Abstract
Despite recent modest improvements in the chemotherapy regimens used to treat acute myeloid leukemia (AML), many patients diagnosed with AML ultimately die of the disease. Commonly occurring genetic alterations have been identified that strongly affect the prognosis for patients with AML. These alterations represent possible targets for investigational therapies that could act to specifically halt the aberrant growth of AML cells while limiting damage to normal cells. One such gene is the Fms-like tyrosine kinase 3 (FLT3) gene, which is mutated in approximately 30% of adult patients with AML and has a significant impact on prognosis. In particular, internal tandem duplications in FLT3 confer a poor prognosis to this large subgroup of patients with AML. Agents that target FLT3 are in development for the treatment of patients who have AML and offer a potential paradigm change in the current standard treatment of AML. For this report, the authors reviewed the prognostic significance of genetic alterations observed in AML with a focus on the therapeutic implications of targeting FLT3. The introduction of such agents may be the next major step toward the era of personalized therapy in AML.
Keywords: acute myeloid leukemia, cytogenetics, FLT3 protein, molecular abnormalities
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by uncontrolled proliferation of myeloid blast cells and interference of the normal hematopoietic process.1 AML accounts for approximately 33% of all cases of leukemia in the United States with an estimated 12,330 new cases of AML and 8950 AML-related deaths in 2010 alone.2 AML is predominantly a disease of the elderly, and the median age at diagnosis is 67 years.3 The standard of care treatment for AML has remained relatively unchanged over the past 4 decades. Therapy typically consists of intensive induction therapy, most commonly with a combination of an anthracycline and cytarabine, followed by postremission treatment, which is usually additional cytarabine-based chemotherapy or stem cell transplantation.4 This treatment is nonspecific and is inadequate for most patients.5 Long-term survival is achieved by approximately 30% to 40% of younger patients with AML (aged < 60 years) but by only 5% to 10% of patients aged > 60 years.5 Improving the outcome of patients with AML will require the development of new, targeted therapies and the definition of prognostic markers to identify patients who are most likely to respond to treatment. Recently, an enhanced understanding of AML biology has led to the elucidation of novel molecular markers, which serve as both prognostic features and potential targets for treatment. Consequently, new agents are under investigation that specifically target pathophysiologic abnormalities that are required for the growth and proliferation of AML cells while aiming to minimally affect normal cells. The era of personalized, targeted therapy has arrived in the treatment of acute leukemias.
Cytogenetic and Molecular Alterations of AML
The prognosis for patients with AML varies dramatically and is strongly influenced by a number of factors, including age, performance status, and cytogenetic and/or molecular alterations.6 The development of selective therapeutic agents and the identification of patients who may benefit from these new treatments requires a good understanding of the mechanisms behind these genetic and molecular alterations as well as their influence on the course of the disease.
Cytogenetic abnormalities
Cytogenetic abnormalities occur in approximately 55% of adult patients with AML and have a major prognostic impact.6 Patients of all ages who have unfavorable cytogenetics have poor 5-year survival rates that range from 2% to 14% compared with rates from 34% to 65% among patients who have favorable cytogenetics.7
Several different cytogenetic abnormalities have been identified in AML, including numerical abnormalities (ie, losses or gains of chromosome segments or entire chromosomes), translocations, and inversions.8 Defining the prognostic impact of individual cytogenetic markers and understanding their relation with other features that impact treatment outcome, such as age and performance status, are complex challenges.
A favorable prognosis is conferred by certain translocations or inversions, for example, the abnormal core-binding factor (CBF) mutations t(8;21)/runt-related transcription factor 1 (RUNX1)-RUNX1 translocated to 1 (cyclin D-related) (RUNX1-RUNX1T1), t(15;17)/promyelocytic leukemia-retinoic acid receptor α (PML-RARA), and inv(16)/core-binding factor β-myosin heavy chain 11 smooth muscle (CBFB-MYH11), which occur in 10% to 15% of patients with AML.1,9–11 In contrast, an unfavorable outcome is associated with most translocations that involve 11q23, which occur in 5% to 10% of adult patients with AML and result in amplification of the mixed-lineage leukemia gene (MLL).12 Trisomy 8, one of the most common genetic abnormalities observed in AML, confers an intermediate prognosis in the absence of a complex karyotype.9 These patients can be stratified further by additional factors, such as age, into low-risk, intermediate-risk, and high-risk groups.13 Other commonly occurring, AML-related numerical abnormalities include monosomy of chromosomes 5 (−5) and/or 7 (−7) and partial deletions of chromosomes 5 (del[5q]) or 7 (del[7q]).14 Typically, this group of abnormalities, known collectively as “−5/−7,” confers a negative prognosis. Outcomes in this group can be stratified further by favorable factors, including simple karyotype or no antecedent hematologic disease.15
Molecular abnormalities
Approximately 50% of patients with AML are cytogenetically normal (ie, they have no detectable chromosomal abnormalities) and, as a group, have an intermediate prognosis.16 Many of these patients (and some with chromosomal abnormalities) carry genetic alterations that lead to changes in gene expression, such as mutations or polymorphisms. These alterations are important for a more differential prognosis to help guide treatment choices, and some specific mutations also may be suitable as therapeutic targets (Table 1).16,17 Two main classes of genetic alterations or mutations have been identified in patients with AML: Class 1 includes genetic alterations or mutations that result in a survival advantage for and/or proliferation of hematopoietic progenitor cells, and Class 2 includes genetic alterations or mutations that cause hematopoietic cells to lose their ability to differentiate and undergo apoptosis. Collaboration between Class 1 and 2 mutations appears to be required for AML development.1 In addition, new subclasses are emerging based on gene expression profiles and microRNA expression signatures.51,52
Table 1.
Genetic Mutations That Affect Prognosis in Patients With Acute Myeloid Leukemia
| Name | Definition | Expression | Prognostic Effect | References |
|---|---|---|---|---|
| NPM-1 | Nucleophosmin-1 | Mutation | Favorable: Higher CR rates; better OS, EFS, and DFS | Thiede 2006,18 Dohner 2005,19 Schnitttger 200520 |
| FLT3-ITD | Fms-like tyrosine kinase receptor internal tandem deletion | Mutation | Unfavorable: Worse DFS and OS | Thiede 2002,21 Whitman 2001,22 Frohling 2002,23 Kainz 2002,24 Ciolli 2004,25 Stirewalt 200626 |
| FLT3-PM | Fms-like tyrosine kinase receptor point mutation | Mutation | Unclear | Frohling 200223 Kiyoi 2006,27 Whitman 2008,28 Mead 200729 |
| FLT3 | Fms-like tyrosine kinase receptor | Overexpression | Unfavorable: Worse OS | Ozeki 2004,30 Kang 201031 |
| BAALC | Brain and acute leukemia, cytoplasmic | Overexpression | Unfavorable: Worse DFS and OS; greater resistant disease | Baldus 2006,32 Baldus 200333 |
| MN1 | Meningioma 1, disrupted in balanced translocation | Overexpression | Unfavorable: Poor response to treatment, high relapse rate, worse risk-free survival and OS | Heuser 200634 |
| MLL-PTD | Mixed-lineage leukemia partial tandem duplication | Mutation/overexpression | Unfavorable: Lower remission durations, worse median survival and relapse-free intervals | Dohner 2002,35 Schnittger 2000,36 Weisser 200537 |
| CEBPα | CCAAT/enhancer-binding protein alpha | Mutation | Favorable: Better EFS, DFS, and OS | Boissel 2005,38 Bienz 2005,39 Barjesteh 2003,40 Frohling 2004,41 Preudhomme 200242 |
| ERG-1 | ETS-related gene-1 | Overexpression | Unfavorable: Worse OS, greater relapse | Marcucci 200543 |
| IDH-1 | Isocitrate dehydrogenase-1 | Mutation | Unfavorable: Worse DFS, higher risk of relapse | Marcucci 2010,44 Boissel 201045 |
| IDH-2 | Isocitrate dehydrogenase-2 | Mutation | Unfavorable: Lower remission rates, shorter OS, higher risk of induction failure | Marcucci 2010,44 Boissel 201045 |
| WT-1 | Wilms’ tumor-1 | Mutation | Unfavorable: Shorter OS, lower CR, higher relapse rates, shorter DFS | Becker 2010,46 Paschka 200847 Renneville 2009,48 Summers 2007,49 Virappane 200850 |
CR indicates complete response; OS, overall survival; EFS, event-free survival; DFS, disease-free survival.
The prognostic impact of a single mutation can be stratified by the presence or absence of mutations in other genes.53 For example, alterations in the most commonly mutated gene in blasts from cytogenetically normal AML patients, nucleophosmin-1 (NPM1), correlate with high cytoplasmic localization of its product, nucleophosmin. After standard therapy, NPM1 mutations confer a favorable prognosis.54 However, nearly 40% of patients with AML whose blasts harbor mutations in NPM1 also have mutations in Fms-like tyrosine kinase 3 (FLT3) that appear to abrogate this prognostic advantage.18,19
Mutations in FLT3 were among the first molecular abnormalities identified in patients with AML. These mutations likely confer the largest single-gene impact on prognosis in AML and serve as potential therapy targets. The remainder of this review is focused on the development of FLT3 inhibitors as an example of a rational, biology-driven, therapeutic approach in AML.
Role of FLT3 in Normal Hematopoiesis
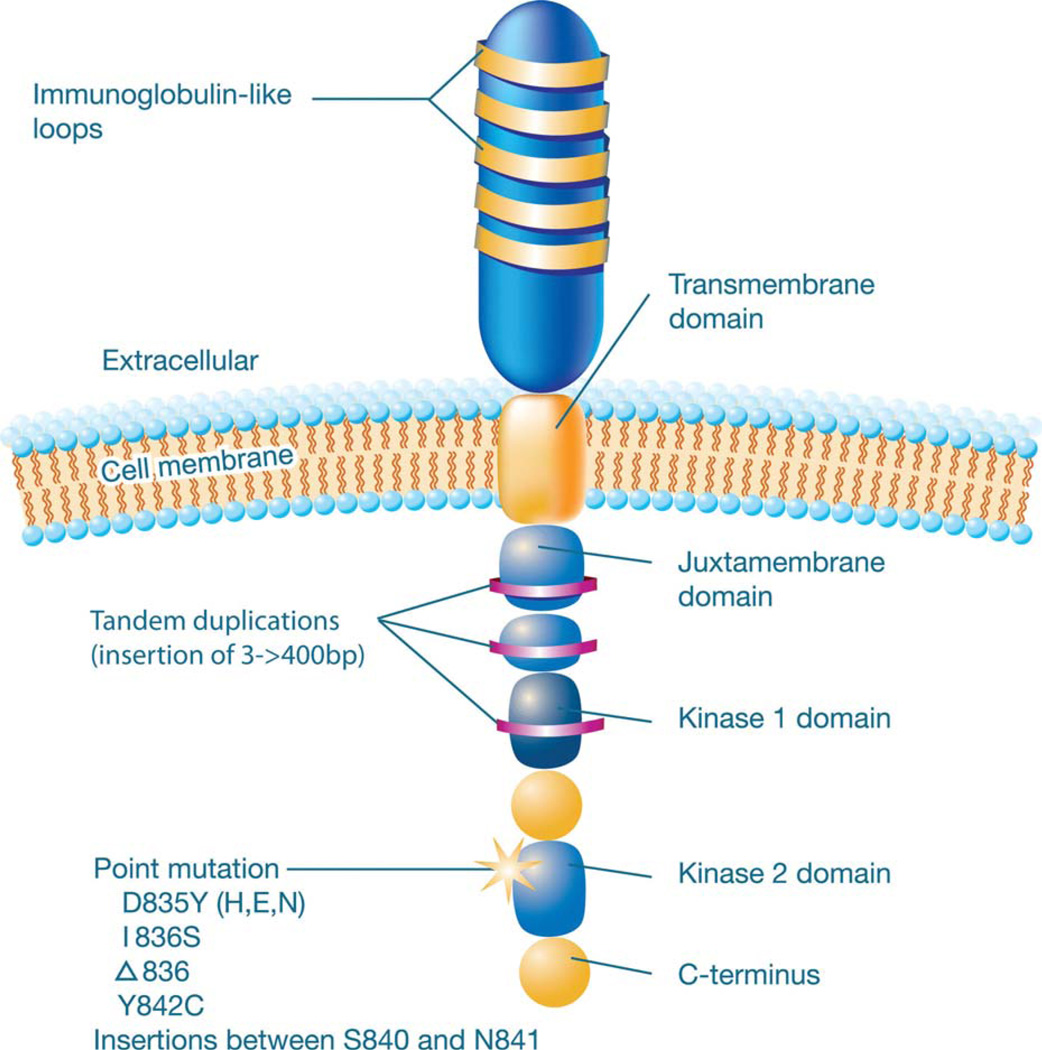
FLT3 encodes a Class III receptor tyrosine kinase that consists of 5 immunoglobulin-like domains, a transmembrane domain, a cytoplasmic juxtamembrane domain, and 2 tyrosine kinase domains (Fig. 1).55–57 FLT3 is expressed normally on bone marrow hematopoietic stem cells, but this expression is lost as these cells differentiate.58 In coordination with other growth factors, FLT3 plays a crucial role in normal hematopoiesis and cellular growth in primitive hematopoietic stem and progenitor cells.58,59 It is noteworthy that FLT3 appears to play this role not only in early progenitor cells but also in progenitors farther along the granulocyte/macrophage pathway, including common myeloid progenitors and granulocyte/macrophage progenitors.60
Figure 1.
This is a schematic depiction of the Fms-like tyrosine kinase 3 (FLT3) receptor. The most common sites of mutations or alterations are indicated (see Thiede et al, 200221; Litzow et al, 200555; Breitenbuecher et al, 200963; and Kayser et al, 2009107). D835Y (H,E,N) indicates substitution of tyrosine, histidine, glutamic acid, or asparagine for aspartic acid at codon 835; I836S, substitution of serine for isoleucine at codon 836; Δ836, delta 836 mutation of 3 base pairs (bp) affecting codon I836; Y842C, substitution of cysteine for tyrosine at codon 842.
Role of FLT3 in AML
FLT3 is expressed on the leukemic cells in 70% to 100% of patients with AML. In addition, activating mutations in FLT3 are observed in approximately 30% of adult patients with AML. Constitutively active, mutant FLT3 results in the proliferation and survival of leukemic blasts.1 Two forms of FLT3-activating mutations are identified commonly in the blasts from patients with AML—internal tandem duplications (ITDs) and point mutations.
Internal tandem duplications (ITDs) are changes that are observed in approximately 25% of all patients with AML.21 The duplications lead to an additional 3 to ≥ 100 amino acids inserted into the receptor,61 resulting in the ligand-independent, constitutive activation of FLT3.62 Until recently, ITDs had been identified only in the juxtamembrane domain of the protein; however, recent work has established that approximately 33% of ITDs occur within the tyrosine kinase domain.63
Point mutations are changes that occur in both the tyrosine kinase domain and the juxtamembrane domain, and they are observed in approximately 5% of all patients with AML.64,65 Both lead to the constitutive activation of FLT3; however, point mutations in the juxtamembrane domain appear to result in less activation compared with kinase domain point mutations and ITDs of the juxtamembrane domain.65
In addition to ITDs and point mutations, overexpression of FLT3 has been detected in both adult and pediatric patients with AML without FLT3 mutations, and this overexpression may have an unfavorable prognostic impact on overall survival (OS).30,31
Prognostic Implications of FLT3 Mutation
The negative impact of FLT3-ITD mutations on prognosis has been confirmed in multiple studies.21,22 AML patients with FLT3-ITD mutations who receive standard treatment may have lower complete response (CR) rates and, significantly, may have significantly shorter disease-free survival (DFS) and OS.22,66 The degree to which the FLT3 mutant allele is expressed also may affect outcome. Patients who had FLT3-ITD mutations with no detectable wild-type FLT3 had shorter DFS and OS compared with patients who had FLT3-ITD mutations with detectable wild-type FLT3.22 Similarly, patients who expressed mutant FLT3/wild-type FLT3 allele ratios > 0.78 had shorter DFS and OS compared with patients who lacked FLT3 mutations.21 The importance of FLT3 allele burden in patients with NPM1-mutated AML recently was determined.67 Typically, patients with NPM1-mutated AML have a favorable prognosis. However, an FLT3-ITD/wild-type ratio of ≥ 0.5 had a significant negative impact on prognosis in patients with NPM1-mutated AML. Thus, although FLT3-ITD status alone is informative in the clinic, the FLT3-ITD allele burden may provide further information. The negative prognostic influence of the FLT3-ITD mutation is observed across age groups, and patients who have FLT3-ITD mutations range in age from infants to elderly adults aged ≥ 60 years, and all have a negative prognosis compared with age-matched patients without FLT3-ITD mutations.68–71
FLT3 Inhibitors in Preclinical Studies
Several FLT3 inhibitors, including monoclonal antibodies and tyrosine kinase inhibitors (TKIs), have demonstrated antitumor activity in preclinical studies. TKIs block the aberrant signal transduction that contributes to tumor cell development. Several TKIs are under clinical development, including midostaurin (PKC-412),72 lestaurtinib (CEP-701),73 sorafenib (BAY 43–9006),74 sunitinib (SU11248),75 KW-2449,76 AC220,77 AP-24534,78 SB1518,79 and ITR-260.80 A recent study analyzed the ability of many of these agents to inhibit both wild-type and FLT3-ITD receptors in vitro and their cytotoxic effects against primary FLT3-ITD mutant blast samples obtained from patients with AML.81 All of the agents were capable of inhibiting both wild-type and FLT3-ITD autophosphorylation, although generally less inhibition was observed against the wild-type receptor. The agents can be broadly classified as highly selective (eg, AC220, sorafenib), intermediate (eg, sunitinib, KW-2449), and less selective (eg, lestaurtinib, midostaurin). It is noteworthy that, in one study, the selective inhibition of FLT3 alone was insufficient for in vitro induction of cytotoxicity in FLT3-ITD blasts obtained from some patients with newly diagnosed AML. The authors suggested that the greatest clinical utility of FLT3 inhibitors may be derived by treating newly diagnosed patients with a less selective agent and treating relapsed patients with a more selective agent.81 In addition, allelic burden was correlated with the in vitro activity of these agents.
Also under development is an anti-FLT3 monoclonal antibody, IMC-EB10, which blocks signaling by binding to the receptor and also induces antibody-dependent cell-mediated cytotoxicity.82 Preclinical studies have confirmed the antiproliferative effects of IMC-EB10 against both wild-type FLT3 and mutant FLT3 AML models.82
Clinical Trials Using FLT3 Inhibitors
FLT3 inhibitors are under investigation as single agents and in combination with other therapies in relapsed and refractory populations as well as newly diagnosed populations. In a retrospective analysis of OS among 213 patients who were treated on several studies at The University of Texas MD Anderson Cancer Center and received FLT3 inhibitors as part of their initial therapy or as salvage therapy, patients with wild-type FLT3 and mutant FLT3 had similar OS despite the expected inferior outcome for the patients with FLT3 mutations.83
Relapsed/refractory/poor risk patients: Single-agent studies
Lestaurtinib
This agent, also known as CEP-701, demonstrated safety, tolerability, and clinical activity in a phase 1/2 study that examined patients with refractory, relapsed, or poor-risk AML who had FLT3 mutations.84 Fourteen patients with a median age of 61 years received lestaurtinib 60 mg twice daily. Clinical activity was observed in 5 patients, including lowered peripheral blood blasts. Some patients also had evidence of stabilized normal hematopoiesis, as evidenced by transfusion independence. Commonly observed toxicities included grade 1/2 nausea and emesis (41% and 29%, respectively), and grade 3/4 generalized weakness (18%). Lestaurtinib at a dose of 60 mg twice daily also was tested as a single agent in a phase 2 study that included older patients with AML (median age, 73 years) who were not considered fit for standard chemotherapy.85 Unlike the prior study, this study was not restricted by FLT3 mutational status. Transient reductions in peripheral blood and bone marrow blasts as well as longer periods of transfusion independence were observed in 3 of 5 patients (60%) with FLT3 mutations and in 5 of 23 patients (23%) with wild-type FLT3. Similar adverse events (AEs) were observed as in the initial phase 1/2 trial. It is noteworthy that the study also included extensive correlative laboratory analyses, which did not reveal a link between baseline FLT3 expression or mutation and the likelihood of achieving a response to lestaurtinib. However, in a separate study, a clear correlation was observed between the results from an ex vivo bioassay, which revealed plasma inhibitory activity for FLT3 in patients who received FLT3 inhibitors versus clinical response.86 Those patients for whom FLT3 inhibitors demonstrated high plasma inhibitory activity ex vivo tended to be the best clinical responders to FLT3 inhibitor therapy. This finding suggests that it may be possible to monitor a particular patient’s plasma drug level to individually tailor the drug dose to achieve optimal FLT3 inhibition.
Midostaurin
The tolerability of midostaurin (PKC412) was determined in a phase 1 study.87 A phase 2 proof-of-concept trial was initiated in which patients (n = 20) with FLT3 mutations who had relapsed/refractory AML or myelodysplastic syndrome (MDS) received single-agent midostaurin at a dose of 75 mg orally 3 times daily.88 Although the trial was open to all patients aged ≥ 18 years, the median patient age was 62 years. A ≥ 50% decrease in peripheral blood and/or bone marrow blast counts was observed in 70% of patients. Seven patients (35%) achieved a > 2-log reduction in blast count for more than 4 weeks. Overall, the drug was well tolerated, and the most commonly observed AEs were grade 1/2 nausea and vomiting. A phase 2b study of midostaurin in patients with AML and MDS who had either wild-type FLT3 (n = 60) or mutant FLT3 (n = 35) also has been reported in which the patients received midostaurin 50 mg or 100 mg twice daily as a single agent.89 In that study, 64% of patients were aged ≥ 65 years. Among the 92 patients who were evaluable for response, 71% of patients who had mutant FLT3 had a ≥ 50% reduction in peripheral blood or bone marrow blasts compared with 42% of patients who had wild-type FLT3. One partial response was observed in a patient with mutant FLT3 on the 100-mg dose regimen. Grade 1/2 nausea and vomiting were the most common AEs and were observed in 60% and 48% of patients, respectively. It is noteworthy that there was no association between AEs and dose or mutational status. FLT3 autophosphorylation was inhibited in most of the responding patients, indicating in vivo target inhibition at the dose schedule used in that study.88
Sorafenib
Sorafenib (BAY 43–9006)—a multikinase inhibitor that targets the v-raf murine sarcoma viral oncogene homolog B1 (B-RAF), platelet-derived growth factor (PDGFR), fibroblast growth factor receptor (FGFR), and FLT3,74,90—currently is approved for the treatment of metastatic renal cancer and advanced hepatocellular carcinoma.91 FLT3 tyrosine kinase inhibition by sorafenib was established in primary human AML cells and in a mouse leukemia xenograft model and had a negligible effect on leukemic cells with wild-type FLT3, FLT3 kinase domain mutations, and normal hematopoietic progenitors.90 Sorafenib has been investigated as a targeted agent against FLT3-mutated AML. It was demonstrated that sorafenib was safe and had clinical activity in 2 phase 1 trials that examined the effects of both dose and schedule of sorafenib in patients with relapsed or refractory AML.92,93 In the first trial, 15 heavily pretreated patients were recruited, and 10 of those patients received a full cycle of sorafenib (3 patients discontinued the study because of disease progression, and 2 discontinued because of complications from infections). Sorafenib was well tolerated, and 6 of 10 patients (60%) who received at least 1 cycle of sorafenib achieved a response, which most frequently was manifested by a profound decrease in peripheral blood and/or bone marrow blasts. However, responses were transient; and, predictably, they were observed only in patients who had FLT3-ITD mutations.92 The second phase 1 trial explored greater dose escalation in a 15-patient cohort with refractory leukemias, because the maximum tolerated dose (MTD) was not achieved in the previous trial. An MTD of sorafenib 400 mg twice daily for a 21-day cycle was determined. The most common grade 3 or higher AEs consisted of fatigue (16%) and hypokalemia (13%). Stable disease was achieved by 11 of 15 patients (73%), and 6 of those patients (40%) had a reduction in bone marrow blasts after only 1 cycle of treatment. However, once again, the responses were transient in nature.93
A randomized phase 1 trial that examined 2 schedules of sorafenib, either continuous or intermittent, at 4 dose levels (100 mg, 200 mg, 300 mg, or 400 mg; all given twice daily) was conducted by the National Cancer Institute of Canada in patients with relapsed or refractory AML (n = 38) or untreated MDS (n = 4).94 Dose-limiting toxicity (DLT) was prevalent at the 400 mg twice-daily dose, but no DLTs were observed at the 300 mg twice-daily dose. Furthermore, sorafenib was well tolerated on a continuous schedule, resulting in the recommended schedule of 300 mg twice daily continuously for 28-day cycles in that patient population. The vast majority of drug-related AEs in that study were gastrointestinal-related events: Thirty-eight percent of all patients experienced grade 1/2 diarrhea or abdominal pain (13 patients had grade 1/2 AEs; and 3 patients had grade 3, 4, or 5 AEs). One CR was noted in a patient with FLT3-ITD–positiveAML.94
Sorafenib administered either before or after allogeneic stem cell transplantation (allo-SCT) also has been explored in a small group of patients with relapsed or refractory, FLT3-ITD–positive AML.95 Sorafenib treatment occurred before allo-SCT (n = 2), after allo-SCT (n = 3), or both before and after allo-SCT (n = 1). Sorafenib-induced remission allowed for allo-SCT in 2 of the 3 refractory patients. Two of the 4 patients who received sorafenib after allo-SCT survived for 216 days and 221 days, respectively, whereas the other 2 patients remained in ongoing complete molecular remission.95
A recent case study also reported the use of sorafenib in an FLT3-ITD–positive woman aged 43 years with extramedullary disease who relapsed after she underwent allo-SCT.96 The antitumor activity of sorafenib was demonstrated in this patient, who achieved a molecular remission.
Sunitinib
Two phase 1 trials have been conducted in AML patient cohorts examining sunitinib (SU11248), a small-molecule inhibitor of RAF, vascular endothelial growth factor 2, c-KIT, and FLT3.97 Sunitinib is approved for use in patients with advanced renal cancer or gastrointestinal stromal tumors who are resistant or intolerant of imatinib. A phase 1, single-agent, dose-finding study of sunitinib was conducted in 16 patients with AML who were either refractory or not amenable to conventional therapy.98 The majority of those patients (67%) were aged > 65 years. No DLTs were observed in the 13 patients who received a starting dose of sunitinib 50 mg daily; the most frequent grade 2 AEs consisted of mild edema, fatigue, and oral ulcerations. The 75-mg daily dose received by 2 patients resulted in 1 episode each of grade 4 fatigue, hypertension, and cardiac failure, which resulted in the elimination of this dose level from further evaluation. All 4 patients with FLT3 mutations had morphologic responses (absence of leukemic blasts from peripheral blood, < 5% blasts in bone marrow, peripheral level of hemoglobin > 90 g/L, and an absolute neutrophil count > 1 × 109/L) or partial responses (a reduction of the absolute blast count in peripheral blood and bone marrow blast percentage by 50%comparedwith baseline) compared with only 2 of 10 evaluable patients who had wild-type FLT3. All responses were of short duration, although the responses were longer in patients who had mutated FLT3.
AC220
AC220 is a TKI that targets KIT, colony-stimulating factor 1 receptor (CSF1R), rearranged during transfection (RET), and PDGFR, but its greatest activity is against FLT3. AC220 has been explored in a phase 1 dose-finding and safety trial in 76 elderly patients with either relapsed or refractory AML or untreated AML.99 The MTD was identified as AC220 200 mg once daily on a continuous dosing schedule. Pharmacokinetic evaluation of treated patients revealed that AC220 potently inhibited phosphorylated FLT3 in FLT3-ITD cells at this dose. AC220 had significant clinical activity, inducing not only a reduction in blast counts but a full CR in some patients. Overall, 10 of 76 patients (13%) patients achieved a CR (2 CRs, 6 CRs with incomplete blood count recovery, and 2 CRs with incomplete platelet recovery) and 13 patients (17%) achieved a partial response. Most responses were observed during the first 28-day cycle of treatment. The median duration of response was 14 weeks with response durations up to 67 weeks and beyond. Higher overall response rates and CR rates were observed in patients who had FLT3-ITD mutations (56% and 28%, respectively) compared with patients who lacked those mutations (20% and 7%, respectively). The most commonly reported AEs that may have been drug-related were mainly grade 2 and included peripheral edema, dysgeusia, and nausea.99
KW-2449
A phase 1, ascending-dose study of KW-2449 was conducted in patients with acute leukemias.100 In vitro studies determined that the cytotoxic effect of KW-2449 ocurred at concentrations sufficient to inhibit FLT3 autophosphorylation to < 20% of baseline. Transient reductions in peripheral blast counts were observed in the patients, and quantitative measurements of FLT3 inhibition determined that FLT3 itself was inhibited only transiently to < 20% of baseline. This study was terminated because the chosen dose was insufficient. A second, phase 1/2 trial aimed at determining the optimal dose of KW-2449 was recently also terminated because of failure to establish a tolerable dose that had potential efficacy.101
Other agents that have demonstrated preclinical activity currently are being explored in phase 1/2 trials, including AP-24534 and the monoclonal antibody IMC-EB10 (Table 2).
Table 2.
Currently Ongoing Clinical Trials Using fms-Like Tyrosine Kinase 3 Receptor Inhibitors for the Treatment of Acute Myeloid Leukemia
| Moleculeh | Phase | Patient Population | FLT3 Mutational Status |
Combination | Recruitment Status |
NCT Identifiera |
|---|---|---|---|---|---|---|
| Midostaurin | 3 | Newly diagnosed, aged <60 y | Mutants only | Daunorubicin and cytarabine | Recruiting | NCT00651261 |
| AC220 | 2 | Relapsed/refractory, aged ≥18 y | ITD mutants only | NA | Recruiting | NCT00989261 |
| Sorafenib | 2 | Newly diagnosed, ages 18–60 y | All | Standard chemotherapy | Recruiting | NCT00893373 |
| Lestaurtinib | 2 | Relapsed, aged ≥18 y | Mutants only | Induction chemotherapy | Not recruiting | NCT00079482 |
| Midostaurin | 1/2 | Poor-risk, aged ≥60 y/aged >70 y | All | Azacitidine | Recruiting | NCT 01093573 |
| Sorafenib | 1/2 | Aged ≥60 y | All | Cytarabine | Recruiting | NCT00516828 |
| Sorafenib | 1/2 | Ages 15–60 y | All | Idarubicin and cytarabine | Not recruiting | NCT00542971 |
| Midostaurin | 1/2 | Relapsed/refractory, ages 3 mo–18 y | Mutants only | NA | Recruiting | NCT00866281 |
| Sunitinib | 1/2 | Aged ≥60 y | Mutants only | Standard chemotherapy | Recruiting | NCT00783653 |
| Midostaurin | 1 | Newly diagnosed, ages 18–60 y | All | Daunorubicin and cytarabine | Not recruiting | NCT00093600 |
| Midostaurin | 1 | Relapsed/refractory or poor prognosis, aged ≥18 y | All | RAD001 | Recruiting | NCT00819546 |
| AC220 | 1 | Relapsed/refractory, aged ≥18 y | All | NA | Not recruiting | NCT00462761 |
| Sorafenib | 1 | Relapsed/refractory, ages 2–20 y | All | NA | Recruiting | NCT00343694 |
| Sorafenib | 1 | Relapsed/refractory, aged ≥18 y | All | NA | Recruiting | NCT00217646 |
| Sorafenib | 1 | Relapsed/refractory, aged ≥18 y | All | NA | Not recruiting | NCT00131989 |
| Sorafenib | 1 | Relapsed/refractory, ages 0–31 y | All | Cytarabine and clofarabine | Recruiting | NCT00908167 |
| Sorafenib | 1 | Poor-risk AML, aged ≥18 yrs | All | Vorinostat | Recruiting | NCT00875745 |
| Sorafenib | 1 | Poor-risk, aged ≥18 y | Mutants only | G-CSF and plerixafor | Not recruiting | NCT00943943 |
| AP-24534 | 1 | Relapsed/refractory, aged ≥18 y | All | NA | Recruiting | NCT00660920 |
| IMC-EB10 | 1 | Relapsed/refractory, aged ≥18 y | All | NA | Recruiting | NCT00887926 |
FLT3, fms-like tyrosine kinase 3 receptor; NCT, National Clinical Trials; ITD, internal tandem duplication; NA, not applicable; G-CSF, granulocyte colony-stimulating factor.
Available at http://www.clinicaltrials.gov.
Combination with chemotherapy
Single-agent studies of FLT3-inhibitory TKIs have revealed that these agents generally are well tolerated but usually are limited by transient clinical activity. Therefore, several agents are being explored in combination with other therapies.
Relapsed/refactory patients
Lestaurtinib
In vitro studies demonstrated a synergistic cytotoxic effect when lestaurtinib was given after chemotherapy in FLT3-mutant AML cells.102 A phase 2 randomized trial was initiated in which patients with mutated FLT3 aged ≥ 18 years in their first relapse were assigned randomly to receive either chemotherapy alone or chemotherapy followed by lestaurtinib 80 mg twice daily. The chemotherapy regimen used was determined according to the duration of initial remission; if the initial remission lasted from 1 to 6 months, then patients received mitoxantrone, etoposide, and cytarabine; whereas patients who had an initial remission of 6 to 24 months received high-dose cytarabine. Overall, the addition of lestaurtinib failed to increase response rates or to prolong the survival of patients with FLT3-mutant AML in first relapse. However, cytotoxicity analyses also were performed on the blasts from patients who received lestaurtinib to gauge their in vitro sensitivity to the drug with a target of > 85% FLT3 inhibition defined from previous studies. Of 79 patients who were tested, 46 patients (58%) achieved this degree of FLT3 inhibition on Day 15 of treatment. Of these 46 “biologic responders,” 18 (39%) achieved a CR, whereas only 3 of 32 patients (9%) who exhibited FLT3 inhibition < 85% achieved a CR.103 The authors concluded that FLT3 inhibition by lestaurtinib, when achieved, was correlated with better CR rates but that this benefit was negated by a poor CR rate in those patients who did not achieve the FLT3 inhibition target. However, these results suggest that, if a more profound and sustained FLT3 inhibition could be achieved, then a favorable effect in long-term outcome might be expected.
Newly diagnosed patients
Midostaurin
A phase 1b study in previously untreated adult patients with AML aged ≤ 60 years was conducted with midostaurin added either in sequence or concomitantly with daunorubicin and cytarabine induction chemotherapy.104 Patients with both mutant and wild-type FLT3 were included. During the trial, the dose and schedule were amended twice to identify a well tolerated option that did not result in a high discontinuation rate. The final dosage regimen was midostaurin 50 mg twice daily on Days 1 through 8 and 15 through 21 (concomitant) or on Days 8 through 21. Of the 40 patients enrolled at the 50 mg twice daily dose, 27 patients had wild-type FLT3, and 13 patients had mutant FLT3. The overall CR rate was not affected by the midostaurin administration schedule: A CR was observed in 32 of 40 patients (80%) in the entire cohort, including 20 of 77 all patients (74%) with wild-type FLT3 and 12 of 13 patients (92%) with mutant FLT3. It is noteworthy that OS was similar between the mutant FLT3 and wild-type FLT3 groups, suggesting to the authors that the addition of midostaurin may have overcome the adverse prognostic effect of FLT3 mutations. The encouraging results observed in the patients with FLT3mutations led to further pursuit of this strategy in an ongoing phase 3, randomized trial (Table 2) that included induction (daunorubicin/cytarabine) and consolidation (high-dose cytarabine) with either midostaurin or placebo in patients with newly diagnosed FLT3-mutated AML aged < 60 years who were stratified according to their FLT3 allele burden into 3 groups: ITD allelic ratio < 0.7, ITD allelic ratio ≥ 0.7, and those with point mutations.
Sorafenib
Sorafenib is currently under investigation in a phase 1/2 trial combined concomitantly with idarubicin and cytarabine in newly diagnosed patients aged < 65 years.105 In the phase 1 portion of that trial, patients received escalating doses and various schedules of sorafenib, and continuous dosing at 400 mg twice daily was established as the phase 2 dose. In the phase 1 portion, 10 patients were treated, including 7 who had FLT3-ITD mutations and had received a median of 2 previous regimens. A CR was achieved by 4 patients, including 3 who had FLT3-ITD mutations. The phase 2 portion included 51 patients (15 with FLT3-ITD mutations and 2 with FLT3 point mutations) with previously untreated AML. Their median age was 53 years. Of 51 evaluable patients, 38 (75%) achieved a CR, including 12 of 13 patients (92%) who had FLT3-ITD mutations, 2 of 2 patients (100%) who had FLT3 point mutations, and 24 of 36 patients (66%) who had wild-type FLT3. The difference in the CR rate between patients with mutated FLT3 and patients with wild-type FLT3 was statistically significant (P = .033). The regimen was well tolerated. The grade 3 AEs, possibly related to the addition of sorafenib to induction chemotherapy, included hyperbilirubinemia in 4 patients, elevated transaminases in 5 patients, and diarrhea in 4 patients, most of which were generally transient. After at a median follow-up of 54 weeks, the probability of survival was 83% at 6 months and 74% at 12 months. Among the patients who had mutated FLT3, 10 patients had relapsed and 5 remained in CR at a median follow-up of 62 weeks (range, 10–76 weeks). Correlative studies determined that, in addition to suppressing the activity of mutant FLT3 in these patients, sorafenib effectively down-regulated the expression of phosphorylated extracellular signal-related kinase, which acts downstream of FLT3 to promote cell survival and proliferation in AML cells.106
Conclusions
Recent advances in the understanding of AML biology have led to significant contributions in the prognostic classification of patients. Most important, this deeper understanding has given us the opportunity for therapeutic intervention. An understanding of the molecular complexity of cytogenetically normal patients with AML and the associated heterogeneity in their outcomes has led to efforts to develop rationally designed, personalized therapy for these specific subgroups of patients, such as FLT3 inhibitors. Growing evidence suggests that these agents have clear clinical value for patients with AML, and particularly for those who have FLT3 mutations. Considering the complex molecular events that characterize AML and the variable characteristics of these agents and their interaction with other agents, our challenge will be to better understand and properly manage these agents to achieve their full potential. The introduction of FLT3 inhibitors for the treatment of AML may well be the start of a new era in the management of this disease after many frustrating years of exclusive dependency on cytotoxic chemotherapy.
Acknowledgments
We thank Michelle Boehm, PhD, for her medical editorial assistance with this manuscript through financial support for editorial assistance from Novartis Pharmaceuticals.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
REFERENCES
- 1.Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–198. doi: 10.1146/annurev.genom.3.032802.115046. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Web site. [Accessed August 8, 2010]; http:\\www.cancer.org.
- 3.Horner MJ, Ries LAG, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; 2009. SEER Cancer Statistics Review, 1975–2006 [based on the November 2008 SEER data submission, posted to the SEER website 2009. http:\\seer.cancer.gov/csr/1975_2006/. Access date. [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia. Vol. 1. Jenkentown, PA: NCCN; 2010. [Google Scholar]
- 5.Schiffer CA. Hematopoietic growth factors and the future of therapeutic research on acute myeloid leukemia. N Engl J Med. 2003;349:727–729. doi: 10.1056/NEJMp030076. [DOI] [PubMed] [Google Scholar]
- 6.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, Burnett AK, Agura ED, Kantarjian HM. Progress in the treatment of acute myeloid leukemia. Cancer. 2007;110:1900–1910. doi: 10.1002/cncr.23000. [DOI] [PubMed] [Google Scholar]
- 8.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 9.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 10.Downing JR. The core-binding factor leukemias: lessons learned from murine models. Curr Opin Genet Dev. 2003;13:48–54. doi: 10.1016/s0959-437x(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 12.Krauter J, Wagner K, Schafer I, et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27:3000–3006. doi: 10.1200/JCO.2008.16.7981. [DOI] [PubMed] [Google Scholar]
- 13.Schaich M, Schlenk RF, Al-Ali HK, et al. Prognosis of acute myeloid leukemia patients up to 60 years of age exhibiting trisomy 8 within a non-complex karyotype: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. Haematologica. 2007;92:763–770. doi: 10.3324/haematol.11100. [DOI] [PubMed] [Google Scholar]
- 14.Kebriaei P, de Lima M, Estey E. Management of acute leukemias. In: DeVita V, Lawrence T, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 8th ed. Vol. 2. Philadelphia, PA: Lippencott Williams & Williams; 2008. pp. 2232–2265. [Google Scholar]
- 15.Estey EH, Pierce S, Keating MJ. Identification of a group of AML/MDS patients with a relatively favorable prognosis who have chromosome 5 and/or 7 abnormalities. Haematologica. 2000;85:246–249. [PubMed] [Google Scholar]
- 16.Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory TK, Wald D, Chen Y, Vermaat JM, Xiong Y, Tse W. Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics [serial online] J Hematol Oncol. 2009;2:23. doi: 10.1186/1756-8722-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006;107:4011–4020. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 19.Dohner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–3746. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 20.Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–3739. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 21.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 22.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 23.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 24.Kainz B, Heintel D, Marculescu R, et al. Variable prognostic value of FLT3 internal tandem duplications in patients with de novo AML and a normal karyotype, t(15;17), t(8;21) or inv(16) Hematol J. 2002;3:283–289. doi: 10.1038/sj.thj.6200196. [DOI] [PubMed] [Google Scholar]
- 25.Ciolli S, Vannucchi AM, Leoni F, et al. Internal tandem duplications of Flt3 gene (Flt3/ITD) predicts a poor postremission outcome in adult patients with acute non-promyelocytic leukemia. Leuk Lymphoma. 2004;45:73–78. doi: 10.1080/1042819031000151851. [DOI] [PubMed] [Google Scholar]
- 26.Stirewalt DL, Kopecky KJ, Meshinchi S, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–3726. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiyoi H, Naoe T. Biology, clinical relevance, and molecularly targeted therapy in acute leukemia with FLT3 mutation. Int J Hematol. 2006;83:301–308. doi: 10.1532/IJH97.06071. [DOI] [PubMed] [Google Scholar]
- 28.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–1270. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 30.Ozeki K, Kiyoi H, Hirose Y, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103:1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- 31.Kang HJ, Lee JW, Kho SH, et al. High transcript level of FLT3 associated with high risk of relapse in pediatric acute myeloid leukemia. J Korean Med Sci. 2010;25:841–845. doi: 10.3346/jkms.2010.25.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldus CD, Thiede C, Soucek S, Bloomfield CD, Thiel E, Ehninger G. BAALC expression and FLT3 internal tandem duplication mutations in acute myeloid leukemia patients with normal cytogenetics: prognostic implications. J Clin Oncol. 2006;24:790–797. doi: 10.1200/JCO.2005.01.6253. [DOI] [PubMed] [Google Scholar]
- 33.Baldus CD, Tanner SM, Ruppert AS, et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: a Cancer and Leukemia Group B study. Blood. 2003;102:1613–1618. doi: 10.1182/blood-2003-02-0359. [DOI] [PubMed] [Google Scholar]
- 34.Heuser M, Beutel G, Krauter J, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108:3898–3905. doi: 10.1182/blood-2006-04-014845. [DOI] [PubMed] [Google Scholar]
- 35.Dohner K, Tobis K, Ulrich R, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–3261. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 36.Schnittger S, Kinkelin U, Schoch C, et al. Screening for MLL tandem duplication in 387 unselected patients with AML identify a prognostically unfavorable subset of AML. Leukemia. 2000;14:796–804. doi: 10.1038/sj.leu.2401773. [DOI] [PubMed] [Google Scholar]
- 37.Weisser M, Kern W, Schoch C, Hiddemann W, Haferlach T, Schnittger S. Risk assessment by monitoring expression levels of partial tandem duplications in the MLL gene in acute myeloid leukemia during therapy. Haematologica. 2005;90:881–889. [PubMed] [Google Scholar]
- 38.Boissel N, Renneville A, Biggio V, et al. Prevalence, clinical profile, and prognosis of NPM mutations in AML with normal karyotype. Blood. 2005;106:3618–3620. doi: 10.1182/blood-2005-05-2174. [DOI] [PubMed] [Google Scholar]
- 39.Bienz M, Ludwig M, Leibundgut EO, et al. Risk assessment in patients with acute myeloid leukemia and a normal karyotype. Clin Cancer Res. 2005;11:1416–1424. doi: 10.1158/1078-0432.CCR-04-1552. [DOI] [PubMed] [Google Scholar]
- 40.Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, Meijer J, et al. Biallelic mutations in the CEBPA gene and low CEBPA expression levels as prognostic markers in intermediate-risk AML. Hematol J. 2003;4:31–40. doi: 10.1038/sj.thj.6200216. [DOI] [PubMed] [Google Scholar]
- 41.Frohling S, Schlenk RF, Stolze I, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22:624–633. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 42.Preudhomme C, Sagot C, Boissel N, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA) Blood. 2002;100:2717–2723. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]
- 43.Marcucci G, Baldus CD, Ruppert AS, et al. Overexpression of the ETS-related gene, ERG, predicts a worse outcome in acute myeloid leukemia with normal karyotype: a Cancer and Leukemia Group B study. J ClinOncol. 2005;23:9234–9242. doi: 10.1200/JCO.2005.03.6137. [DOI] [PubMed] [Google Scholar]
- 44.Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boissel N, Nibourel O, Renneville A, et al. Prognostic impact of isocitrate dehydrogenase enzyme isoforms 1 and 2 mutations in acute myeloid leukemia: a study by the Acute Leukemia French Association Group. J Clin Oncol. 2010;28:3717–3723. doi: 10.1200/JCO.2010.28.2285. [DOI] [PubMed] [Google Scholar]
- 46.Becker H, Marcucci G, Maharry K, et al. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:788–792. doi: 10.1182/blood-2010-01-262543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paschka P, Marcucci G, Ruppert AS, et al. Wilms’ tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:4595–4602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renneville A, Boissel N, Zurawski V, et al. Wilms tumor 1 gene mutations are associated with a higher risk of recurrence in young adults with acute myeloid leukemia: a study from the Acute Leukemia French Association. Cancer. 2009;115:3719–3727. doi: 10.1002/cncr.24442. [DOI] [PubMed] [Google Scholar]
- 49.Summers K, Stevens J, Kakkas I, et al. Wilms’ tumour 1 mutations are associated with FLT3-ITD and failure of standard induction chemotherapy in patients with normal karyotype AML. Leukemia. 2007;21:550–551. doi: 10.1038/sj.leu.2404514. author reply 552. [DOI] [PubMed] [Google Scholar]
- 50.Virappane P, Gale R, Hills R, et al. Mutation of the Wilms’ tumor 1 gene is a poor prognostic factor associated with chemotherapy resistance in normal karyotype acute myeloid leukemia: the United Kingdom Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2008;26:5429–5435. doi: 10.1200/JCO.2008.16.0333. [DOI] [PubMed] [Google Scholar]
- 51.Bacher U, Kohlmann A, Haferlach C, Haferlach T. Gene expression profiling in acute myeloid leukaemia (AML) Best Pract Res Clin Haematol. 2009;22:169–180. doi: 10.1016/j.beha.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Larson RA. Micro-RNAs and copy number changes: new levels of gene regulation in acute myeloid leukemia. Chem Biol Interact. 2010;184:21–25. doi: 10.1016/j.cbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 54.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 55.Litzow MR. More flitting about FLT3. Blood. 2005;106:3331–3332. [Google Scholar]
- 56.Rosnet O, Schiff C, Pebusque MJ, et al. Human FLT3/FLK2 gene: CDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–1119. [PubMed] [Google Scholar]
- 57.Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991;6:1641–1650. [PubMed] [Google Scholar]
- 58.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 60.Kikushige Y, Yoshimoto G, Miyamoto T, et al. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J Immunol. 2008;180:7358–7367. doi: 10.4049/jimmunol.180.11.7358. [DOI] [PubMed] [Google Scholar]
- 61.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 62.Grundler R, Miething C, Thiede C, Peschel C, Duyster J. FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood. 2005;105:4792–4799. doi: 10.1182/blood-2004-11-4430. [DOI] [PubMed] [Google Scholar]
- 63.Breitenbuecher F, Schnittger S, Grundler R, et al. Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. 2009;113:4074–4077. doi: 10.1182/blood-2007-11-125476. [DOI] [PubMed] [Google Scholar]
- 64.Frohling S, Skelin S, Liebisch C, et al. Comparison of cytogenetic and molecular cytogenetic detection of chromosome abnormalities in 240 consecutive adult patients with acute myeloid leukemia. J Clin Oncol. 2002;20:2480–2485. doi: 10.1200/JCO.2002.08.155. [DOI] [PubMed] [Google Scholar]
- 65.Reindl C, Bagrintseva K, Vempati S, et al. Point mutations in the juxtamembrane domain of FLT3 define a new class of activating mutations in AML. Blood. 2006;107:3700–3707. doi: 10.1182/blood-2005-06-2596. [DOI] [PubMed] [Google Scholar]
- 66.Kottaridis PD, Gale RE, Frew ME, et al. The presence of an FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 67.Schnittger S, Weiss T, Haferlach C, Kern W, Haferlach T. Prognostic impact of FLT3 mutation load in NPM1 mutated AML [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 826. [Google Scholar]
- 68.Breccia M, Frustaci AM, Cannella L, et al. Comorbidities and FLT3-ITD abnormalities as independent prognostic indicators of survival in elderly acute myeloid leukaemia patients. Hematol Oncol. 2009;27:148–153. doi: 10.1002/hon.889. [DOI] [PubMed] [Google Scholar]
- 69.Emerenciano M, Menezes J, Vasquez ML, et al. Clinical relevance of FLT3 gene abnormalities in Brazilian patients with infant leukemia. Leuk Lymphoma. 2008;49:2291–2297. doi: 10.1080/10428190802491698. [DOI] [PubMed] [Google Scholar]
- 70.Shimada A, Taki T, Tabuchi K, et al. Tandem duplications of MLL and FLT3 are correlated with poor prognoses in pediatric acute myeloid leukemia: a study of the Japanese Childhood AML Cooperative Study Group. Pediatr Blood Cancer. 2008;50:264–269. doi: 10.1002/pbc.21318. [DOI] [PubMed] [Google Scholar]
- 71.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 73.Hexner EO, Serdikoff C, Jan M, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 75.O’Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 76.Shiotsu Y, Kiyoi H, Ishikawa Y, et al. KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I-mutated BCR/ABL translocation. Blood. 2009;114:1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- 77.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivera VM, Xu Q, Berk L, et al. Potent antitumor activity of AP24534, an orally active inhibitor of bcr-abl, Flt3 and other kinases, in both in vitro and in vivo models of acute myeloid leukemia (AML) [abstract] Blood (ASH Annual Meeting Abstracts) 2008;112 Abstract 2932. [Google Scholar]
- 79.Hart S, Goh KC, Tan YC, Amalini C, Wood JM. SB1518, a novel oral JAK2-FLT3 inhibitor, is efficacious in models of acute myeloid leukemia and primary leukemic cells from patients [abstract]. Proceedings of the 101st Annual Meeting of the American Association for Cancer Res; AARC; April 17–21, 2010; Washingtonm, DC. Philadelphia, PA: AARC; 2010. Abstract 2524. [Google Scholar]
- 80.Liu C-P, Liu HE, Lee O, et al. ITRI-260, a promising candidate for treatment of acute myeloid leukemia [abstract]. Proceedings of the 101st Annual Meeting of the American Association for Cancer Res; AARC; April 17–21, 2010; Washington, DC. Philadelphia, PA: AARC; 2010. Abstract LB-160. [Google Scholar]
- 81.Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115:1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Youssoufian H, Rowinsky EK, Tonra J, Li Y. Targeting FMS-related tyrosine kinase receptor 3 with the human immunoglobulin G1 monoclonal antibody IMC-EB10. Cancer. 2010;116:1013–1017. doi: 10.1002/cncr.24787. [DOI] [PubMed] [Google Scholar]
- 83.Pemmaraju N, Kantarjian HM, Ravandi F, et al. FLT3 inhibitor therapy for patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML): impact on survival according to FLT3 status [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 1026. [Google Scholar]
- 84.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 85.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 86.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108:3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485–1492. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- 88.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 89.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 91.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 92.Quintas-Cardama A, Kantarjian H, Andreef M, et al. Phase I trial of intermittent administration of sorafenib (BAY 43–9006) for patients (pts) with refractory/relapsed acute myelogenous leukemia (AML) [abstract] J Clin Oncol. 2007;25(18S) suppl Abstract 7018. [Google Scholar]
- 93.Pratz KW, Cho E, Karp J, et al. Phase I dose escalation trial of sorafenib as a single agent for adults with relapsed and refractory acute leukemias [abstract] J Clin Oncol. 2009;27(15S) Abstract 7065. [Google Scholar]
- 94.Crump M, Hedley D, Kamel-Reid S, et al. A randomized phase I clinical and biologic study of 2 schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: an NCIC (National Cancer Institute of Canada) Clinical Trials Group study. Leuk Lymphoma. 2010;51:252–260. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- 95.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 96.Safaian NN, Czibere A, Bruns I, et al. Sorafenib (nexavar) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leuk Res. 2009;33:348–350. doi: 10.1016/j.leukres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 97.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 98.Fiedler W, Serve H, Dohner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 99.Cortes J, Foran J, Ghirdaladze D, et al. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a first-in-human (FIH) phase 1 AML study [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 636. [Google Scholar]
- 100.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113:3938–3946. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kyowa Pharmaceutical, Inc. Safety, Tolerability, and Pharmacokinetic/Pharmacodynamic Study of KW-2449 in Acute Myelogenous Leukemia. [Accessed April 26, 2010];National Clinical Trials identifier NTC00779480. http://clinicaltrials.gov/ct2/show/NCT00779480?term=kw-2449&rank=2.
- 102.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 103.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for FLT3 mutant AML patients in first relapse [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114 doi: 10.1182/blood-2010-08-301796. Abstract 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stone RM, Fischer T, Paquette R, et al. A phase 1b study of midostaurin (PKC412) in combination with daunorubicin and cytarabine induction and high-dose cytarabine consolidation in patients under age 61 with newly diagnosed de novo acute myeloid leukemia: overall survival of patients whose blasts have FLT3 mutations is similar to those with wild-type FLT3 [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 634. [Google Scholar]
- 105.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 107.Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–2392. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]