating combination investigated the role of the molecule indoleamine 2, 3-dioxygenase (IDO) which catabolizes tryptophan and thus limits T cell proliferation (Mellor and Munn 2001, Munn, et al. 1998). While the presence of T cells and an alllogeneic mating was required for pregnancy loss in response to inhibition of IDO, no T cell antigen specificity was demonstrated (e.g. specific attack of antigen-expressing fetal-placental units versus non antigen expressing units in the same uterus) suggesting that the T cell response may have been a ‘bystander’ effect but not the primary mover of the immune response. It should also be noted that deficiency in IDO does not negate pregnancy in other strain combinations (Riella, et al. 2013).
While there is no direct evidence that inhibition of this molecule increases resorption in the CBA/J X DBA/2 model, the idea that altered IDO expression could be the basis for pregnancy loss in the CBA/J X DBA/2 model led to experiments showing that enhanced exposure to molecules such as CTLA-4 correlates with decreased resorption and increased IDO expression (Li, et al. 2009). Here again however, while these models presume that it is an anti-allogeneic response that leads to resorption the exact antigen has not been delineated, and the specific response has not been demonstrated.
Generation of “protective immunity” against pregnancy loss
Another critical element suggesting the role of immune modulation in the CBA/J x DBA/2 model was developed either concurrent to or in response to the finding that a history of recurrent miscarriage could be ameliorated by transfusion with allogeneic leukocytes (Taylor and Faulk 1981). Investigators discovered that while administration of DBA/2 or CBA/J spleen cells about one week prior to mating did not decrease the resorption rate, administration of ‘third party’ Balb/c spleen cells did (Bobe, et al. 1986, Chaouat, et al. 1983), as long as it occurred within 8 weeks before mating with a DBA/2 male (Baines, et al. 1996). Pre-immunization with spleen cells from DBA/2 by Balb/c recombinant strains led to differing results, with some strains decreasing and other increasing the resorbtion rate (Kiger, et al. 1985). Early studies suggested that exposure to castrated Balb/c males or even dirty Balb/c male bedding in was protective DBA/2- mated CBA/J females (see Table 2 for representative manipulations of the model and associated references). These studies were precursors to others providing the observation that exposure to seminal plasma fluids and likely seminal plasmid-related antigenic peptides presented in the context of MHC mediate decreased resorption (Clark, et al. 2013). Prior mating to a Balb/c male is also protective, as long as subsequent pregnancy with a DBA/2 male occurred within 6 weeks (Baines, et al. 1996).
Table 2.
Representative manipulations of CBA × DBA matings
| Baseline Resorption Rate* (%) | Manipulation (Injection unless noted) | Resulting Resorption Rate* (%) | References |
|---|---|---|---|
| Decreased resorption rate | |||
| 31 | Second pregnancy | 1 | (Chavez, et al. 1987) |
| 30-35 | Second pregnancy | 15 | (Gendron, et al. 1992) |
| 23 | Add normal Balb/c male to cage on day 6 of 1st pregnancy | 4 | (Baines, et al. 1994) |
| 23 | Add castrated Balb/c male to cage on day 6 of 1st pregnancy | 9 | (Baines, et al. 1994) |
| 23 | Add Balb/c male bedding to cage on day 6 of 1st pregnancy | 9 | (Baines, et al. 1994) |
| 30 | Specific Pathogen free room | 8 | (Hamilton and Hamilton 1987) |
| 20-35 | Balb/c male spleen cells prior to mating# | 5-15 | (Kiger, et al. 1985) |
| 35 | Balb/c male spleen cells prior to mating | 20 | (Clark, et al. 2008a) |
| 20-35 | Balb/c male spleen cells prior to mating | 10 | (Clark, et al. 2013) |
| 20-35 | Balb/c spleen cells pre- incubated with Balb/c seminal plasma prior to mating | 3 | (Clark, et al. 2013) |
| 20-35 | DBA spleen cells pre-incubated with Balb/c seminal plasma prior to mating | 10 | (Clark, et al. 2013) |
| 30 | Spleen cells from Balb/c × DBA recombinant inbred strains K,N,C prior to mating | 5-17 | (Kiger, et al. 1985) |
| 24 | CBA bone marrow-derived dendritic cells pulsed with lysate of DBA male spleen cells | 5 | (Blois, et al. 2004) |
| 24 | Anti CD11b antibody iv on day 6 | 12 | (Duclos, et al. 1994) |
| 27 | Anti- CD8 and anti- CD86 | 9 | (Jin, et al. 2005) |
| 24 | Anti- CD86 | 8 | (Zhao, et al. 2007) |
| 28 | Anti-CD86 | 7 | (Zhu, et al. 2005) |
| 20 | 5mg/kg Cyclosporin A | 3 | (Du, et al. 2007) |
| 27 | 5mg/kg Cyclosporin A | 16 | (Li, et al. 2009) |
| 27-30 | Adenovirus –driven overexpression of CTLA-4 day 5 | 12 | (Li, et al. 2009) |
| 23 | i.vag. injection TGF-β day 0.5 | 12 | (Clark, et al. 2008a) |
| 70 | Interleukin (IL) 10 from culture supernatant days 6,8,10 | 5 | (Chaouat, et al. 1995) |
| 35 | Tumor Necrosis Factor(TNF) inhibitor (Pentoxifillin) days 6,8,10 | 20 | (Chaouat, et al. 1995) |
| 35 | Anti γ-interferon early in gestation | 15 | (Chaouat, et al. 1995) |
| 35 | TNF inhibitor + anti γ-IFN early in gestation | 10 | (Chaouat, et al. 1995) |
| 46-55 | IL-3 days 6.5-10.5 | 19-28 | (Chaouat, et al. 1990) |
| 35-40 | Interferon-τ | 5-10 | (Chaouat, et al. 1995) |
| 43 | IL-2 daily for 10 day from 4 days prior to mating | 15 | (Chen, et al. 2013) |
| 31 | C57Bl/6 male spleen and thymus cells | 8 | (Chavez, et al. 1987) |
| 31 | DBA male spleen and thymus cells | 12 | (Chavez, et al. 1987) |
| 27 | T cells from anti-B7 treated non-pregnant mice on day 4 gestation | 11 | (Jin, et al. 2004) |
| 18-20 | CD4+ CD25+ T cells from day 14 CBA × Balb/c females on day 0-2 of pregnancy | ~0 | (Zenclussen, et al. 2005) (Wafula, et al. 2009) |
| 20 | CD4+ CD25+ T cells from day 14 CBA × Balb/c females on day 0-2 of pregnancy + anti CTLA4 day 0,3,6,9 pregnancy | ~0 | (Wafula, et al. 2009) |
| 29 | Fresh CD4+CD25+ T cells from non-pregnant CBA females day 1-4 of pregnancy | 19-22 | (Yin, et al. 2012) |
| 29 | In vitro expanded CD4+CD25+ T cells from non-pregnant CBA females day 1-4 of pregnancy | 10-12 | (Yin, et al. 2012) |
| 30 | 3 × 105 regulatory B cells day 0 of pregnancy | ~0 | (Jensen, et al. 2013b) |
| 20 | Lipopolysaccharide (LPS) 2 weeks before mating | 9 | (Baines, et al. 1996) |
| 20 | Complete Freund's Adjuvant 2 weeks before mating | 10 | (Baines, et al. 1996) |
| 43 | Flt-3$ 6 days before mating | 14 | (Chen, et al. 2013) |
| 29 | Anti Crry Ig | 10 | (Girardi, et al. 2006) |
| 29 | Anti C5a | 10 | (Girardi, et al. 2006) |
| 29 | C5a receptor antagonist peptide | 10 | (Girardi, et al. 2006) |
| 30 | Prevastatin | 10 | (Redecha, et al. 2009) |
| Increased resorption rate | |||
| 19 | Second experiment, same publication (change housing) | 37 | (Clark, et al. 1994) |
| 19 | Third experiment, same publication (change housing) | 45 | (Clark, et al. 1994) |
| 43 | TNF ~ day 5.5 | 52 | (Chaouat, et al. 1990) |
| 28 | TNF ~day 7 | 98 | (Chaouat 1994) |
| 43 | TNF ~9.5 | 88 | (Chaouat, et al. 1990) |
| 43 | TNF ~13.5 | 67 | (Chaouat, et al. 1990) |
| 28 | γ-IFN ~day 7 | 75 | (Chaouat 1994) |
| 30 | IL-2 ~day 7 | 65 | (Chaouat 1994) |
| 50 | Anti IL-10 days 6,8,10 | 80 | (Chaouat, et al. 1995) |
| 19 | Anti GMCSF day 7.5 | 38 | (Clark, et al. 1994) |
| ? | LPS on day 0.5 | 18 | (Clark, et al. 2008a) |
| 38 | LPS ~day 7 | 100 | (Chaouat, et al. 1990) |
| 28-30 | LPS ~day 7 | 55-58 | (Chaouat 1994) (Clark, et al. 2004) |
| 24 | Poly I :C | 65 | (Duclos, et al. 1994) |
| 30 | Balb/c × DBA recombinant inbred strains L,E | 50-62 | (Kiger, et al. 1985) |
| 10-23 | Sound stress for 24 hours on day 5.5 | 44 | (Prados, et al. 2011) |
| 10 | Sound stress for 24 hours on day 5.5 | 30 | (Arck, et al. 1999) |
| 33 | Anti CD25 antibody day 1 | 67 | (Chen, et al. 2013) |
| 10 | Anti- T cell receptor antibody day 5.5 | ~100 | (Arck, et al. 1997) |
| 11 | Anti-γδ T cell antibody day 8.5 | 44-48 | (Arck, et al. 1999) |
| 19 | Anti- CD8 antibody day 6.5 | 60 | (Clark, et al. 1994) |
| 45 | Anti- CD8 antibody day 6.5 | 78 | (Clark, et al. 1994) |
| 20 | CD4+ CD25+ T cells from day 14 CBA × Balb/c females on day 0-2 of pregnancy + anti PD1 day 0,3,6,9 pregnancy | ~30 | (Wafula, et al. 2009) |
| No change resorption rate | |||
| 25 | Prior delivery with Balb/c males 6 weeks before mating | (Baines, et al. 1996) | |
| 20 | Second pregnancy with rapid re-mating (seven days) after delivery | (Baines, et al. 1996) | |
| 20-35 | CBA male spleen cells 7 days prior to mating | (Clark, et al. 2013) | |
| 20-35 | DBA male spleen cells 7 days prior to mating | (Clark, et al. 2013) | |
| 20-35 | Non-pregnant female Balb/c spleen cells ip 7 days prior to mating | (Kiger, et al. 1985) | |
| 20-35 | Male Balb/b or Balb/k spleen cells ip 7 days prior to mating | (Kiger, et al. 1985) | |
| 27 | Balb/c spleen cells 8 weeks before mating | (Baines, et al. 1996) | |
| 20-35 | Balb/c spleen cells pre-incubated with DBA seminal plasma | (Clark, et al. 2013) | |
| 11 | Anti Vgamma1.1, Anti Vdelta 6 antibody day 5.5 | (Arck, et al. 1999) | |
| 30 | Spleen cells from Balb/c × DBA recombinant inbred Strains M,J,D | (Kiger, et al. 1985) | |
It appears that mostly male cells were given, although this is not always easily discernible, and in some cases it was observed that administration of female spleen cells did not decrease resorption (Kiger, et al. 1985). Moreover, the number of spleen cells given varied, with some giving up to 50 million male spleen cells (Chaouat, et al. 1995). A rather unique immunization protocol using C57BL/6 (not Balb/c) male spleen and thymus cells an multiple immunizations both prior to pregnancy up to day 5 of gestation produced decreased resorptions in DBA/2- mated CBA/J females. However, continued exposure into a second pregnancy increased the resorption rate to that seen in unmanipulated CBA/J x DBA/2 pregnancies (Chavez, et al. 1987), again suggesting fluidity in the protective effect.
While attention was focused on finding the mechanisms leading to enhanced suppression of anti-DBA/2 immunity in CBA/J mothers, the results of pre-pregnancy immunization protocols led to differing findings in peripheral tissues versus decidua. For example, very early sets of observations suggested that although pre-pregnancy immunization apparently decreased the rate of resorbtion, it did not correlate with suppression of maternal spleen T cell proliferation, or generation of anti-DBA/2 cytotoxic T cell activity in vitro in some cases (Bobe, et al. 1986), while in others it apparently did (Chaouat, et al. 1985). Moreover, while pre-pregnancy immunization of CBA/J females before mating with DBA/2 males increased the apparent ability for placental cells to suppress NK cell mediated lysis in this model, several normal mating combinations expressed similar low levels of such suppression as the unmanipulated CBA/J mothers (Chaouat, et al. 1985). An additional finding related to the protective effect of immunization was the elaboration of an anti-paternal MHC antibody or serum protein that possessed a suppressive factor, underlying the complexity of the ‘protective’ effect of immunization (Chaouat, et al. 1985).
T helper cytokine biology and the ‘CBA/J x DBA’ model
Very early studies of the CBA/J x DBA/2 model had suggested that two types of immune response were present in the placenta and decidua, one protective and the other harmful. The idea that successful pregnancy was dependent on limitation of maternal immunity evolved to include the idea that pregnancy was dependent on regulating/suppressing the expression of T helper (TH)1-type immunity and enhancing TH2-type immune responses (Krishnan, et al. 1996, Lin, et al. 1993). This idea was supported by several studies in the CBA/J x DBA/2model where investigators observed deficiency in cytokines such as IL-4 and IL-10 in fetal-placental units of CBA/J X DBA/2pregnancies as compared to normal pregnancies in unrelated strains (Chaouat, et al. 1995) and increased TH1-type systemic responsiveness of CBA/J maternal T cells (Jin, et al. 2006). Moreover administration of TH1 cytokines alone, such as Tumor Necrosis Factor (TNF) and γ-interferon greatly increased resorption rates (Chaouat 1994). Further, the investigators observed that post-implantation antibody-mediated depletion of interleukin 10 (IL-10) resulted in increased pregnancy loss while administration of exogenous IL-10 had the opposite effect (Chaouat, et al. 1995). Related studies also suggested that enhanced expression of these cytokines is a mechanism by which pre pregnancy immunization of CBA/J females decreases the resorption rate subsequently observed (Chaouat, et al. 1995). The issue continued to be studied and yielded data suggesting that CBA/J X DBA/2 pregnancies, as compared to ‘non-resorbing’ CBA/J X Balb/c produced CD4 T cells with adhesion molecules consistent with a TH2-like phenotype (Jiang, et al. 2009). Demonstration of normal pregnancy, despite immunization against paternal antigens in mice deficient in IL-4 (Bonney 2001), and IL-10 (Bonney and Onyekwuluje 2004) (among others) and several studies showing the importance of TH1-type cytokines in normal placental development and decidual function e.g. (Ashkar, et al. 2000, Chaouat, et al. 2002, Monk, et al. 2005) suggested that a TH1/ TH2 paradigm alone was too simplistic to explain resorption in the CBA/J X DBA/2 model in particular and maternal tolerance in general (Chaouat, et al. 2004, Mas, et al. 2008).
Alternative models of immune system activation and the ‘CBA/J x DBA’ model
Although the CBA/J X DBA/2 model delineated complexities of the cytokine milieu present at the maternal-fetal interface use of the model continued to support the fundamental theory of self/non-self discrimination and the critical dependence of successful pregnancy on the regulation, limitation and deviation (towards certain classes of response) of local or systemic maternal immunity. The mid ‘90s brought forth alternative theories about the role of tissues, antigens and the immune system in the generation of T cell activation. One model (Matzinger 1994) suggested that it was not non-self recognition, but the presence of metabolic dysfunction, necrosis, or other fundamental tissue or cellular-level dysregulation that generated the signals to initiate the immune response. These ‘Danger signals’, in the course of this process could lead to activation of local cells whose primary purpose was processing and presentation of antigen (professional antigen presenting cells (Matzinger 1994) and the generation of both primary and costimulatory signals used by T cells to achieve full activation. A critical assertion of the theory was that as long as a tissue was functioning normally, and not undergoing any stress, damage or fundamental dysregulation, there was no need to suppress, limit or deviate the immune system in order to prevent autoreactive T cell-mediated damage. This theory suggested that this should be true for the fetus, even though the fetus might be ‘semi-allogeneic’ to the mother (Bonney 2007). An alternative to the Danger theory suggested that immune system activation was primarily generated through recognition of evolutionary or infectious non-self’ (Janeway 1992) but kept many tenets of self/non self discrimination theory. This later alternative posited that immune system activation occurred based on recognition of molecular patterns that comprised bacterial products (Janeway Jr, et al. 1996). Investigators working with the CBA/J x DBA/2 model appeared to meld the specifics of the two models to explain some of the peculiar findings observed in DBA/2-mated CBA/J mice.
One such finding was the wide variation in resorption rate which appeared to be increased in dirtier conventional housing versus “clean” housing (Clark, et al. 1997). It was further observed that injection of lipopolysaccharide (LPS) also induced resorption when given intravenously 7 days after mating (Chaouat, et al. 1990), and that injection of inflammatory cytokines such as TNF mimicked (Chaouat, et al. 1990) and augmented (Chaouat 1994, Clark, et al. 2004) the LPS response. However this effect was also observed in non abortion-prone matings, even if to a lesser extent (Chaouat, et al. 1990). When it was later observed that the effect of LPS and other molecules on resorptions was indeed dependent on Toll- like receptor (TLR) signaling (Clark, et al. 2003), this and related mechanisms were thought to comprise a ‘third signal’ or pathway by which resorptions could occur in this mating combination. The finding that immunization with Balb/c spleen could counteract resorption due to early LPS or TNF-related administration, but not preterm delivery due to late administration of the molecules in DBA/2- mated CBA/J mice (Chaouat 1994) suggested to some that resorption was in part due to mechanisms that were unique to ‘Danger’ and fell within the context of failed maternal tolerance in the classical (self/nonself) sense.
Another observation generated by this mating combination yet difficult to explain by classic immune theory was that exposure to sound stress for 24 hours on day 5.5 of pregnancy increased the resorption rate (Arck, et al. 1995). This was associated with expression of inflammatory cytokines (Arck, et al. 1995), adhesion molecules important in lymphocyte trafficking (Prados, et al. 2011) and a decrease in suppressive molecules mediating T cell metabolism (Blois, et al. 2005), and was ameliorated by administration of Balb/c spleen cells. As these studies developed, it was hypothesized that sound stress changed intestinal permeability and was similar to systemic injection with LPS (Clark, et al. 2004). While this was supported by other studies in the literature (Bijlsma, et al. 2001) it was not definitively proven in this model. It could be said that the investigators using the CBA/J X DBA/2 model did not see these observations as strongly arguing against either self/non-self discrimination theory or its corollary, that lack of inherent suppression or limitation of the maternal immune system was the primary pathway by which resorptions occurred in this model. Work continued to find other mechanisms supportive of a suppressive process.
However, according to the ‘Danger’ theory, signals through TLR and other molecules recognizing pathogen-associated molecular patterns comprise a particular subset of the wide range of possible ‘Danger’ signals. Potentially any molecule that is deregulated in expression, location or configuration (folding, Seong and Matzinger 2004) could, in the right context during pregnancy (Bonney 2007), signal ‘Danger’ is occurring in a cell or tissue and activate local antigen presenting cells. Even complement itself could serve as a Danger signal (Kwan, et al. 2012). From this perspective, the task of understanding the high resorption rate present in the CBA/J x DBA/2 model might be framed as having two components: i) the effort to incorporate existing data that suggest fundamental dysregulation in the critical tissues present at the maternal-fetal interface and ii) the search to find mechanisms by which this dysregulation occurs.
Published observations have noted abnormal decidual vascular modification (Dixon, et al. 2006) altered expression of VEGF and its receptor (Girardi, et al. 2006) decreased blood perfusion (Redecha, et al. 2009), decreased trophoblast giant cells (Girardi, et al. 2006), increased fibrin deposition (Redecha, et al. 2009), increased Tissue factor expression (Redecha, et al. 2009), and increased thrombin activity disregulation (Clark, et al. 1998). Increased placental/decidual expression of complement component C3 is also an element of the model that is thought to promote increased influx of macrophages and neutrophils and local expression of TNF (Girardi, et al. 2006). Many of these abnormalities suggest comparison with the innate immune response following ischemia-reperfusion injury (Zhang and Carroll 2007). Moreover, this mating combination suffers from increased oxidative stress that can be remediated by over expression of heme-oxygenase-I (Zenclussen, et al. 2006). In addition, there is evidence suggesting a differential expression of a placental ATPase in this combination as compared to normal or syngeneic Balb/c matings (Jaiswal, et al. 2011), and this is correlated with altered macrophage function in the placenta. However, while LPS can cause this altered ATPase expression, it is not clear whether altered macrophage function drives or is driven by altered ATPase expression in this abortion-prone model (Jaiswal, et al. 2011). Early (Muzikova and Clark 1995) and later studies (Brown, et al. 2013) also suggest that abnormalities existing in the CBA/J decidua and its interaction with the developing embryo are the prime movers of the pathology related to these pregnancies, and this idea is supported by the fact that “reverse” matings, between DBA/2 females and CBA/J males are considered normal (Dixon, et al. 2006). Finally, this mating combination suffers from a fundamental disorganization of DNA methylation (Brown, et al. 2013). Thus the CBA/J x DBA/2mating combination is abnormal on many levels potentially independent of primary immune system disregulation.
Since the original description of increased pregnancy failure in CBA/J dams mated to DBA/2 males, most reports have focused on the pregnancy failure aspect of the model and have not commented about a maternal phenotype. A 2010 report (Ahmed, et al. 2010) notes that DBA/2-mated CBA/J females develop significant proteinuria by day 12 of gestation, indicating some sort of pregnancy-induced renal damage. Light and electron microscopic analysis of the maternal kidneys of these dams showed evidence of endothelial injury and diminished blood flow, consistent with the proteinuria. Although DBA/2-mated CBA/J dams did not develop elevated blood pressure during pregnancy, they were markedly more sensitive to a challenge with angiotensin II than were control animals, suggesting a generalized effect of pregnancy on the maternal vasculature. This was further corroborated by ex-vivo studies showing a stronger response of the aortic ring to angiotensin II in DBA/2mated CBA/J dams. These studies highlight a potential fundamental and systemic dysregulation underlying this mating.
Regulatory T cells: resurgence of immune modulation and the ‘CBA/J x DBA’ model
The resurrection of the suppressor T cell as a CD4+ CD25+ regulatory T cell expressing the Forkhead transcription factor Foxp3 (Fontenot, et al. 2003) opened a new avenue for those who sought to explain, in the context of self/non-self discrimination, why there was increased resorption in the CBA/J x DBA/2 model and why pre-pregnancy immunization with Balb/c spleen cells, exposure to Balb/c seminal plasma (Clark, et al. 2013) or administration of specific cytokines such as TGF beta (Clark, et al. 2008a) decreased the resorption rate.
Several lines of evidence suggested that ‘naturally’ occurring T regs were a specific lineage, bearing FoxP3, highly dependent on IL-2, and expressing molecules such as CD25 and CTLA4. It is thought that these cells resulted from having a high affinity for and seeing ‘self’ antigen in the thymus. The now-named ‘tT-reg’ (Abbas, et al. 2013) were thought to leave the thymus into the periphery as fully mature and functional cells, many expressing the memory cell markers (e.g. CD44hi, CD62Llo), that could suppress other subsets of self-reactive T cells (reviewed in Sakaguchi, et al. 2008). In addition, investigators have observed that exposure of naïve T cells in the periphery to antigen in the context of TGF-beta, IL-2, retinoic acid or signals via Fms-related tyrosine kinase 3 ligand, could produce a ‘pT reg’ (Abbas, et al. 2013) T cell with regulatory function (Sakaguchi, et al. 2008).
In contrast, exposure to antigen in the context of TGF-beta and IL-6 could generate TH17 T cells. The stability of T-reg and TH17 phenotypes may not be strong (Sakaguchi, et al. 2013), as conversion can occur in the context of hormonal disregulation (Li, et al. 2013) severe infection (Rowe, et al. 2012a, Zhang, et al. 2012) and TLR signaling (Nyirenda, et al. 2011). Understanding the various mechanisms by which thymic or peripheral T regs exert their effects on T cells is ongoing, and can include suppression of cytokine production and proliferation (Thornton and Shevach 1998) direct killing (Abdulahad, et al. 2011) and modulation of function(Collison, et al. 2009) (Zelinskyy, et al. 2013).
Regulatory T cell dysfunction has been implicated for many years in mouse models of premature ovarian failure and autoimmune oophoritis and other reproductive track-related autoimmune diseases (Bonomo, et al. 1995, Tung and Teuscher 1995). Investigators observed that administration of CD4+ CD25+ spleen and thymus cells from a day 14 normally pregnant (Balb/c-mated) CBA/J female to a DBA/2-mated CBA/J female on day 0-2 of pregnancy decreased the resorption rate (Wafula, et al. 2009, Zenclussen, et al. 2005), while antibody to CD25(Chen, et al. 2013) increased the rate of resorption and blockade of PD-1 (Wafula, et al. 2009) and abrogated the protective effect of T reg administration. These experiments did not delineate the specificity of the regulatory T cell pool, and indeed it has been shown that CD4+ CD25+ cells with regulatory capacity, potentially naturally occurring, thymus derived T regs, expand during syngeneic as well as allogeneic pregnancies (Aluvihare, et al. 2004, Teles, et al. 2013). Moreover, like many of the experiments done in this model, the T regulatory administration was not done in a pregnancy where neither the role of the specificity nor the level of target paternal antigen expression (for example a CBA/J x (CBA/J x DBA/2 F1) backcross) on resorption could be assessed.
Experiments in this model have suggested that in vitro activated and expanded CD25+ CD4+ versus naïve T regs were effective in decreasing resorption (Yin, et al. 2012). In other models, it has also been observed that shared expression of antigen in the maternal thymus and the fetus markedly enhances antigen-specific T reg proliferation in the uterine draining lymph nodes (Chen, et al. 2013). However, these observations do not prove that exposure to specific antigen is directly related to generation of T reg function and decreased resorptions specifically in antigen–expressing sites. Moreover, while expansion of such cells is thought to occur at the level of the uterine draining lymph nodes (Teles, et al. 2013), it is not clear where and to what extent the transferred C25+CD4+ cells traffic in the process of decreasing resorptions in this model.
Does the presence of T regs and the effects of their modification prove self/non-self theory, or that pregnancy is critically dependent on suppression, deviation or limitation of the maternal immune system? Are T regs critical to successful semi-allogeneic pregnancy? While many users and proponents of the CBA/J x DBA/2 model may still hold to this thinking (Chen, et al. 2013), there is an alternative. For example, it is known that T cells with regulatory function and memory are generated by antigen exposure in the right context (TGF-beta etc) during pregnancy (Rowe, et al. 2012b). It could be said that these cells are essentially another flavor of T cells which have been revealed by the experimental context generated. Moreover, they, along with their partner, TH17 cells represent a similar balance of reactivity, dependent on tissue need and tendency (Matzinger and Kamala 2011) as that ascribed to the TH2 –TH1 paradigm of some years ago. It is even possible, if not likely that there are several flavors of T reg cells perhaps ones that specifically regulate certain types of inflammation (Bizargity, et al. 2009). These cells are not the ‘Sang Real’ of tolerance. Danger, in the form for example of intracellular infection, can decrease the presence and activity of T regs, and generate anti-fetal immunity (Rowe, et al. 2012a). While such a situation is obviously harmful to the fetus, it is protects the mother against infection and other losses associated with carrying a potentially abnormal fetus to term.
Another possibility, that includes naturally occurring T regs, is that these cells exist to limit “collateral damage” (Thangavelu, et al. 2013) or ‘bystander effect (Anderson 2006)”. In this vein the original Danger model dealt with the generation of auto-reactive T cells in the context of an immune response to infection by suggesting that tissues, especially large and fast growing tissues could essentially “out run” the effects of auto-reactive T cells (Matzinger 1994), while small, slow growing tissues could not (Anderson, et al. 2001). It is possible then to see T regs, especially the naturally occurring ones which tend to be tissue specific (del Rio, et al. 2011), as a possible mechanism by which specific tissues may ‘out run’ bystander auto-reactive T cell generation. Self/non-self theory may strongly depend on the existence of T regs. While they are not critical, they can be incorporated into to this alternative theory.
Driving where? The future of the ‘CBA/J x DBA’ model
The recent observation that there is a very pronounced maternal renal/vascular phenotype in the CBA/J x DBA/2 model (Ahmed, et al. 2010) and that the model relates only to first pregnancies (Singh, et al. 2011) has several important implications. First, the fact that the mothers are so profoundly affected by the abnormal pregnancies strongly implies that the CBA/J x DBA/2 model should not be interpreted as being a model for most human first trimester miscarriages. In the majority of human miscarriages, there is no evidence of any sort of maternal renal/vascular disease although pregnancy loss can be the first manifestation of disease (Yin, et al. 2013). In fact, pregnancy failure secondary to severe maternal illness caused by the pregnancy itself is relatively rare, and the pregnancy “failures” are usually the consequence of deliberate termination procedures. Second, the fact that the CBA/J x DBA/2 model relates almost entirely to first pregnancies, makes one question its relevance to recurrent pregnancy loss in humans. The generally understood concept behind recurrent pregnancy loss is that there is some process at the maternal-fetal interface that similarly affects all pregnancies. If the pathophysiological mechanism in the CBA/J x DBA/2 model were similar to the human situation, then women who had had one pregnancy loss would be expected to have a diminished probability for a subsequent pregnancy to end in miscarriage. In fact, the evidence suggests that women who have had one miscarriage have a somewhat increased probability for loss in a subsequent pregnancy (Regan, et al. 1989). On this basis, there may be concern regarding the potential to gain general understanding of most human recurrent miscarriages.
However, the observations generated in the CBA/J x DBA/2model show the capacity for the maternal immune system to respond to signals presented by its environment. Taken together the observations that i) T-regs can be increased either through administration of third-party spleen cells or other exposure to seminal plasma antigens, ii) there is a significant amount of tissue level metabolic and other dysregulation, iii) resorption is associated with a significant innate immune response: neutrophils, macrophages, dendritic cells, inflammatory cytokines, suggest that a likely role for T cells with regulatory function in this model are as modulators of innate immunity. This is in keeping with what has been observed in the non pregnant state (Maloy, et al. 2003), suggested by studies of the regulation of LPS-induced preterm birth in a syngeneic mouse mating (Bizargity, et al. 2009), and inferred from studies of human preterm birth (Ito, et al. 2010). In this light the model could be very useful in understanding the intricate metabolic and developmental programming particular to the maternal-fetal interface, further delineation of the ‘Danger’ signal or ‘signal 0’, and further understanding of the complex and intricate mechanisms by which adaptive immunity might exert feedback control of innate immunity. This model could also examine the role of the recently ‘re-recognized’ innate lymphoid cells, including Natural Killer T cells (Boyson, et al. 2006) and the non-T cell receptor expressing counterparts of known T helper cell subsets (Lane, et al. 2012, Spits and Cupedo 2012). Moreover, recent data suggest that transfer of CD19+CD5+CD1d+ B cells iv, with concomitant increase in such cells in the spleen, also decreases resorptions (Jensen, et al. 2013a), and further examination of this cell type is warranted.
The mid 90s witnessed the use T cell receptor transgenic mice in the study of maternal tolerance (Tafuri, et al. 1995). Since then several investigators have produced evidence in these systems that the maternal T cells are “aware” that the host is pregnant (Bonney, et al. 2011), undergo proliferation and death (Norton, et al. 2010) and are able to respond to fetal antigens (Erlebacher, et al. 2007, Norton, et al. 2010). For most mice, neither the increased presence of T cells specific for fetal antigen nor manipulations to increase fetal antigen specific T cell proliferation or function has resulted in specific pregnancy loss (see review by Moldenhauer, et al. 2010). The CBA/J x DBA/2 model has perhaps encouraged the expanded use of TCR transgenic mice. In one particular model, baseline levels the overall fetal resorption rate is low, despite a high frequency of maternal T cells specific for an antigen transgenically expressed on the fetus (Chen, et al. 2013). Immunization against this antigen along with depletion of T regs with antibody to CD25 increased overall resorption several fold, however, the role of the level of fetal antigen expression in resorbed versus nonresorbed fetuses is yet to be delineated (Chen, et al. 2013). It might be very useful to generate a TCR transgenic mouse model within the CBA/J (expressing maternal specific anti-fetal T cells), DBA/2 (expressing specific fetal antigen) and Balb/c (expressing fetal antigen) backgrounds. If there is a specific fetal antigen that drives the resorptions in the CBA/J x DBA/2model, it needs to be found in order to do similar experiments based on responsiveness to that particular antigen.
More important than the generation of specific tools however is the willingness to engage in experimentation with the model in the context of evolving theory of how the immune system chooses between activation and tolerance. While several theories that might be considered (Anderson 2006, Anderson, et al. 2001, Cohn 2013, Zinkernagel and Hengartner 2004), we have herein give one possible interpretation of the data derived from the CBA/J x DBA/2 model that would allow for alternatives to fundamental self/non self discrimination theory as the basis for maternal tolerance. We posit that with minds open to this and other alternatives, we will be able to use the CBA/J x DBA/2 model to continue to produce observations that enhance our understanding of the critically important biology of successful pregnancy and its complications.
Acknowledgement
The authors acknowledge all of the years of hard work done by our colleagues and apologize for observations not cited due to space considerations. We thank Jonathon E Boyson for review of this work and for helpful suggestions.
Funding
This work was in part funded by NIH RO1 HD043185, R21AI081000, and P20 RR021905, the University of Vermont College of Medicine Department of Obstetrics, Gynecology and Reproductive Sciences, and the Vermont Center for Immunology and Infectious Disease.
Footnotes
Declaration of Interest
The authors report no conflict of interest that could be perceived as prejudicing the impartiality of research reported.
References
- Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DAA, Ziegler SF. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- Abdulahad WH, Boots AMH, Kallenberg CGM. FoxP3+ CD4+ T cells in systemic autoimmune diseases: the delicate balance between true regulatory T cells and effector Th-17 cells. Rheumatology. 2011;50:646–656. doi: 10.1093/rheumatology/keq328. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A new mouse model to explore therapies for preeclampsia. PLoS One. 2010;5:e13663. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Anderson CC. Time, Space and Contextual Models of the Immunity Tolerance Decision: Bridging the Geographical Divide of Zinkernagel and Hengartner's ‘Credo 2004’. Scandinavian Journal of Immunology. 2006;63:249–256. doi: 10.1111/j.1365-3083.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Anderson CC, Carroll JM, Gallucci S, Ridge JP, Cheever AW, Matzinger P. Testing time-, ignorance-, and danger-based models of tolerance. J Immunol. 2001;166:3663–3671. doi: 10.4049/jimmunol.166.6.3663. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Benirschke K. Maternal Tolerance of Foetal Tissue. Br Med J. 1964;1:1534–1535. doi: 10.1136/bmj.1.5397.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck PC, Ferrick DA, Steele-Norwood D, Croitoru K, Clark DA. Murine T cell determination of pregnancy outcome: I. Effects of strain, alphabeta T cell receptor, gammadelta T cell receptor, and gammadelta T cell subsets. Am J Reprod Immunol. 1997;37:492–502. doi: 10.1111/j.1600-0897.1997.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Ferrick DA, Steele-Norwood D, Egan PJ, Croitoru K, Carding SR, Dietl J, Clark DA. Murine T cell determination of pregnancy outcome. Cell Immunol. 1999;196:71–79. doi: 10.1006/cimm.1999.1535. [DOI] [PubMed] [Google Scholar]
- Arck PC, Merali FS, Manuel J, Chaouat G, Clark DA. Stress-triggered abortion: inhibition of protective suppression and promotion of tumor necrosis factor-alpha (TNF-alpha) release as a mechanism triggering resorptions in mice. Am J Reprod Immunol. 1995;33:74–80. doi: 10.1111/j.1600-0897.1995.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines MG, Duclos AJ, de Fougerolles AR, Gendron RL. Immunological prevention of spontaneous early embryo resorption is mediated by non-specific immunosimulation. Am J Reprod Immunol. 1996;35:34–42. doi: 10.1111/j.1600-0897.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Baines MG, Haddad EK, Pomerantz DK, Duclos AJ. Effects of sensory stimuli on the incidence of fetal resorption in a murine model of spontaneous abortion: the presence of an alien male and postimplantation embryo survival. J Reprod Fertil. 1994;102:221–228. doi: 10.1530/jrf.0.1020221. [DOI] [PubMed] [Google Scholar]
- Bijlsma PB, van Raaij MT, Dobbe CJ, Timmerman A, Kiliaan AJ, Taminiau JA, Groot JA. Subchronic mild noise stress increases HRP permeability in rat small intestine in vitro. Physiol Behav. 2001;73:43–49. doi: 10.1016/s0031-9384(01)00424-3. [DOI] [PubMed] [Google Scholar]
- Bizargity P, Del Rio R, Phillippe M, Teuscher C, Bonney EA. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol Reprod. 2009;80:874–881. doi: 10.1095/biolreprod.108.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois S, Alba Soto CD, Olmos S, Chuluyan E, Gentile T, Arck PC, Margni RA. Therapy with dendritic cells influences the spontaneous resorption rate in the CBA/J × DBA/2J mouse model. Am J Reprod Immunol. 2004;51:40–48. doi: 10.1046/j.8755-8920.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- Blois S, Tometten M, Kandil J, Hagen E, Klapp BF, Margni RA, Arck PC. Intercellular adhesion molecule-1/LFA-1 cross talk is a proximate mediator capable of disrupting immune integration and tolerance mechanism at the feto-maternal interface in murine pregnancies. J Immunol. 2005;174:1820–1829. doi: 10.4049/jimmunol.174.4.1820. [DOI] [PubMed] [Google Scholar]
- Bobe P, Chaouat G, Stanislawski M, Kiger N. Immunogenetic studies of spontaneous abortion in mice. II. Antiabortive effects are independent of systemic regulatory mechanisms. Cell Immunol. 1986;98:477–485. doi: 10.1016/0008-8749(86)90306-0. [DOI] [PubMed] [Google Scholar]
- Bobe P, Kiger N. Immunogenetic studies of spontaneous abortion in mice. III. Non-H-2 antigens and gestation. J Immunogenet. 1989;16:223–231. doi: 10.1111/j.1744-313x.1989.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Bonney EA. Maternal tolerance is not critically dependent on IL-4. Immunology. 2001;103:382–389. doi: 10.1046/j.1365-2567.2001.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA. Preeclampsia: a view through the danger model. Journal of Reproductive Immunology. 2007;76:68–74. doi: 10.1016/j.jri.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA. Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside. Am J Reprod Immunol. 2013;69:567–584. doi: 10.1111/aji.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA, Onyekwuluje J. Maternal tolerance to H-Y is independent of IL-10. Immunological Investigations. 2004;33:385–395. doi: 10.1081/imm-200032732. [DOI] [PubMed] [Google Scholar]
- Bonney EA, Shepard MT, Bizargity P. Transient modification within a pool of CD4 T cells in the maternal spleen. Immunology. 2011;134:270–280. doi: 10.1111/j.1365-2567.2011.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo A, Kehn PJ, Payer E, Rizzo L, Cheever AW, Shevach EM. Pathogenesis of post-thymectomy autoimmunity. Role of syngeneic MLR-reactive T cells. J Immunol. 1995;154:6602–6611. [PubMed] [Google Scholar]
- Boyson JE, Nagarkatti N, Nizam L, Exley MA, Strominger JL. Gestation stage-dependent mechanisms of invariant natural killer T cell-mediated pregnancy loss. Proc Natl Acad Sci U S A. 2006;103:4580–4585. doi: 10.1073/pnas.0511025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch DW, Gibson M, Silver RM. Recurrent Miscarriage. New England Journal of Medicine. 2010;363:1740–1747. doi: 10.1056/NEJMcp1005330. [DOI] [PubMed] [Google Scholar]
- Brown LY, Bonney EA, Raj RS, Nielsen B, Brown S. Generalized Disturbance of DNA Methylation in the Uterine Decidua in the CBA/J × DBA/2 Mouse Model of Pregnancy Failure. Biol Reprod. 2013;11:11. doi: 10.1095/biolreprod.113.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- Burnet FM. The immunological significance of the thymus: an extension of the clonal selection theory of immunity. Australas Ann Med. 1962;11:79–91. doi: 10.1111/imj.1962.11.2.79. [DOI] [PubMed] [Google Scholar]
- Chaouat G. Synergy of lipopolysaccharide and inflammatory cytokines in murine pregnancy: alloimmunization prevents abortion but does not affect the induction of preterm delivery. Cell Immunol. 1994;157:328–340. doi: 10.1006/cimm.1994.1231. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBA × DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol. 1995;154:4261–4268. [PubMed] [Google Scholar]
- Chaouat G, Kiger N, Wegmann TG. Vaccination against spontaneous abortion in mice. J Reprod Immunol. 1983;5:389–392. doi: 10.1016/0165-0378(83)90248-6. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Kolb JP, Kiger N, Stanislawski M, Wegmann TG. Immunologic consequences of vaccination against abortion in mice. J Immunol. 1985;134:1594–1598. [PubMed] [Google Scholar]
- Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. Reproductive immunology 2003: reassessing the Th1/Th2 paradigm? Immunol Lett. 2004;92:207–214. doi: 10.1016/j.imlet.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBA × DBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990;89:447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Zourbas S, Ostojic S, Lappree-Delage G, Dubanchet S, Ledee N, Martal J. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. Journal of Reproductive Immunology. 2002;53:241–256. doi: 10.1016/s0165-0378(01)00119-x. [DOI] [PubMed] [Google Scholar]
- Chavez DJ, McIntyre JA, Colliver JA, Faulk WP. Allogeneic matings and immunization have different effects on nulliparous and multiparous mice. J Immunol. 1987;139:85–88. [PubMed] [Google Scholar]
- Chen T, Darrasse-Jeze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G, Klatzmann D. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191:2273–2281. doi: 10.4049/jimmunol.1202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen OB, Kolte AM, Dahl M, Larsen EC, Steffensen R, Nielsen HS, Hviid TV. Maternal homozygocity for a 14 base pair insertion in exon 8 of the HLA-G gene and carriage of HLA class II alleles restricting HY immunity predispose to unexplained secondary recurrent miscarriage and low birth weight in children born to these patients. Hum Immunol. 2012;73:699–705. doi: 10.1016/j.humimm.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Steffensen R, Nielsen HS, Varming K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol Obstet Invest. 2008;66:257–267. doi: 10.1159/000149575. [DOI] [PubMed] [Google Scholar]
- Clark DA, Brierley J, Banwatt D, Chaouat G. Hormone-induced preimplantation Lyt 2+ murine uterine suppressor cells persist after implantation and may reduce the spontaneous abortion rate in CBA/J mice. Cell Immunol. 1989;123:334–343. doi: 10.1016/0008-8749(89)90294-3. [DOI] [PubMed] [Google Scholar]
- Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA. Cytokine-dependent abortion in CBA x DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase [correction of prothombinase]. J Immunol. 1998;160:545–549. [PubMed] [Google Scholar]
- Clark DA, Chaouat G, Mogil R, Wegmann TG. Prevention of spontaneous abortion in DBA/2-mated CBA/J mice by GM-CSF involves CD8+ T cell-dependent suppression of natural effector cell cytotoxicity against trophoblast target cells. Cell Immunol. 1994;154:143–152. doi: 10.1006/cimm.1994.1064. [DOI] [PubMed] [Google Scholar]
- Clark DA, Fernandes J, Banwatt D. Prevention of spontaneous abortion in the CBA x DBA/2 mouse model by intravaginal TGF-beta and local recruitment of CD4+8+ FOXP3+ cells. Am J Reprod Immunol. 2008a;59:525–534. doi: 10.1111/j.1600-0897.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- Clark DA, Manuel J, Lee L, Chaouat G, Gorczynski RM, Levy GA. Ecology of danger-dependent cytokine-boosted spontaneous abortion in the CBA x DBA/2 mouse model. I. Synergistic effect of LPS and (TNF-alpha + IFN-gamma) on pregnancy loss. Am J Reprod Immunol. 2004;52:370–378. doi: 10.1111/j.1600-0897.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Clark DA, McDermott MR. Impairment of host vs graft reaction in pregnant mice. I. Suppression of cytotoxic T cell generation in lymph nodes draining the uterus. J Immunol. 1978;121:1389–1393. [PubMed] [Google Scholar]
- Clark DA, McDermott MR, Szewczuk MR. Impairment of host-versus-graft reaction in pregnant mice. II. Selective suppression of cytotoxic T-cell generation correlates with soluble suppressor activity and with successful allogeneic pregnancy. Cell Immunol. 1980;52:106–118. [PubMed] [Google Scholar]
- Clark DA, Merali FS, Hoskin DW, Steel-Norwood D, Arck PC, Croitoru K, Murgita RA, Hirte H. Decidua-associated suppressor cells in abortion-prone DBA/2-mated CBA/J mice that release bioactive transforming growth factor beta2-related immunosuppressive molecules express a bone marrow-derived natural suppressor cell marker and gamma delta T-cell receptor. Biol Reprod. 1997;56:1351–1360. doi: 10.1095/biolreprod56.5.1351. [DOI] [PubMed] [Google Scholar]
- Clark DA, Petitbarat M, Chaouat G. How should data on murine spontaneous abortion rates be expressed and analyzed? Am J Reprod Immunol. 2008b;60:192–196. doi: 10.1111/j.1600-0897.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- Clark DA, Rahmati M, Gohner C, Bensussan A, Markert UR, Chaouat G. Seminal plasma peptides may determine maternal immune response that alters success or failure of pregnancy in the abortion-prone CBAxDBA/2 model. J Reprod Immunol. 2013;20:00052–00051. doi: 10.1016/j.jri.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Clark DA, Yu G, Arck PC, Levy GA, Gorczynski RM. MD-1 is a critical part of the mechanism causing Th1-cytokine-triggered murine fetal loss syndrome. Am J Reprod Immunol. 2003;49:297–307. doi: 10.1034/j.1600-0897.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Cohn M. Challenging the Tritope Model of T cell receptor structure-function relationships with classical data on ‘super’ and ‘allo-MHC’ antigens. Scand J Immunol. 2013;78:313–324. doi: 10.1111/sji.12092. [DOI] [PubMed] [Google Scholar]
- Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles AR, Baines MG. Modulation of the natural killer cell activity in pregnant mice alters the spontaneous abortion rate. J Reprod Immunol. 1987;11:147–153. doi: 10.1016/0165-0378(87)90018-0. [DOI] [PubMed] [Google Scholar]
- del Rio R, Sun Y, Alard P, Tung KS, Teuscher C. H2 control of natural T regulatory cell frequency in the lymph node correlates with susceptibility to day 3 thymectomy-induced autoimmune disease. J Immunol. 2011;186:382–389. doi: 10.4049/jimmunol.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ME, Chien EK, Osol G, Callas PW, Bonney EA. Failure of decidual arteriolar remodeling in the CBA/J x DBA/2 murine model of recurrent pregnancy loss is linked to increased expression of tissue inhibitor of metalloproteinase 2 (TIMP-2). Am J Obstet Gynecol. 2006;194:113–119. doi: 10.1016/j.ajog.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Du MR, Dong L, Zhou WH, Yan FT, Li DJ. Cyclosporin a improves pregnancy outcome by promoting functions of trophoblasts and inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in the mouse. Biol Reprod. 2007;76:906–914. doi: 10.1095/biolreprod.106.056648. [DOI] [PubMed] [Google Scholar]
- Duclos AJ, Pomerantz DK, Baines MG. Relationship between decidual leukocyte infiltration and spontaneous abortion in a murine model of early fetal resorption. Cell Immunol. 1994;159:184–193. doi: 10.1006/cimm.1994.1306. [DOI] [PubMed] [Google Scholar]
- Ecker JL, Laufer MR, Hill JA. Measurement of embryotoxic factors is predictive of pregnancy outcome in women with a history of recurrent abortion. Obstet Gynecol. 1993;81:84–87. [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk WP, McIntyre JA. Immunological studies of human trophoblast: markers, subsets and functions. Immunol Rev. 1983;75:139–175. doi: 10.1111/j.1600-065x.1983.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Faulk WP, Temple A, Lovins RE, Smith N. Antigens of human trophoblasts: a working hypothesis for their role in normal and abnormal pregnancies. Proc Natl Acad Sci U S A. 1978;75:1947–1951. doi: 10.1073/pnas.75.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R, St Hill CA, Govan AJ, Ralfs IG, Gurney FJ, Denye V. Immunological responses in pregnancy and survival of fetal homograft. Br Med J. 1972;3:150–152. doi: 10.1136/bmj.3.5819.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firkin BG, Howard MA, Radford N. Possible relationship between Lupus Inhibitor and recurrent abortion in young women. The Lancet. 1980;316:366. doi: 10.1016/s0140-6736(80)90361-x. [DOI] [PubMed] [Google Scholar]
- Fischer Lindahl K. On naming H2 haplotypes: functional significance of MHC class Ib alleles. Immunogenetics. 1997;46:53–62. doi: 10.1007/s002510050242. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Gendron RL, Baines MG. Infiltrating decidual natural killer cells are associated with spontaneous abortion in mice. Cell Immunol. 1988;113:261–267. doi: 10.1016/0008-8749(88)90025-1. [DOI] [PubMed] [Google Scholar]
- Gendron RL, Baines MG. Morphometric analysis of the histology of spontaneous fetal resorption in a murine pregnancy. Placenta. 1989;10:309–318. doi: 10.1016/0143-4004(89)90031-3. [DOI] [PubMed] [Google Scholar]
- Gendron RL, Farookhi R, Baines MG. Resorption of CBA/J × DBA/2 mouse conceptuses in CBA/J uteri correlates with failure of the feto-placental unit to suppress natural killer cell activity. J Reprod Fertil. 1990;89:277–284. doi: 10.1530/jrf.0.0890277. [DOI] [PubMed] [Google Scholar]
- Gendron RL, Farookhi R, Baines MG. Murine pregnancies predisposed to spontaneous resorption show alterations in the concentrations of leukotriene B4 and prostaglandin E2. Biol Reprod. 1992;47:72–75. doi: 10.1095/biolreprod47.1.72. [DOI] [PubMed] [Google Scholar]
- Girardi G. Role of tissue factor in feto-maternal development: a xiphos. J Thromb Haemost. 2011;9:250–256. doi: 10.1111/j.1538-7836.2010.04135.x. [DOI] [PubMed] [Google Scholar]
- Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RD, Simpson E, Samelson LE. In vitro cell-mediated immune responses to the male specific(H-Y) antigen in mice. J Exp Med. 1975;142:1108–1120. doi: 10.1084/jem.142.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MS, Hamilton BL. Environmental influences on immunologically associated spontaneous abortion in CBA/J mice. J Reprod Immunol. 1987;11:237–241. doi: 10.1016/0165-0378(87)90060-x. [DOI] [PubMed] [Google Scholar]
- Heine O, Neppert J, Mueller-Eckhardt G. Influence of immunization with allogeneic spleen cells on the number of viable neonates in mice. J Reprod Immunol. 1989;15:169–173. doi: 10.1016/0165-0378(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Ho HN, Chen SU, Yang YS, Huang SC, Lee TY, Gill TJ., 3rd Age, environment, and lymphocyte immunization influence the spontaneous resorption rate in the CBA/J × DBA/2J mouse model. Am J Reprod Immunol. 1994;31:47–51. doi: 10.1111/j.1600-0897.1994.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Inada K, Shima T, Nakashima A, Aoki K, Ito M, Saito S. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J Reprod Immunol. 2013;97:104–111. doi: 10.1016/j.jri.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K, Nikaido T, Saito S. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84:75–85. doi: 10.1016/j.jri.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Jaiswal MK, Gilman-Sachs A, Chaouat G, Beaman KD. Placental ATPase expression is a link between multiple causes of spontaneous abortion in mice. Biol Reprod. 2011;85:626–634. doi: 10.1095/biolreprod.111.092494. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Goodnow CC, Medzhitov R. Immunological tolerance: Danger – pathogen on the premises! Current Biology. 1996;6:519–522. doi: 10.1016/s0960-9822(02)00531-6. [DOI] [PubMed] [Google Scholar]
- Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 Cells Restore Pregnancy Tolerance in a Mouse Model. Biol Reprod. 2013a doi: 10.1095/biolreprod.113.110791. [DOI] [PubMed] [Google Scholar]
- Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 Cells Restore Pregnancy Tolerance in a Mouse Model. Biol Reprod. 2013b;28:28. doi: 10.1095/biolreprod.113.110791. [DOI] [PubMed] [Google Scholar]
- Jiang PJ, Lin QD, Bao SM, Zhao AM, Zhang Y, Xiao SJ. Relationship between expression of chemokine receptors CCR3, CCR5 and CXCR3 on CD4(+) T cells and spontaneous abortion in mice. Chin Med J (Engl) 2009;122:390–395. [PubMed] [Google Scholar]
- Jin LP, Li DJ, Zhang JP, Wang MY, Zhu XY, Zhu Y, Meng Y, Yuan MM. Adoptive transfer of paternal antigen-hyporesponsive T cells induces maternal tolerance to the allogeneic fetus in abortion-prone matings. J Immunol. 2004;173:3612–3619. doi: 10.4049/jimmunol.173.6.3612. [DOI] [PubMed] [Google Scholar]
- LP Jin, Zhou YH, Wang MY, Zhu XY, Li DJ. Blockade of CD80 and CD86 at the time of implantation inhibits maternal rejection to the allogeneic fetus in abortion-prone matings. J Reprod Immunol. 2005;65:133–146. doi: 10.1016/j.jri.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Jin LP, Zhou YH, Zhu XY, Wang MY, Li DJ. Adoptive transfer of paternal antigen-hyporesponsive T cells facilitates a Th2 bias in peripheral lymphocytes and at materno-fetal interface in murine abortion-prone matings. Am J Reprod Immunol. 2006;56:258–266. doi: 10.1111/j.1600-0897.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988;332:35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kiger N, Chaouat G, Kolb JP, Wegmann TG, Guenet JL. Immunogenetic studies of spontaneous abortion in mice. Preimmunization of females with allogeneic cells. J Immunol. 1985;134:2966–2970. [PubMed] [Google Scholar]
- Kim HJ, Hsu LYF, Paciuc S, Cristian S, Quintana A, Hirschhorn K. Cytogenetics of Fetal Wastage. New England Journal of Medicine. 1975;293:844–847. doi: 10.1056/NEJM197510232931703. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy Impairs resistance of C57Bl.6 mice to Leishmania major infection and causes decreases antigen-specific IFN-g responses and increased production of T Helper cytokines. J.Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- Kwan WH, van der Touw W, Heeger PS. Complement regulation of T cell immunity. Immunol Res. 2012;54:247–253. doi: 10.1007/s12026-012-8327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen T, Lokki ML, Tulppala M, Ylikorkala O, Koskimies S. Increased frequency of complement C4 ‘null’ alleles in recurrent spontaneous abortions. Hum Reprod. 1991;6:1384–1387. doi: 10.1093/oxfordjournals.humrep.a137273. [DOI] [PubMed] [Google Scholar]
- Lane PJ, Gaspal FM, McConnell FM, Withers DR, Anderson G. Lymphoid tissue inducer cells: pivotal cells in the evolution of CD4 immunity and tolerance? Front Immunol. 2012;3:24. doi: 10.3389/fimmu.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li B, Fan W, Geng L, Li X, Li L, Huang Z, Li S. CTLA4Ig gene transfer alleviates abortion in mice by expanding CD4+CD25+ regulatory T cells and inducing indoleamine 2,3-dioxygenase. J Reprod Immunol. 2009;80:1–11. doi: 10.1016/j.jri.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Li X, Wang B, Li Y, Wang L, Zhao X, Zhou X, Guo Y, Jiang G, Yao C. The Th1/Th2/Th17/Treg paradigm induced by stachydrine hydrochloride reduces uterine bleeding in RU486-induced abortion mice. J Ethnopharmacol. 2013;145:241–253. doi: 10.1016/j.jep.2012.10.059. [DOI] [PubMed] [Google Scholar]
- Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T-Helper 2-Type Cytokines at the Maternal-Fetal interface. J.Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ TR Cells Suppress Innate Immune Pathology Through Cytokine-dependent Mechanisms. The Journal of Experimental Medicine. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas AE, Petitbarat M, Dubanchet S, Fay S, Ledee N, Chaouat G. Immune regulation at the interface during early steps of murine implantation: involvement of two new cytokines of the IL-12 family (IL-23 and IL-27) and of TWEAK. Am J Reprod Immunol. 2008;59:323–338. doi: 10.1111/j.1600-0897.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- McIntyre JA, Coulam CB, Faulk WP. Recurrent spontaneous abortion. Am J Reprod Immunol. 1989;21:100–104. doi: 10.1111/j.1600-0897.1989.tb01011.x. [DOI] [PubMed] [Google Scholar]
- McIntyre JA, Faulk WP. Trophoblast modulation of maternal allogeneic recognition. Proc Natl Acad Sci U S A. 1979;76:4029–4032. doi: 10.1073/pnas.76.8.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JA, Faulk WP. Recurrent spontaneous abortion in human pregnancy: results of immunogenetical, cellular, and humoral studies. Am J Reprod Immunol. 1983;4:165–170. doi: 10.1111/j.1600-0897.1983.tb00272.x. [DOI] [PubMed] [Google Scholar]
- McIntyre JA, Faulk WP, Verhulst SJ, Colliver JA. Human trophoblast-lymphocyte cross-reactive (TLX) antigens define a new alloantigen system. Science. 1983;222:1135–1137. doi: 10.1126/science.6648525. [DOI] [PubMed] [Google Scholar]
- Medawar PB. Some Immunological and Endocrinological Problems Raised by the Evolution of Viviparity in Vetebrates. Symposia for the Soc.Exp.Biol. 1954;7:320–328. [Google Scholar]
- Mellor AL, Munn DH. Tryptophan catabolism prevents maternal T cells from activating lethal anti-fetal immune responses. Journal of Reproductive Immunology. 2001;52:5–13. doi: 10.1016/s0165-0378(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Mohlin FC, Mercier E, Fremeaux-Bacchi V, Liszewski MK, Atkinson JP, Gris JC, Blom AM. Analysis of genes coding for CD46, CD55, and C4b-binding protein in patients with idiopathic, recurrent, spontaneous pregnancy loss. Eur J Immunol. 2013;43:1617–1629. doi: 10.1002/eji.201243196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldenhauer LM, Hayball JD, Robertson SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J Reprod Immunol. 2010;87:1–13. doi: 10.1016/j.jri.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Monk JM, Leonard S, McBey BA, Croy BA. Induction of murine spiral artery modification by recombinant human interferon-gamma. Placenta. 2005;26:835–838. doi: 10.1016/j.placenta.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Muzikova E, Clark DA. Is spontaneous resorption in the DBA/2-mated CBA/J mouse due to a defect in “seed” or in “soil”? Am J Reprod Immunol. 1995;33:81–85. doi: 10.1111/j.1600-0897.1995.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Shima T, Inada K, Ito M, Saito S. The Balance of the Immune System between T Cells and NK Cells in Miscarriage. American Journal of Reproductive Immunology. 2012;67:304–310. doi: 10.1111/j.1600-0897.2012.01115.x. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Steffensen R, Varming K, Van Halteren AG, Spierings E, Ryder LP, Goulmy E, Christiansen OB. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum Mol Genet. 2009;18:1684–1691. doi: 10.1093/hmg/ddp077. [DOI] [PubMed] [Google Scholar]
- Norton MT, Fortner KA, Oppenheimer KH, Bonney EA. Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology. 2010;131:426–437. doi: 10.1111/j.1365-2567.2010.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirenda MH, Sanvito L, Darlington PJ, O'Brien K, Zhang GX, Constantinescu CS, Bar-Or A, Gran B. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- Ober C, Karrison T, Odem RR, Barnes RB, Branch DW, Stephenson MD, Baron B, Walker MA, Scott JR, Schreiber JR. Mononuclear-cell immunisation in prevention of recurrent miscarriages: a randomised trial. The Lancet. 1999;354:365–369. doi: 10.1016/S0140-6736(98)12055-X. [DOI] [PubMed] [Google Scholar]
- Oku K, Atsumi T, Bohgaki M, Amengual O, Kataoka H, Horita T, Yasuda S, Koike T. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis. 2009;68:1030–1035. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- Porter TF, LaCoursiere Y, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2006;19:CD000112. doi: 10.1002/14651858.CD000112.pub2. [DOI] [PubMed] [Google Scholar]
- Prados MB, Solano ME, Friebe A, Blois S, Arck P, Miranda S. Stress increases VCAM-1 expression at the fetomaternal interface in an abortion-prone mouse model. J Reprod Immunol. 2011;89:207–211. doi: 10.1016/j.jri.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Redecha P, van Rooijen N, Torry D, Girardi G. Pravastatin prevents miscarriages in mice: role of tissue factor in placental and fetal injury. Blood. 2009;113:4101–4109. doi: 10.1182/blood-2008-12-194258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. Bmj. 1989;299:541–545. doi: 10.1136/bmj.299.6698.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riella LV, Dada S, Chabtini L, Smith B, Huang L, Dakle P, Mfarrej B, D'Addio F, Adams LT, Kochupurakkal N, Vergani A, Fiorina P, Mellor AL, Sharpe AH, Yagita H, Guleria I. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am J Pathol. 2013;182:2204–2213. doi: 10.1016/j.ajpath.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Listeria monocytogenes cytoplasmic entry induces fetal wastage by disrupting maternal Foxp3+ regulatory T cell-sustained fetal tolerance. PLoS Pathog. 2012a;8:e1002873. doi: 10.1371/journal.ppat.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012b;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Scott DM, Ehrmann JE, Ellis PS, Bishop CE, Agulnik AI, Simpson E, Mitchell MJ. Indetification of a mouse male-specific transplantation antigen, H-Y. Nature. 1997;376:695–698. doi: 10.1038/376695a0. [DOI] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Shearman RP, Garrett WJ. Double-blind study of effect of 17-hydroxyprogesterone caproate on abortion rate. Br Med J. 1963;1:292–295. doi: 10.1136/bmj.1.5326.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: A history of transplantation, immune response genes, sex determination, and expression cloning. Annu.Rev.Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension. 2011;58:716–724. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T Cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- Tang AW, Alfirevic Z, Quenby S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod. 2011;26:1971–1980. doi: 10.1093/humrep/der164. [DOI] [PubMed] [Google Scholar]
- Tang AW, Alfirevic Z, Turner MA, Drury JA, Small R, Quenby S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum Reprod. 2013;28:1743–1752. doi: 10.1093/humrep/det117. [DOI] [PubMed] [Google Scholar]
- Taylor C, Faulk WP. Prevention of recurrent abortion with leucocyte transfusions. Lancet. 1981;2:68–70. doi: 10.1016/s0140-6736(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Teles A, Thuere C, Wafula PO, El-Mousleh T, Zenclussen ML, Zenclussen AC. Origin of Foxp3(+) cells during pregnancy. Am J Clin Exp Immunol. 2013;2:222–233. [PMC free article] [PubMed] [Google Scholar]
- Thangavelu G, Gill RG, Boon L, Ellestad KK, Anderson CC. Control of In Vivo Collateral Damage Generated by T Cell Immunity. The Journal of Immunology. 2013;191:1686–1691. doi: 10.4049/jimmunol.1203240. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ Immunoregulatory T Cells Suppress Polyclonal T Cell Activation In Vitro by Inhibiting Interleukin 2 Production. The Journal of Experimental Medicine. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung KS, Teuscher C. Mechanisms of autoimmune disease in the testis and ovary. Hum Reprod Update. 1995;1:35–50. doi: 10.1093/humupd/1.1.35. [DOI] [PubMed] [Google Scholar]
- Vance RE, Jamieson AM, Cado D, Raulet DH. Implications of CD94 deficiency and monoallelic NKG2A expression for natural killer cell development and repertoire formation. Proc Natl Acad Sci U S A. 2002;99:868–873. doi: 10.1073/pnas.022500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H, Haas W, Pohlit H. Cytotoxic T cells recognize male antigen and H-2 as distinct entities. J Exp Med. 1978;147:1291–1295. doi: 10.1084/jem.147.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafula PO, Teles A, Schumacher A, Pohl K, Yagita H, Volk HD, Zenclussen AC. PD-1 but not CTLA-4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am J Reprod Immunol. 2009;62:283–292. doi: 10.1111/j.1600-0897.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- Weil RJ, Tupper C. Personality, Life Situation, and Communication: A Study of Habitual Abortion. Psychosomatic Medicine. 1960;22:448–455. doi: 10.1097/00006842-196011000-00005. [DOI] [PubMed] [Google Scholar]
- Yin T, Huang F, Ren J, Liu W, Chen X, Li L, Xie D, Lu Y. Bilateral sudden hearing loss following habitual abortion: a case report and review of literature. Int J Clin Exp Med. 2013;6:720–723. [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Han X, Shi Q, Zhao Y, He Y. Adoptive transfer of CD4+CD25+ regulatory T cells for prevention and treatment of spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2012;161:177–181. doi: 10.1016/j.ejogrb.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Zelinskyy G, Werner T, Dittmer U. Natural regulatory T cells inhibit production of cytotoxic molecules in CD8+ T cells during low-level Friend retrovirus infection. Retrovirology. 2013;10:109. doi: 10.1186/1742-4690-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenclussen AC, Blois S, Stumpo R, Olmos S, Arias K, Malan Borel I, Roux ME, Margni RA. Murine abortion is associated with enhanced interleukin-6 levels at the feto-maternal interface. Cytokine. 2003;24:150–160. doi: 10.1016/j.cyto.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenclussen ML, Anegon I, Bertoja AZ, Chauveau C, Vogt K, Gerlof K, Sollwedel A, Volk HD, Ritter T, Zenclussen AC. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol. 2006;69:35–52. doi: 10.1016/j.jri.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu X, Liu X, Zhang R, Fu Q, Xu X. The Treg/Th17 imbalance in Toxoplasma gondii-infected pregnant mice. Am J Reprod Immunol. 2012;67:112–121. doi: 10.1111/j.1600-0897.2011.01065.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44:103–110. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Zhao FX, Zhang YY, Liu RH, Li SM. Effect of blockage of costimulatory signal on murine abortion-prone model. Chin Med J (Engl) 2007;120:1247–1250. [PubMed] [Google Scholar]
- Zhou WH, Dong L, Du MR, Zhu XY, Li DJ. Cyclosporin A improves murine pregnancy outcome in abortion-prone matings: involvement of CD80/86 and CD28/CTLA-4. Reproduction. 2008;135:385–395. doi: 10.1530/REP-07-0063. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Zhou YH, Wang MY, Jin LP, Yuan MM, Li DJ. Blockade of CD86 signaling facilitates a Th2 bias at the maternal-fetal interface and expands peripheral CD4+CD25+ regulatory T cells to rescue abortion-prone fetuses. Biol Reprod. 2005;72:338–345. doi: 10.1095/biolreprod.104.034108. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Hengartner H. On immunity against infections and vaccines: credo 2004. Scand J Immunol. 2004;60:9–13. doi: 10.1111/j.0300-9475.2004.01460.x. [DOI] [PubMed] [Google Scholar]