Electronic device therapies, including implantable pacemakers and defibrillators, have revolutionized the management of cardiovascular disease (1). For example, patients with certain slow cardiac rhythms (bradycardia) can experience exercise intolerance, easy fatigability, or circulatory collapse. Given that currently available drugs cannot safely and sustainably elevate heart rate, the only proven treatment for symptomatic bradycardia is permanent pacemaker implantation. Contemporary electronic pacemakers have an extended battery life, contain leads that minimize inflammation and scarring, and possess advanced algorithms to contend with heart rate elevations during exercise. These features allow pacemakers to improve longevity and quality of life in patients who require them. But electronic pacemakers cannot recapitulate all aspects of the endogenous sinoatrial node, the dominant pacemaker in the uninjured heart. In this regard, a recent study by Hu et al. (2) demonstrates the feasibility of a somatic cell reprogramming strategy for creating a biological pacemaker in a large animal preclinical model, raising prospects for clinical translation.
Although the efficacy of electronic pacemakers is clear, there remain specific clinical situations where a biological pacemaker might be advantageous. For example, pacemaker infections necessitate removal of all device-associated equipment (i.e., battery and leads) while the patient is treated with antibiotics (3). However, this is problematic for pacemaker-dependent patients during the period of treatment (typically 2 to 6 weeks). Currently, a temporary pacemaker is placed for this indication, but implantation of additional hardware is not an ideal solution given the high likelihood for re-infection. Many view this scenario as the perfect niche for a temporary biological pacemaker (4). A biological pacemaker should theoretically exhibit more physiological autonomic responses and accommodate for growth, which is especially important in pediatric patients. Such reasoning provides strong rationale for developing a biological pacemaker.
Previous attempts to create a biological pacemaker have focused on three general approaches: introduction of specific ion channels into heart muscle cells (cardiomyocytes) by gene transfer (transduction), ion channel expression in non-cardiomyocytes followed by cell fusion to native cardiomyocytes in situ, and the introduction of stem cells that have been previously differentiated into cardiomyocytes. In an early iteration of ion channel transduction, a dominant negative potassium channel (which antagonizes the native channel) was introduced directly into ventricular myocardium in a small animal preclinical model, resulting in a transient escape rhythm (5). Because such an approach required direct delivery of the gene encoding the potassium channel into cardiomyocytes, cell fusion strategies were developed to optimize the properties of heterologous “delivery” cells prior to their introduction into preclinical animal models (6–8). However, these approaches may not recapitulate additional as-yet uncharacterized features of pacemaker cells. To circumvent this limitation, embryonic stem cells were differentiated in vitro into cardiomyocytes with pacemaker activity and then introduced directly into the heart of pigs or guinea pigs (9, 10). Although this strategy proved successful, the teratogenic potential and heterogeneity of differentiated embryonic stem cells have slowed clinical translation.
Recently, direct lineage reprogramming has emerged as a means for converting one cell type into another cell type, including pluripotent stem cells, neurons, and cardiomyocytes (11). The conversion of fibroblasts into mouse and human cardiomyocyte-like cells has been well-documented (12, 13), although it remains inefficient. However, a method for generating pacemaker cells in this way has not yet been identified. Alternatively, interconversion of distinct cardiomyocyte cell types (e.g., ventricular to pacemaker and atrial to pacemaker) has been observed in vitro and in vivo (14, 15). Specifically, the human developmental transcription factor T-box 18 (Tbx18) can reprogram ventricular myocytes directly into induced sinoatrial node (iSAN) cells with many features characteristic of endogenous pacemaker cells. Moreover, this somatic reprogramming into iSAN cells has been achieved in vivo in a small animal preclinical model, resulting in durable pacemaker activity.
The study by Hu et al. represents the next logical step toward clinical translation of somatic reprogramming to create a biological pacemaker. The authors used an adenovirus vector to transduce the gene encoding human Tbx18 into ventricular cardiomyocytes of pigs. The procedure involved viral delivery via a specialized catheter threaded through a vein into the right ventricle. They observed meaningful pacemaker activity in the context of experimentally induced complete heart block, a clinical condition that would normally require pacemaker implantation in humans. From a functional standpoint, relative to control animals, the pigs receiving Tbx18 showed an increased heart rate with less dependence on a backup electronic pacemaker. Pacemaker activity in pigs that received Tbx18 showed diurnal variation (higher rate during the day and lower rate at night) and appropriate autonomic responses (e.g., increased rate in response to isoproterenol infusion), relative to control animals. Furthermore, the authors provide evidence that pacemaker activity emanates from the injection site, indicating that reprogrammed cardiomyocytes are indeed responsible for the observed clinical improvements. No increase in arrhythmias was observed in Tbx18-recipient animals, and all measured clinical parameters appeared similar to those of control animals.
The demonstration of stable pacemaker activity induced through percutaneous venous delivery in a clinically relevant large-animal model suggests that somatic reprogramming may be a viable strategy for creating a biological pacemaker. Prior to nonhuman primate studies and possible phase I clinical trials in humans, however, several hurdles will need to be cleared. Although Hu et al. suggest that the transient nature of adenoviral infection might be advantageous for a temporary biological pacemaker after removal of an infected device, it remains unclear whether the observed durability will be sufficient for prolonged antibiotic regimens (e.g., 6 weeks for pacemaker-related endocarditis). The Tbx18-induced biological pacemaker was effective for up to 14 days in the pig model. The lack of clinically adverse effects in the study is encouraging, but it remains to be seen whether viral dispersion leads to multiple pacemaker foci within the heart or whether the low-level infection in the lung and spleen that was observed might have long-term consequences. Moreover, extended studies will be needed to evaluate whether adenoviral infection leads to untoward inflammation or fibrosis at the site of injection, which may lead to dangerous ventricular arrhythmias or depressed myocardial function. Given these potential limitations, the development of alternative delivery systems capable of more durable gene expression may expand the possible indications for a biological pacemaker in the future.
Because Tbx18 is a pleiotropic developmental regulator, it could induce alternative cell fates in vivo that have escaped detection and are potentially dangerous. Nonetheless, the results of Hu et al. represent a critical step toward potentially filling an important clinical niche and provide an encouraging indication that a biological pacemaker might eventually be ready for human translation.
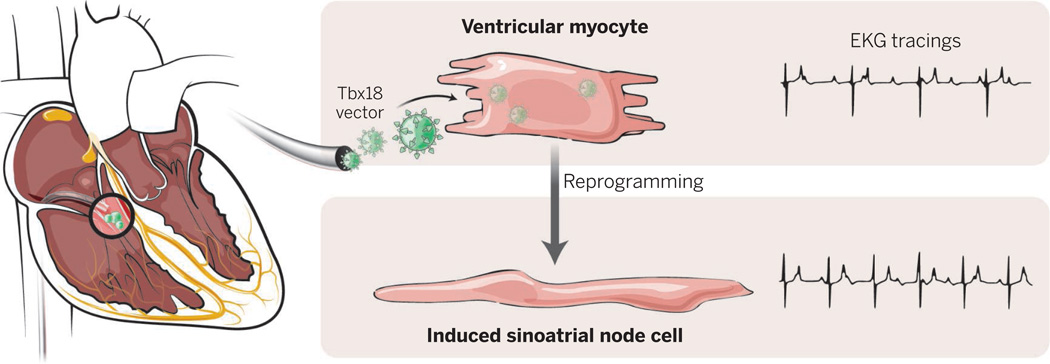
Biological pacemaker.
Tbx18-containing adenoviral particles can be delivered percutaneously in pigs that have a slow cardiac rhythm. Over a 1-week period, cardiomyocytes are reprogrammed into pacemaker cells at the site of virus injection and animals show increased heart rate, clinical improvement, and no obvious adverse sequelae.
Contributor Information
Nikhil V. Munshi, Email: nikhil.munshi@utsouthwestern.edu.
Eric N. Olson, Email: eric.olson@utsouthwestern.edu.
REFERENCES
- 1.Lampert R. Circulation. 2013;128:1576. doi: 10.1161/CIRCULATIONAHA.113.001555. [DOI] [PubMed] [Google Scholar]
- 2.Hu YF, Dawkins JF, Cho HC, Marbán E, Cingolani E. Sci. Transl. Med. 2014;6:245ra94. doi: 10.1126/scitranslmed.3008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baddour LM, Cha YM, Wilson WR. N. Engl. J. Med. 2012;367:842. doi: 10.1056/NEJMcp1107675. [DOI] [PubMed] [Google Scholar]
- 4.Cho HC, Marbán E. Circ. Res. 2010;106:674. doi: 10.1161/CIRCRESAHA.109.212936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miake J, Marbán E, Nuss HB. Nature. 2002;419:132. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 6.Potapova I, et al. Circ. Res. 2004;94:952. doi: 10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- 7.Plotnikov AN, et al. Circulation. 2007;116:706. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 8.Cho HC, Kashiwakura Y, Marbán E. Circ. Res. 2007;100:1112. doi: 10.1161/01.RES.0000265845.04439.78. [DOI] [PubMed] [Google Scholar]
- 9.Kehat I, et al. Nat. Biotechnol. 2004;22:1282. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 10.Xue T, et al. Circulation. 2005;111:11. doi: 10.1161/01.CIR.0000159092.92366.CD. [DOI] [PubMed] [Google Scholar]
- 11.Chambers SM, Studer L. Cell. 2011;145:827. doi: 10.1016/j.cell.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Ieda M, et al. Cell. 2010;142:375. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam YJ, et al. Proc. Acad. Natl. Sci. U.S.A. 2013;110:5588. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor N, Liang W, Marbán E, Cho HC. Nat. Biotechnol. 2013;31:54. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakker ML, et al. Cardiovasc. Res. 2012;94:439. doi: 10.1093/cvr/cvs120. [DOI] [PubMed] [Google Scholar]