Abstract
Retinal ganglion cells (RGCs), the output neurons of the retina, have axons that project via the optic nerve to diverse targets in the brain. Typically, RGC axons do not branch before exiting the retina and thus do not provide it with synaptic feedback. Although a small subset of RGCs with intraretinal axon collaterals has been previously observed in human, monkey, cat, and turtle, their function remains unknown. A small, more recently identified population of RGCs expresses the photopigment melanopsin. These intrinsically photosensitive retinal ganglion cells (ipRGCs) transmit an irradiance-coding signal to visual nuclei in the brain, contributing both to image-forming vision and to several non-image forming functions including circadian photoentrainment and the pupillary light reflex. In this study, using melanopsin immunolabeling in monkey and a genetic method to sparsely label melanopsin cells in mouse, we show that a subgroup of ipRGCs have axons that branch en route to the optic disc, forming intraretinal axon collaterals that terminate in the inner plexiform layer of the retina. The previously described collateral-bearing population identified by intracellular dye injection is anatomically indistinguishable from the collateral-bearing melanopsin cells identified here, suggesting they are a subset of the melanopsin-expressing RGC type and may therefore share its functional properties. Identification of an anatomically distinct subpopulation in mouse, monkey and human suggests this pathway may be conserved in these and other species (turtle, cat) with intraretinal axon collaterals. We speculate that ipRGC axon collaterals constitute a likely synaptic pathway for feedback of an irradiance signal to modulate retinal light responses.
Keywords: melanopsin, collaterals, retina, primate, mouse
Introduction
Visual processing in the retina proceeds via excitatory synaptic transmission from photoreceptors to bipolar cells to retinal ganglion cells. Ganglion cells, the output neurons of the retina, often branch to terminate in multiple central targets. They are distinct from other long projection neurons in that they typically lack recurrent collaterals terminating in the vicinity of the cell of origin. However, a small number of ganglion cells with recurrent axon collaterals have been rarely but consistently reported in human, monkey, cat, and turtle retina (Peterson & Dacey, 1998). In addition to a primary axon that exits the eye via the optic disk, these cells have axon collateral branches terminating within the inner plexiform layer (IPL) of the retina itself. These collateral-bearing cells have very large, sparsely branching dendritic trees stratified in the inner half of the IPL and thin, branching collateral arbors terminating in the outer IPL. Although their consistent anatomy suggests a single cell type, it remains unclear whether the collaterals themselves reflect a functional specialization of a low-density type or a rare, non-functional aberration to which this cell type is uniquely vulnerable.
Recently, physiological evidence has emerged that a small population of atypical ganglion cells provide a positive feedback signal to the retinal circuitry (Barnard et al., 2006; Zhang et al., 2008; Zhang et al., 2012). These cells express the photopigment melanopsin and are intrinsically photosensitive (Berson et al., 2002; Hattar et al., 2002; Panda et al., 2002; Provencio et al., 2002; Lucas et al., 2003), contributing to image-forming vision (Brown et al., 2010; Ecker et al., 2010; Renna et al., 2011) but serving primarily as irradiance detectors for non-image forming functions such as circadian photoentrainment, the pupillary light reflex, and mood (Lucas et al., 2003; Panda et al., 2003; Gamlin et al., 2007; Goz et al., 2008; Guler et al., 2008; Hatori et al., 2008; Chen et al., 2011; LeGates et al., 2012). A melanopsin-based feedback signal appears to entrain the retinal circadian clock, modulating sensitivity of cone circuitry according to time of day (Barnard et al., 2006). In addition, a melanopsin-dependent intrinsic light response has been discovered in dopaminergic amacrine cells (Zhang et al., 2008; Zhang et al., 2012) via an excitatory synapse either directly or serially from melanopsin ganglion cells. However, no direct anatomical evidence of synaptic input from melanopsin expressing cells to other retinal cell types has been found.
Using genetic and immunohistochemical methods, we describe a novel subpopulation of melanopsin cells with intraretinal axon collaterals that terminate in the IPL of both mouse and macaque monkey retina. We present anatomical evidence suggesting that previously described collateral-bearing cells belong to the melanopsin cell population, indicating a functional role consistent with that of melanopsin cells. We hypothesize that the axon collaterals of melanopsin cells constitute at least one pathway for the positive feedback of irradiance information to the retina, possibly via synaptic contact with the dopaminergic amacrine cell.
Materials and Methods
Mouse
All mice were of mixed background (BL/6;129SvJ) and sacrificed at ~2–4 months. Mice were housed and treated in accordance with National Institutes of Health (NIH) and Institutional Animal Care and Use Committee guidelines. Animal care and use protocols were approved by the Johns Hopkins University Animal Care and Use Committee. To sparsely label melanopsin-expressing cells, 0.5–2.0 mg of tamoxifen was injected intraperitoneally into Opn4CreERT2; Z/AP mice two weeks prior to alkaline phosphatase (AP) staining as previously described (Lobe et al., 1999; Ecker et al., 2010; Chen et al., 2011; Percival et al., 2011). In brief, mice were perfused with 45 ml of 4% paraformaldehyde (PFA) and retinas were dissected out. After staining, whole mount retinas were post fixed in 4% PFA overnight and washed with ethanol series overnight before mounting. Stack images were taken with Zeiss Axio Imager 1 microscope with 1 or 2 μm intervals and imported into Adobe Photoshop for detailed tracing using a Wacom tracing tablet or traced by hand from printed images of composited stacks. Total retinal area, soma size, and dendritic and axonal field areas (defined as the area of a convex polygon enclosing the outermost processes) were measured in ImageJ (NIH). When a collateral branched into two large arbors, axonal field area was determined for each arbor separately. Total collateral length was measured in ImageJ from print images of individual cells. Cells were traced by hand from print images.
Primate
Macaque tissue was obtained from the Regional Primate Center at the University of Washington. Human eyes were obtained from donors to the Lions Eye Bank at the University of Washington. The anti-melanopsin immunolabeling has been described elsewhere (Dacey et al., 2005). In brief, macaque ganglion cells were labeled in whole mount retina using a rabbit polyclonal antibody derived from cloned full-length c-DNA for human melanopsin (hNA; provided by Dr. King-Wai Yau) and biotinylated goat anti-rabbit (BA-1000; Vector Labs, Burlingame, CA), and processed for HRP histochemistry (ABC Elite kit, Vector Labs). In separate experiments, macaque and human giant cells with axon collaterals were intracellularly injected with Neurobiotin (SP-1120; Vector Labs, Burlingame, CA) in the in vitro retina and processed for HRP histochemistry as previously described (Peterson & Dacey, 1998). Cells were traced by hand using a camera lucida.
Results
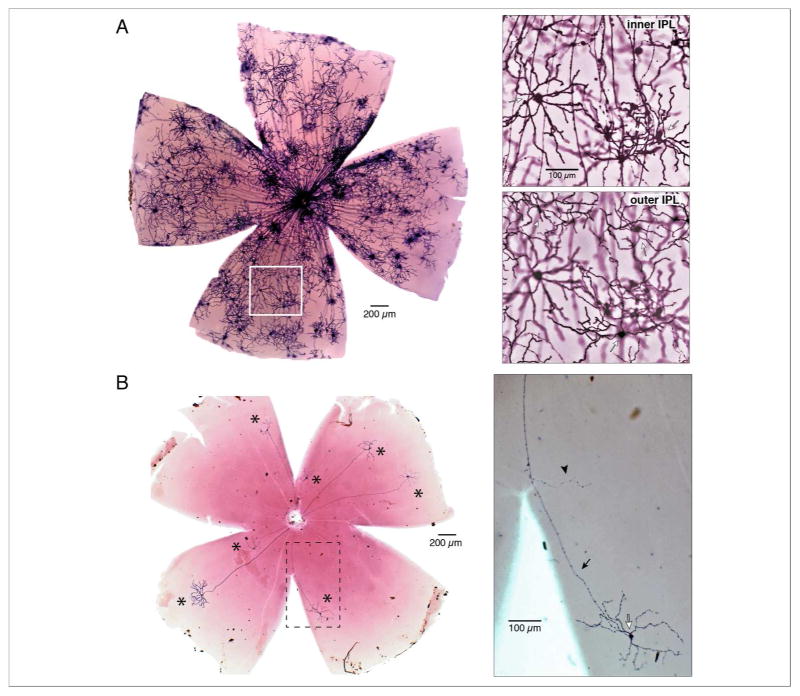
To study the axonal targeting of melanopsin cells in mouse, we crossed the Opn4-driven tamoxifen-inducible Cre mouse line (Opn4CreERT2) with the Z/AP reporter line (Indra et al., 1999; Lobe et al., 1999; Chen et al., 2011; Percival et al., 2011). This allowed controlled expression of alkaline phosphatase (AP) on the plasma membrane of melanopsin-expressing cells. The number of cells labeled was roughly proportional to the volume of tamoxifen injected. High-volume tamoxifen injection densely labeled melanopsin-expressing cells (Fig. 1A, left panel). Both inner stratifying M2 (Fig. 1A, right panel, top) and outer stratifying M1 cells (Fig. 1A, right panel, bottom) were identified. Lower volume tamoxifen injection (Fig. 1B, left panel) resulted in much sparser labeling, primarily of M1 cells, making it possible to detect fine-caliber collateral processes that branched from M1 cell axons intraretinally but would be obscured by overlapping processes in densely labeled retinas (Fig. 1B, right panel). Two M1 cells are shown at high magnification in Figures 2A and B. The cell in Figure 2A had a highly branched axon collateral stratified in the outer IPL. The axon of the M1 cell in figure 2B gave rise to a sparsely branched collateral stratified in the inner IPL.
Figure 1.
Titrated tamoxifen injection controls the density of labelled cells in knock-in mice (Opn4CreERT2; Z/AP) to reveal intraretinal collaterals. A, Micrograph of a high-volume tamoxifen-injected retina with dense labelling of melanopsin-expressing cells. Area indicated by white box is shown at right at higher magnification. Top panel: focus is on the dendrites of M2 cells (open arrows) in the inner IPL. Bottom panel: focus is shifted to the dendrites of M1 cells in the outer IPL (open arrows). B, Low tamoxifen injection resulted in a sparsely labeled retina with seven non-overlapping M1 cells (asterisks). Area within dashed lines is shown at right at higher magnification. The axon (closed arrow) of an M1 cell (open arrow) gives off a single collateral process (arrowhead).
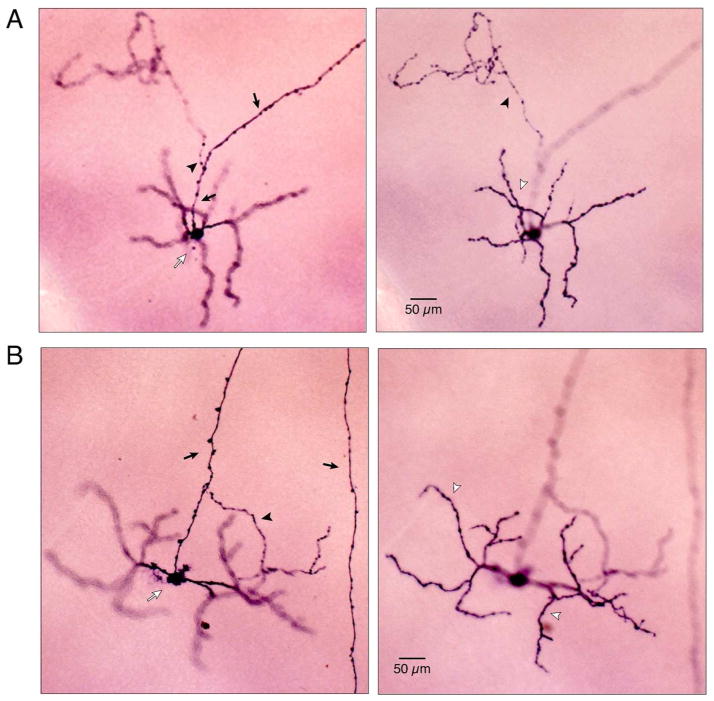
Figure 2.
Photomicrographs of intraretinal axon collateral-bearing M1 cells in mouse. A, Photomicrographs of an M1 cell with outer-stratifying collateral. Left panel: focus is on the soma (open arrow) and axon (closed arrows). A single collateral (arrowhead) branches from the axon. Right panel: focus is shifted to the dendrites (open arrowhead) and axon collateral (closed arrowhead) in the outer IPL. B, Photomicrographs of an M1 cell with inner-stratifying collateral. Left panel: focus is on the soma (open arrow) and axon (closed arrow) which gave rise to a single collateral (closed arrowhead) that terminated in the inner IPL. Right panel: focus is on the dendritic arbor in the outer IPL.
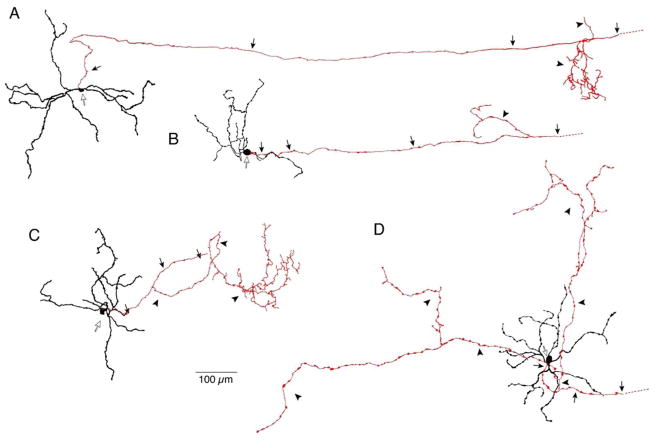
Axon collaterals varied in arbor size and extent of branching (Fig. 3). The cells in Figure 3A-C had relatively small collateral arbors, but the arbors in Figures 3A and 3C were highly branched, while the arbor in Figure 3B had only two branches. The cell in Figure 3D had a very large, sparsely branched collateral arbor. Collaterals extended a variable distance, terminating up to as much as 800 μm away from their cell bodies.
Figure 3.
Mouse axon collateral arbors show variability in size and extent of branching. A–D. Tracings of 4 M1 cells with axon collaterals. Cell bodies indicated by open arrows; primary axons and collaterals are shown in red. Primary axons (arrows) extended to the optic disk and gave rise to collateral processes (arrowheads) that terminated in either the inner (A, B) or outer (C, D) IPL.
To estimate the percentage of M1 cells with axon collaterals, we counted the total number of labeled M1 cells and the number with collaterals in retinas (n=23) in which overlap between cells was minimal enough for individual cells to be distinguished. We estimate the percentage of cells with axon collaterals is ~7% (7 outer, 4 inner, 11 total cells with collaterals; 165 labeled M1 cells). About 4% of the cells had outer stratifying collaterals and ~3% had inner stratifying collaterals.
To estimate the total retinal area covered by collaterals, we calculated area based on the mean collateral field diameter (127.8 ± 16.4 μm, n = 9) and multiplied by the number of collateral-bearing M1 cells (7% of ~700 total M1 cells in adult mouse retina, n = 49). Dividing by mean retinal area (11.0 ± 2.4 mm2, n = 3), we estimated that inner and outer stratifying collaterals together cover ~5.7% of the retina. Inner stratifying collaterals cover ~2.4%, outer collaterals ~3.3%. In these estimates the collateral field diameter was taken as the diameter of a circle with area equal to that covered by a convex polygon enclosing the outermost tips of the collateral arbor. Because collateral arbors were often very sparse (Fig. 3D), the field diameter, and thus the retinal percentage covered, are both likely to be overestimates.
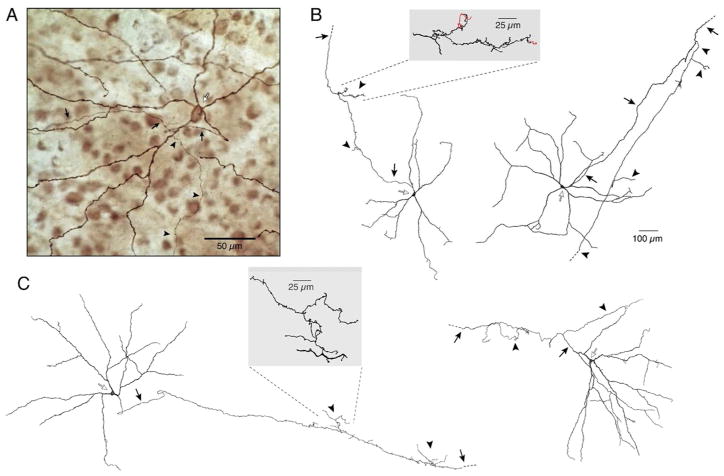
The giant melanopsin-expressing ganglion cells of macaque retina also exhibit axon collaterals. Collaterals were found on both outer (Fig. 4A) and inner (not shown) stratifying melanopsin cells and, in turn, branched in either the inner or outer IPL. In two samples of neighboring melanopsin-expressing cells (21 inner, 82 outer, 103 cells total), ~10.7 % (2 inner, 9 outer, 11 total) had intraretinal axon collaterals, similar to the frequency in mouse retina. The 2 inner cells had inner-stratifying collaterals and the 9 outer cells had outer-stratifying collaterals. A previous study of monkey and human ganglion cells using intracellular dye injection reported inner stratifying cells with outer stratifying intraretinal axon collaterals (Peterson & Dacey, 1998). These cells, re-examined in the present study, had large somas and very large, sparsely branched dendritic trees (Figs. 4B and C), consistent with the anatomy of monkey giant melanopsin-expressing cells. As in mouse, axon collaterals of monkey and human cells often showed secondary and tertiary branching (Figs. 4B and C) and extended up to ~800 μm from the primary axon. It is likely that all the large, axon collateral-bearing cells in both monkey and human are associated with the same low-density ganglion cell population that labels for melanopsin and has been shown to be intrinsically photosensitive (Dacey et al., 2005).
Figure 4.
Primate giant melanopsin ganglion cells exhibit axon collaterals. A, Photomicrograph of an outer stratifying melanopsin immunolabeled giant cell in macaque retina. Soma (open arrow) was displaced to the INL. The axon (closed arrows) gave rise to a thin collateral (arrowheads) studded with varicosities that could be followed for ~800 μm in the outer IPL before fading. B–C, Tracings of inner stratifying giant cells (open arrows) intracellularly filled with Neurobiotin from macaque (B) and human (C) retina. Axons (closed arrows) gave off several collateral branches (arrowheads) en route to the optic disk. Collaterals terminated in the outer IPL with the exception of the cell on left in B, where two short collaterals terminated in the inner IPL (shown in red in inset). Insets show magnified views of collaterals indicated by the dashed lines.
Discussion
In both monkey and mouse, axon collaterals were associated with a relatively small percentage, ~10%, of melanopsin-expressing ganglion cells. However, in macaque, the fine calibre processes and their light immunostaining made the collaterals very difficult to observe and our finding that 11% of monkey melanopsin-expressing cells form collaterals must be considered a minimum estimate. In mouse, the collaterals were much easier to observe due to the clear, sparse labeling. However, we observed intraretinal axon collaterals only for the outer-stratifying M1 cells. This is possibly because our method preferentially labels M1 cells at low-volume tamoxifen injection, possibly due to their relatively high melanopsin expression (Hattar et al., 2006; Baver et al., 2008; Schmidt & Kofuji, 2009; Berson et al., 2010). If, as in primate, mouse inner-stratifying melanopsin cells also form collaterals, the actual percentage of cells with collaterals would be higher in mouse than the estimated 7%.
Regardless of the difficulties in observing collaterals, the relatively low frequency and the variable size and density of the associated terminal arbors suggest that the collaterals do not tile the retina in a regular spatial array (Wässle & Boycott, 1991) and are therefore not associated with a separate and distinctive type of melanopsin-expressing ganglion cell. It is unlikely the collateral-bearing cell is an artifact, however, as it has been seen using different methods in different species. The observation that melanopsin-expressing cells give rise to axon collaterals in both mouse and primate suggests that collaterals are a conserved, functional specialization associated specifically with melanopsin-expressing cells.
The dopaminergic amacrine cell, which costratifies with the dendrites of melanopsin cells in the outer IPL, signals by volume transmission to all retinal cell classes, modulating synaptic input for optimal visual function at varying light levels (Dowling & Ehinger, 1975; Lasater & Dowling, 1985; Nir et al., 2002; Hayashida & Ishida, 2004; Ichinose & Lukasiewicz, 2007). An estimated 21% of DA cells exhibit sustained melanopsin-dependent light responses consistent with glutamatergic input from melanopsin cells (Zhang et al., 2007; Zhang et al., 2008; Zhang et al., 2012). Zhang et al. (2012) propose two potential routes for retrograde transmission from melanopsin cells to DA cells: via dendrodendritric synapses, as have been reported in catfish retina (Sakai et al., 1986), or by recurrent axon collaterals of ganglion cells, as previously described in cat and human retina (Dacey, 1985; Peterson & Dacey, 1998). Although melanopsin and amacrine cell dendrites do cofasciculate extensively and this alternative route for retrograde transmission of the melanopsin signal cannot be ruled out, there is as yet no evidence of synaptic input from melanopsin dendrites to dopaminergic amacrine cell dendrites (Belenky et al., 2003; Dacey et al., 2006; Jusuf et al., 2007; Viney et al., 2007). The data shown here support the alternative hypothesis, that the axon collaterals of melanopsin cells provide the route for transmission of light signals to the DA cells.
In double- (melanopsin, TH) and triple-labelled (melanopsin, TH, presynaptic SNARE complex protein SNAP25) rat and human retina, rare examples of potential collateral input to DA cell dendrites have been observed (Vugler et al., 2007, initial study; personal correspondence suggesting collateral input). Despite forming a relatively sparse plexus, if collaterals selectively target DA cell dendrites, assuming that the entire length of each collateral made contact with DA cell dendrites, we estimate that, as an upper bound, a DA cell could receive input at up to ~12% of its total length, or 300 μm. This estimate is based on dividing the mean collateral length per mouse retina (24956 μm, our measurements) by the mean DA dendrite length per mouse retina, calculated as the product of the mean DA cell dendritic length and the number of DA cells known to receive melanopsin input (21%)(Raven et al., 2003; Badea et al., 2009).
Over a third of the collaterals we observed are stratified at the inner border of the IPL and would therefore not be positioned to contact the dendrites of dopaminergic amacrine cells at the outer border of the IPL. This suggests collateral-bearing cells may have at least two functional roles. In the developing retina, melanopsin cell input has been demonstrated to play a role in the segregation of retinogeniculate projections by modulating the waves of synchronous depolarization in neighboring ganglion cells driven by the starburst amacrine cell network (Renna et al., 2011). Melanopsin cell input to this circuit could be to starburst amacrines or to spike-generating retinal ganglion cells themselves (Renna et al., 2011). Although axon collaterals do not costratify with the ON or OFF starburst amacrine plexus, located at ~30% and ~70% depth, respectively (Famiglietti, 1983; Mariani & Hersh, 1988; Rodieck, 1989), or with known retinal ganglion cell types other than the melanopsin cells themselves, it is possible that melanopsin cells influence retinal waves by collateral-mediated synapses with an intermediate cell type. This does, however, seem less likely than signaling to other ganglion cells by dendrodendritic gap junctions (Sekaran et al., 2003).
Ganglion cells with branching axons have been described in calf, dog, lizard, cat, turtle, mouse, monkey, and human (Gallego & Cruz, 1965; Honrubia & Elliott, 1968; Dacey, 1985; Usai et al., 1991; Peterson & Dacey, 1998). Our results in primate indicate that the previously observed collateral-bearing cells of monkey and human are, in fact, melanopsin cells, and suggests the hypothesis that all previously observed collateral-bearing cells of other species express melanopsin. Further investigation of the development, connectivity, and physiology of the recurrent collaterals will be required to determine the precise role that this unique circuit plays in the regulation of retinal function.
Acknowledgments
This work was supported by the National Institutes of Health grants GM076430 (S.H.), EY06678 (D.M.D.), RR00166 (the National Primate Research Center at the University of Washington), the David and Lucile Packard Foundation (S.H.), the Alfred P. Sloan Foundation (S.H.), and Vision Research Core grants EY01730 (D.M.D.).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Badea T, Hua Z, Smallwood P, Williams J, hRotolo T, Ye X, Nathans J. New Mouse Lines for the Analysis of Neuronal Morphology Using CreER(T)/loxP-Directed Sparse Labeling. Plos one. 2009;4:e7859. doi: 10.1371/journal.pone.0007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard A, Hattar S, Hankins M, Lucas R. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson D, Castrucci A, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Wide-spreading terminal axons in the inner plexiform layer of the cat’s retina: Evidence for intrinsic axon collaterals of ganglion cells. J Comp Neurol. 1985;242:247–262. doi: 10.1002/cne.902420207. [DOI] [PubMed] [Google Scholar]
- Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Liao H-W, Yau K-W. Two types of melanopsin-containing ganglion cell in the primate retina: links to dopaminergic amacrine and DB6 cone bipolar cells. Invest Ophthalmol Vis Sci. 2006:47. [Google Scholar]
- Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retina. Science. 1975;188:270–273. doi: 10.1126/science.804181. [DOI] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, Legates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV., Jr ‘Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 1983;261:138–144. doi: 10.1016/0006-8993(83)91293-3. [DOI] [PubMed] [Google Scholar]
- Gallego A, Cruz J. Mammalian retina: associational nerve cells in ganglion cell layer. Science. 1965;150:1313–1314. doi: 10.1126/science.150.3701.1313. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. Plos one. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. Plos one. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau K, Berson D. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Ishida AT. Dopamine receptor activation can reduce voltage-gated Na+ current by modulating both entry into and recovery from inactivation. Journal of Neurophysiology. 2004;92:3134–3141. doi: 10.1152/jn.00526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honrubia FM, Elliott JH. Efferent innervation of the retina I. Morphologic study of the human retina. Arch Ophthalmol. 1968;80:98–103. doi: 10.1001/archopht.1968.00980050100017. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Ambient Light Regulates Sodium Channel Activity to Dynamically Control Retinal Signaling. J Neurosci. 2007;27(17):4756–4764. doi: 10.1523/JNEUROSCI.0183-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci. 2007;26:2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA. 1985;82:3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Mariani AP, Hersh LB. Synaptic organization of cholinergic amacrine cells in the Rhesus monkey retina. J Comp Neurol. 1988;267:269–280. doi: 10.1002/cne.902670209. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison J, Haque R, Low M, Grandy D, Rubinstein M, Iuvone P. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. Journal of Neuroscience. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Percival KA, Martin PR, Grunert U. Synaptic inputs to two types of koniocellular pathway ganglion cells in marmoset retina. J Comp Neurol. 2011;519:2135–2153. doi: 10.1002/cne.22586. [DOI] [PubMed] [Google Scholar]
- Peterson BB, Dacey DM. Morphology of human retinal ganglion cells with intraretinal axon collaterals. Vis Neurosci. 1998;15:377–387. doi: 10.1017/s0952523898152161. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. Nature. 2002;415:493–494. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Raven MA, Eglen SJ, Ohab JJ, BER Determinants of the Exclusion Zone in Dopaminergic Amacrine Cell Mosaics. THE JOURNAL OF COMPARATIVE NEUROLOGY. 2003;461:123–136. doi: 10.1002/cne.10693. [DOI] [PubMed] [Google Scholar]
- Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci. 2011;14:827–829. doi: 10.1038/nn.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. Starburst amacrine cells of the primate retina. J Comp Neurol. 1989;285:18–37. doi: 10.1002/cne.902850104. [DOI] [PubMed] [Google Scholar]
- Sakai HM, Naka KI, Dowling JE. Ganglion cell dendrites are presynaptic in catfish retina. Nature. 1986;319:495–497. doi: 10.1038/319495a0. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and Morphological Differences among Intrinsically Photosensitive Retinal Ganglion Cells. Journal of Neuroscience. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- Usai C, Ratto GM, Bisti S. Two systems of branching axons in monkey’s retina. J Comp Neurol. 1991;308:149–161. doi: 10.1002/cne.903080202. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Vugler AA, Redgrave P, Semo M, Lawrence J, Greenwood J, Coffey PJ. Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol. 2007 doi: 10.1016/j.expneurol.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, McMahon DG. Melanopsin mediates retrograde visual signaling in the retina. PLoS ONE. 2012;7:e42647. doi: 10.1371/journal.pone.0042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Zhou TR, McMahon DG. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. Journal of Neuroscience. 2007;27:692–699. doi: 10.1523/JNEUROSCI.4478-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]