Abstract
Objective
To examine familial aggregation of gout and to estimate the heritability and environmental contributions to gout susceptibility in the general population.
Methods
Using data from the National Health Insurance (NHI) Research Database in Taiwan, we conducted a nationwide cross-sectional study of data collected from 22 643 748 beneficiaries of the NHI in 2004; among them 1 045 059 individuals had physician-diagnosed gout. We estimated relative risks (RR) of gout in individuals with affected first-degree and second-degree relatives and relative contributions of genes (heritability), common environment shared by family members and non-shared environment to gout susceptibility.
Results
RRs for gout were significantly higher in individuals with affected first-degree relatives (men, 1.91 (95% CI 1.90 to 1.93); women, 1.97 (95% CI 1.94 to 1.99)) and also in those with affected second-degree relatives (men, 1.27 (95% CI 1.23 to 1.31); women, 1.40 (95% CI 1.35 to 1.46)). RRs (95% CIs) for individuals with an affected twin, sibling, offspring, parent, grandchild, nephew/niece, uncle/aunt and grandparent were 8.02 (6.95 to 9.26), 2.59 (2.54 to 2.63), 1.96 (1.95 to 1.97), 1.93 (1.91 to 1.94), 1.48 (1.43 to 1.53), 1.40 (1.32 to 1.47), 1.31 (1.24 to 1.39), and 1.26 (1.21 to 1.30), respectively. The relative contributions of heritability, common and non-shared environmental factors to phenotypic variance of gout were 35.1, 28.1 and 36.8% in men and 17.0, 18.5 and 64.5% in women, respectively.
Conclusions
This population-based study confirms that gout aggregates within families. The risk of gout is higher in people with a family history. Genetic and environmental factors contribute to gout aetiology, and the relative contributions are sexually dimorphic.
Keywords: Gout, Epidemiology, Arthritis
Introduction
Gout is the most common inflammatory joint disease1–4 with an impact on morbidity5–7 and premature mortality.8–10 The disease is heritable, as suggested by familial clustering of the disease;11–20 however, the existence of many known risk factors, such as male gender, increasing age,21 22 obesity,23 chronic renal impairment,24 hypertension,25 26 long-term use of diuretics27 and certain diets with high purine28 and alcohol,29 also supports a strong environmental contribution. Currently, the balance between genetic and environmental contributions is still unclear.
High heritability of hyperuricaemia,30 the main driver of urate crystal deposition and the development of gout, has led to efforts to identify susceptibility genes. A large familial segregation study has demonstrated significant heritability for hyperuricaemia30 and specific genetic associations, particularly genes involved in renal urate clearance, have been identified that mechanistically might explain genetic susceptibility to hyperuricaemia.31–34 Despite the strong evidence supporting a genetic contribution to hyperuricaemia, studies concerning the relative contributions of genetic and environmental factors to gout are rare. A complex segregation analysis conducted in aborigines in Taiwan showed a substantial genetic component for gout,35 but a recent classic twin study, with 514 all-male twin pairs in the US, paradoxically found significant heritability for hyperuricaemia but not for clinical gout.36 Additionally, efforts largely failed to identify susceptibility genes to gout beyond genes controlling serum urate concentration, thus questioning the role of genetic factors in gout.34
Therefore, we undertook the first nationwide population-based study to estimate the degree of familial aggregation of gout and the extent to which heritability and a common familial environment might each account for familial aggregation. We studied this in Taiwan because, first, Taiwan has one of the highest reported estimates of gout prevalence worldwide37 and, second, there is an established nationwide health insurance database containing sufficient demographic, family history and medical data on the entire Taiwanese population to allow us to address these questions.
Methods
The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (approval number 101-2178C).
Source of data
The primary data source came from the National Health Insurance Research Database (NHIRD), which contains registration information and original claims data on all beneficiaries of NHI in Taiwan since its establishment in 1995. All entries for an individual are linked by a unique personal identifier assigned to each Taiwanese resident, which allows accurate linkage of records from the registration files and from the original claims data. Before release for research, personal identifiers are deidentified to ensure confidentiality.
The registry of beneficiaries, one of the registration files, contains details of demographics, residence, kinship relationships, occupation categories, insurance status and insurance amount of all beneficiaries of NHI. Claims data on all outpatient visits, inpatient care and pharmacy dispensing were recorded in specific datasets with information, such as dates of events, medical diagnoses, medical expenditure and details of prescriptions, operations, examinations and procedures.
Study population and classification
The study population consisted of all NHI beneficiaries (11 360 576 men; 11 283 172 women) in 2004, representing 99.8% of the total population of Taiwan at the end of 2004.38 Enrolled individuals were classified according to the affection status of gout of their first-degree and second-degree relatives who were registered in the NHI before 2004.
Identification of cases with gout
The primary case definition of gout was having a physician-recorded diagnosis of gout (International Classification of Diseases, Ninth Revision [ICD-9] code: 274.x) together with at least one prescription containing gout-specific medications (colchicine, benzbromarone, allopurinol, probenecid, sulfinpyrazone) at either an outpatient or emergency visit during 2000–2004. An alternative definition, used for sensitivity analysis, was having two outpatient or emergency visits with a physician-recorded diagnosis of gout during 2000–2004. An identical case definition of gout was used for all individuals and their relatives.
Identification of first-degree and second-degree relatives and family ascertainment
The registry of beneficiaries specifies relationships between the insured person who pays the fee, and his/her dependents, allowing parent-offspring relationships and spouses to be identified directly. Among 28 402 865 individuals registered with the NHI during 1996–2010, 21 009 551 pairs of parent-offspring relationships were identified. Full siblings were identified as individuals who shared the same parents. Twins were full siblings who have the same date of birth (±1 day). Second-degree relatives were ascertained based on the aforementioned relationships. These links allowed the identification of 4 191 274 families spanning 2–5 generations.
Demographics and socioeconomic information
We also incorporated socioeconomic factors, including residence, occupations and income levels, to reflect population stratification with the aboriginals (with significantly higher prevalence of gout39) and Han people in Taiwan. For details of these factors, please refer to the online supplementary materials.
Statistical analysis
The prevalence of gout was calculated for the general population and for individuals who had an affected spouse and/or affected relatives. Any individual fulfilling the case definitions of gout was defined as a prevalent case. For prevalence of gout in individuals with affected first-degree and second-degree relatives, age and sex were taken into account and age-standardised and sex-standardised prevalence (95% CI) was determined. The standard population used was the general population of Taiwan in 2004.
The degree of familial aggregation of gout was estimated using the relative risk (RR), which was calculated as the adjusted prevalence ratio between individuals with affected relatives and the entire population of Taiwan in 200440 The marginal Cox proportional hazard model with an equal follow-up time for all subjects with robust sandwich estimate,41 42 adjusted for age, place of residence, income, occupation and family size, was used to optimise the estimate of the RR. Because case clustering within a family may occur, the robust sandwich estimate was used when calculating confidence bounds.41 The RR was estimated for individuals with different family relatives affected with gout, including first-degree and second-degree relatives affected, and for the number of affected first-degree relatives (father, mother, son, daughter, brother, sister).
We used the standard ACE model to examine the influences of additive genetic (A), common environmental factors shared by family members (C) and non-shared environmental factors (E) accounting for variance in a phenotype (P). This model can be expressed as:
where σp2=total phenotypic variance; σc2=common environmental variance; σc2=common environmental variance and σE2=non-shared environmental variance.
The heritability was defined as the proportion of phenotypic variance that is attributable to genetic factors and can be expressed as 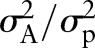 and the familial transmission was expressed as
and the familial transmission was expressed as 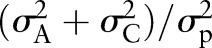 , which is the sum of heritability and common environmental variances.
, which is the sum of heritability and common environmental variances.
We used the polygenic liability model to calculate both measures.43–45 For details of this model, please see the online supplementary material. We used the sibling RR, spouse RR and the prevalence of gout in the general population (p) to calculate the familial transmission and the heritability, which were expressed as
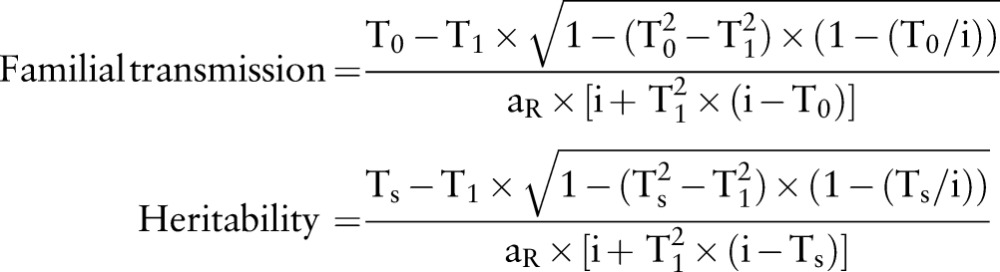 |
where 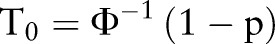 ;
; 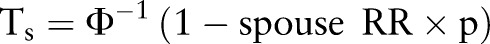 ;
; 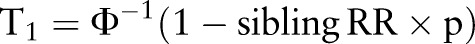 ; p=prevalence of gout in the normal population); aR: the additive genetic relationship between the relatives, for full sibling, aR=0.5; i=z/p; z, the height of the standard normal curve pertaining to gout prevalence, and Φ, standard normal cumulative distribution function.46
; p=prevalence of gout in the normal population); aR: the additive genetic relationship between the relatives, for full sibling, aR=0.5; i=z/p; z, the height of the standard normal curve pertaining to gout prevalence, and Φ, standard normal cumulative distribution function.46
Therefore, the common environmental component was the difference between familial transmission and heritability. Since the epidemiologic and clinical features of gout are sexually dimorphic, and hence, equal genetic variances in both sexes may not hold true,47 we estimated sex-specific familial transmission and heritability using respective sex-specific populations.
All analyses were performed for primary and alternative case definitions of gout. A 2-sided p value 0.05 was considered statistically significant. All analyses were performed using SAS V.9.3 (SAS institute, Cary, North Carolina, USA).
Results
Gout prevalence in individuals with affected family members versus the general population
We identified 802 765 men and 242 294 women with gout in 2004 giving a crude prevalence of gout of 4.62% (95% CI 4.61% to 4.63%) (see online supplementary table S1). Men had a significantly higher prevalence (7.07%, 95% CI 7.05% to 7.08%) than women (2.15%, 95% CI 2.14% to 2.16%). We identified 1 663 904 individuals with at least one affected first-degree relative, and 604 468 individuals with at least one affected second-degree relative. The standardised prevalence of gout in individuals with affected first-degree and second-degree relatives were 13.37% (95% CI 13.35% to 13.39%) and 10.05% (95% CI 10.03% to 10.06%) in men, and 4.16% (95% CI 4.15% to 4.18%) and 3.01% (95% CI 3.00% to 3.02%) in women, respectively. Figure 1a and 1b show age-specific and sex-specific prevalence of gout in men and women which, at all ages, is higher in individuals with affected first-degree relatives than in those with second-degree relatives and the general population.
Figure 1.
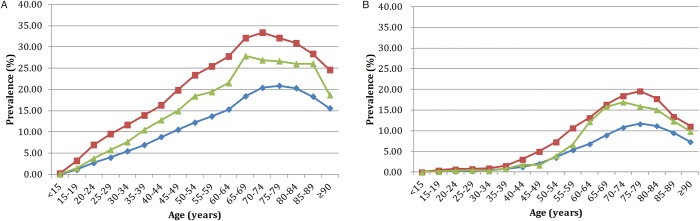
Age-specific prevalence of gout in men (A) and women (B) according to the affection status of relatives (red, individuals with affected first-degree relatives; green, individuals with affected second-degree relatives; blue, the general population).
Family exposure and risk of gout
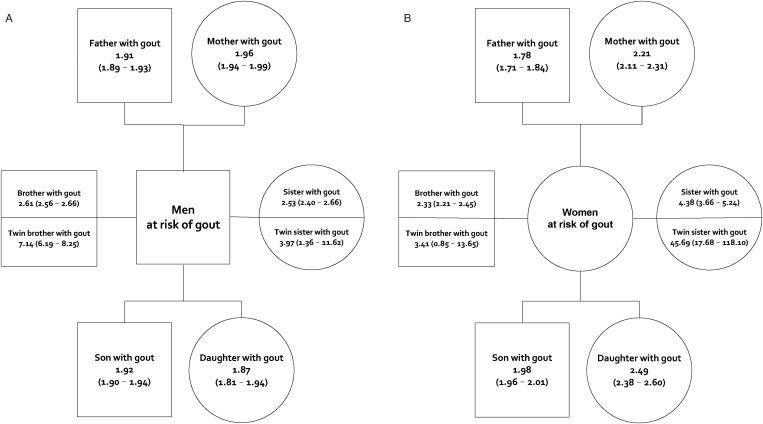
The risk of gout was significantly higher in individuals with affected first-degree relatives than in the general population, the RRs being 1.91 (95% CI 1.90 to 1.93) in men and 1.97 (95% CI 1.94 to 1.99) in women (see online supplementary table S2). Individuals with affected second-degree relatives also had an increased risk of gout, albeit significantly lower than those with affected first-degree relatives, with RRs of 1.27 (95% CI 1.23 to 1.31) in men and 1.40 (95% CI 1.35 to 1.46) in women. Figure 2 shows that individuals with an affected twin had the highest risk, followed by individuals with an affected sibling, then individuals with an affected offspring and, finally, individuals with an affected parent. Same-sex twins had the highest RR, being higher in female-female twin pairs than male-male twin pairs. The RRs for gout in individuals with any category of affected second-degree relative (table 1) were lower than RRs in those with affected first-degree relatives (figure 2). The RRs also increased with the number of affected first-degree relatives. Compared with the general population, individuals with one, two or three or more categories of affected first-degree relatives had RRs (95% CIs) of 1.87 (1.86 to 1.89), 3.22 (3.15 to 3.29) and 4.96 (4.64 to 5.30), respectively. This trend was more prominent in women (figure 3).
Figure 2.
Relative risks (95% CI) of gout among (A) men and (B) women with affected first-degree relatives (square, male; circle, female) in comparison with the general population in Taiwan in 2004.
Table 1.
Relative risk of gout among individuals with affected second-degree relatives in comparison with the general population in Taiwan in 2004
| Affected second-degree relatives | Men at risk | Women at risk | ||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| Grandparent | ||||
| Grandfather | 1.18 | 1.12 to 1.25 | 1.29 | 1.10 to 1.51 |
| Grandmother | 1.31 | 1.25 to 1.37 | 1.45 | 1.24 to 1.68 |
| Grandchild | ||||
| Grandson | 1.25 | 1.20 to 1.31 | 1.45 | 1.39 to 1.52 |
| Granddaughter | 1.39 | 1.21 to 1.59 | 1.54 | 1.33 to 1.78 |
| Uncle or aunt | ||||
| Uncle | 1.32 | 1.24 to 1.40 | 1.19 | 0.96 to 1.45 |
| Aunt | 1.21 | 0.98 to 1.48 | 0.91 | 0.41 to 2.03 |
| Nephew or niece | ||||
| Nephew | 1.42 | 1.34 to 1.51 | 1.16 | 0.95 to 1.41 |
| Niece | 1.42 | 1.16 to 1.74 | 0.90 | 0.41 to 2.00 |
Figure 3.
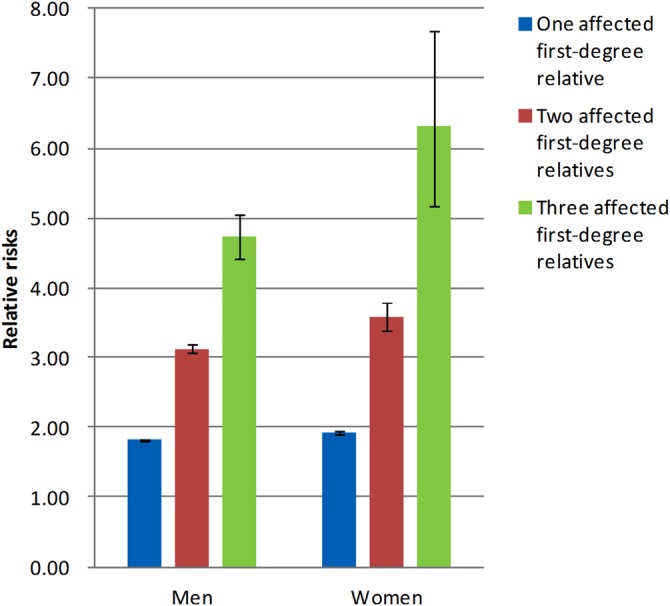
The ‘dose-response’ relationship between the numbers of affected first-degree relatives and relative risk of gout (blue: one; red: two; green: three first-degree relatives).
Familial aggregation of gout was evident in individuals with affected biological relatives, and also in those with affected spouses. The RRs were 1.66 (1.65–1.68) in men with an affected wife and 1.65 (95% CI 1.64 to 1.67) in women with an affected husband.
Relative contributions of genetic, common and non-shared environmental factors
To separate the influences of genes and environment, we calculated heritability and familial transmission. In men, heritability was 35.1% (95% CI 34.1% to 36.0%) and familial transmission was 63.2% (95% CI 61.8% to 64.7%); whereas in women, they were 17.0% (95% CI 15.0% to 19.0%) and 35.5% (95% CI 33.1% to 37.8%), respectively. Figure 4 shows the relative contributions of genetic (heritability), common environmental and non-shared environmental components to the phenotypic variances of gout.
Figure 4.
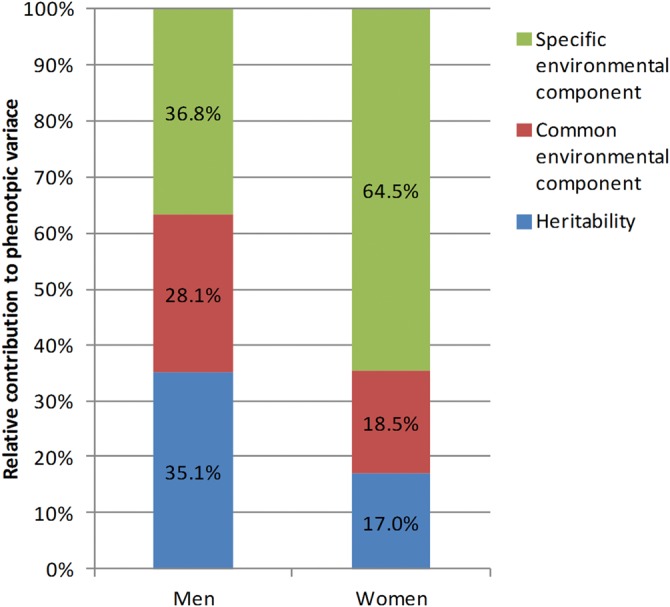
Relative contributions of heritability (blue), common environmental (red) and specific environmental factors (green) to phenotypic variation of gout.
Sensitivity analysis
We also used alternative case definition of gout to do sensitivity analysis. The results were very similar to our primary analysis (please see online supplementary table S3, figures S1 and S2).
Discussion
This nationwide population study has confirmed familial aggregation of gout by demonstrating a greater prevalence and RR of gout for individuals with affected family members compared to the general population. The risk of gout is increased more by having affected first-degree relatives than having affected second-degree relatives, and appears ‘dose-dependent’ in that the risk increases with the number of affected relatives. These results confirm the long-held belief that gout clusters within families and supports an important contribution of common familial factors in predisposing to the development of gout.
However, biological relatives tend to share similar environmental and lifestyle risk factors in addition to genes; both contribute to familial aggregation. Therefore, we examined the risk associated with having a spouse who has gout on the assumption that any increased risk from this predominantly reflects predisposition from environmental and lifestyle factors common to family members. We found that the relative contributions differ between men and women; however, overall it appears that genetic factors play a smaller, but still substantial, role than environmental factors in the aetiology of gout. Our findings are consistent with the relative paucity of gout susceptibility genes identified by genome-wide association studies in comparison with greater numbers of genes associated with risk of hyperuricaemia, which has a greater heritability.31–34
Consistent with previous studies, our findings provide strong evidence to support the existence of familial aggregation of gout.11–19 However, current evidence concerning the relative contributions of genetic and environmental exposures for gout susceptibility is limited. A complex segregation study conducted in the aborigines of Taiwan supported the existence of a substantial genetic predisposition to gout; however, no heritability estimate was reported.35 By contrast, one recent study of 253 monozygotic and 261 dizygotic North American male twin pairs found a significant heritability for hyperuricaemia (49.6%) but, surprisingly, given that chronic hyperuricaemia is the key mechanism for urate crystal formation, no heritability (0%; 95% CI 0% to 61.8%) for gout.36 Nevertheless, our whole population study provided several lines of evidence to support the existence of genetic predisposition to gout. First, our data on twin pairs showed significantly different risk profiles in same-sex twins compared to opposite-sex twins. Although lack of information on zygosity prevented the calculation of heritability based on twin data, the higher RR shared by same-sex (partly monozygotic) twins compared to opposite-sex (exclusively dizygotic) twins supports a genetic contribution. Second, using the spouse as an indicator of shared environmental risk, we estimated a heritability of 35.1% in men and 17.0% in women. Therefore, although not the sole explanation for familial aggregation, genetic factors in addition to environmental influences, do contribute to the development of gout.
It has long been observed that men are significantly more likely to have gout than women.48 49 Additionally, onset of gout is later in women.50 The cause of this sexual dimorphism is not clear. One explanation is the uricosuric effect of oestrogen which results in lower serum urate levels in premenopausal women.51 Therefore, prevalence of gout is generally low in premenopausal women and increases dramatically after menopause.52 Different exposure to environmental risk factors may also contribute to the sex difference. For instance, dietary calorie intake and alcohol consumption are lower in women than men in Taiwan according to a national nutrition survey.53 54 Our study shows that familial transmission and heritability are both significantly higher in men. These findings suggest that genetic and common environmental factors are the main predisposing factors to gout in men, but not in women. Therefore, the sex difference can be partly attributed to different contributions from family factors. Further study is needed to confirm this finding.
There are several limitations to the study. First, it was confined to Taiwan, so results may not be generalisable to other settings. Second, the NHIRD is primarily a health insurance database that contains limited information on criteria for clinical diagnosis. We did not have data on potential confounding factors, therefore, we cannot test the interactions between family history and other confounders and their independent contributions to the risk of gout. Further, our analysis of relative genetic and environmental contributions was based on the multifactorial liability model, and our results are subject to assumptions, so should be interpreted with caution. However, the published data on other disease, such as schizophrenia46 support the validity of this model. Finally, we cannot account for the effects of assortative mating whereby spouses are more similar for a phenotype than they would be if mating occurred at random in the population. If this assortment is not negligible, a biased estimation of relative genetic and environmental contributions may occur.55
Our main strengths include the use of data from the entire population of approximately 23 million individuals, and systematic methods to identify and ascertain first-degree and second-degree relatives, which allow very precise estimation of prevalence and RRs of gout with minimal selection bias. The virtually complete identification of gout cases, and the use of consistent case definitions for individuals at risk and their relatives, ensured the absence of information bias. Furthermore, we used prospectively recorded data for diagnosis, for construction of family relationships and for ascertaining socioeconomic information, thus minimising recall bias and other errors associated with self-reporting.
The present study provides quantitative estimates of familial RR and heritability for gout in an entire population of Taiwan. Our results confirm the clinical belief that gout clusters within families, and that genetic and environmental components contribute to its aetiology. Studies of familial risk in other populations are required to determine the generalisability of these findings to other populations.
Supplementary Material
Acknowledgments
We would like to thank the National Science Council of Taiwan (project NSC 102-2314-B-182A-104) and Chang Gung Memorial Hospital (project CMRPG3B1671) for financial support and the University of Nottingham for methodological assistance and infrastructure. Sponsors of the study had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. This study is based on the National Health Insurance Research Database, which is provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes, Taiwan. The interpretation and conclusions contained herein do not represent positions of the Bureau of National Health Insurance or the National Health Research Institutes.
Footnotes
Contributors: Study concept and design: C-FK, WZ and MD; acquisition of data: C-FK, L-CS, K-HYu and S-FL; analysis and interpretation of data: C-FK, MJG, L-CS, AMV, WZ and MD; drafting of the manuscript: C-FK and WZ; critical revision of the manuscript for important intellectual content: C-FK, MJG, L-CS, K-HY, S-FL, AMV, WZ and MD; statistical analysis: C-FK, MJG, L-CS and WZ; obtaining funding: C-FK, L-CS, K-HY and S-FL; administrative, technical, or material support: MJG, L-CS, K-HY, S-FL, AMV, WZ and MD; study supervision: WZ, MD and MJG.
Funding: Chang Gung Memorial Hospital, and the National Science Council in Taiwan.
Competing interests: None.
Ethics approval: Chang Gung Memorial Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data and statistical codes are available on request from the corresponding author at zandis@gmail.com
References
- 1.Choi HK, Mount DB, Reginato AM, et al. Pathogenesis of gout. Ann Intern Med 2005;143:499–516. [DOI] [PubMed] [Google Scholar]
- 2.Annemans L, Spaepen E, Gaskin M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis 2008;67:960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011;63:3136–41. [DOI] [PubMed] [Google Scholar]
- 4.Winnard D, Wright C, Taylor WJ, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology (Oxford) 2012;51:901–9. [DOI] [PubMed] [Google Scholar]
- 5.Ab. Usbott RD, Brand FN, Kannel WB, et al. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol 1988;41:237–42. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan E, Baker JF, Furst DE, et al. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006;54:2688–96. [DOI] [PubMed] [Google Scholar]
- 7.Sheane BJ, Cunnane G. Tophaceous gout and chronic kidney disease. J Clin Rheumatol 2007;13:293. [DOI] [PubMed] [Google Scholar]
- 8.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894–900. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan E, Svendsen K, Neaton JD, et al. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med 2008;168:1104–10. [DOI] [PubMed] [Google Scholar]
- 10.Kuo CF, See LC, Luo SF, et al. Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology (Oxford) 2010;49:141–6. [DOI] [PubMed] [Google Scholar]
- 11.Mituszova M, Judak A, Poor G, et al. Clinical and family studies in Hungarian patients with gout. Rheumatol Int 1992;12:165–8. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg BS. Heredity of gout and hyperuricemia. Arthritis Rheum 1965;8:627–47. [DOI] [PubMed] [Google Scholar]
- 13.Emmerson BT. Heredity in primary gout. Australas Ann Med 1960;9:168–75. [DOI] [PubMed] [Google Scholar]
- 14.Hauge M, Harvald B. Heredity in gout and hyperuricemia. Acta Med Scand 1955;152:247–57. [DOI] [PubMed] [Google Scholar]
- 15.Smyth CJ, Cotterman CW, Freyberg RH. The genetics of gout and hyperuricaemia. Ann Rheum Dis 1948;7:248. [PubMed] [Google Scholar]
- 16.Smyth CJ, Cotterman CW, Freyberg RH. The genetics of gout and hyperuricaemia; an analysis of 19 families. J Clin Invest 1948;27:749–59. [PubMed] [Google Scholar]
- 17.Grahame R, Scott JT. Clinical survey of 354 patients with gout. Ann Rheum Dis 1970;29:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copeman WSC. A short history of the gout and the rheumatic diseases. Berkeley: University of California Press, 1964. [Google Scholar]
- 19.Cobb S. The frequency of the rheumatic diseases. Cambridge: Harvard University Press, 1971. [Google Scholar]
- 20.Reginato AM, Mount DB, Yang I, et al. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol 2012;8:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: is the incidence rising? J Rheumatol 2002;29:2403–06. [PubMed] [Google Scholar]
- 22.Mikuls TR, Saag KG. New insights into gout epidemiology. Curr Opin Rheumatol 2006;18:199–203. [DOI] [PubMed] [Google Scholar]
- 23.Choi HK, Atkinson K, Karlson EW, et al. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005;165:742–8. [DOI] [PubMed] [Google Scholar]
- 24.Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med 2008;75(Suppl 5):S13–16. [DOI] [PubMed] [Google Scholar]
- 25.Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two-year followup of a prospective cohort. Arthritis Rheum 2010;62:1069–76. [DOI] [PubMed] [Google Scholar]
- 26.Choi HK, Atkinson K, Karlson EW, et al. Obesity, weight change, hypertension, diuretic use, and risk of gout in men—The health professionals follow-up study. Arch Intern Med 2005;165:742–8. [DOI] [PubMed] [Google Scholar]
- 27.Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, et al. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012;41:879–89. [DOI] [PubMed] [Google Scholar]
- 28.Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol 2005;17:341–5. [PubMed] [Google Scholar]
- 29.Choi HK, Atkinson K, Karlson EW, et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 2004;363:1277–81. [DOI] [PubMed] [Google Scholar]
- 30.Wilk JB, Djousse L, Borecki I, et al. Segregation analysis of serum uric acid in the NHLBI Family Heart Study. Hum Genet 2000;106:355–9. [DOI] [PubMed] [Google Scholar]
- 31.Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437–42. [DOI] [PubMed] [Google Scholar]
- 32.Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 2009;5:e1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 2008;372:1953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kottgen A, Albrecht E, Teumer A, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet 2013;45:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang WH, Chang SJ, Wang TN, et al. Complex segregation and linkage analysis of familial gout in Taiwanese aborigines. Arthritis Rheum 2004;50:242–6. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan E, Lessov-Schlaggar CN, Krasnow RE, et al. Nature versus nurture in gout: a twin study. Am J Med 2012;125:499–504. [DOI] [PubMed] [Google Scholar]
- 37.Lin KC, Lin HY, Chou P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J Rheumatol 2000;27:1045–50. [PubMed] [Google Scholar]
- 38.Directorate General of Budget, Accounting and Statistics, Executive Yuan, Taiwan. Population by sex, rate of population increase, average persons per household, density and natural increase rate. http://eng.stat.gov.tw/lp.asp?ctNode=2265&CtUnit=1072&BaseDSD=36&MP=5 (accessed 15 Aug 2013).
- 39.Chou CT, Lai JS. The epidemiology of hyperuricaemia and gout in Taiwan aborigines. Br J Rheumatol 1998;37:258–62. [DOI] [PubMed] [Google Scholar]
- 40.Risch N. Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet 1990;46:222–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med 1994;13:2233–47. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Chia KS. Estimation of prevalence rate ratios for cross sectional data: an example in occupational epidemiology. Br J Ind Med 1993;50:861–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falconer DS. The inheritance of liability to diseases with variable age of onset, with particular reference to diabetes mellitus. Ann Hum Genet 1967;31:1–20. [DOI] [PubMed] [Google Scholar]
- 44.Reich T, James JW, Morris CA. The use of multiple thresholds in determining the mode of transmission of semi-continuous traits. Ann Hum Genet 1972;36:163–84. [DOI] [PubMed] [Google Scholar]
- 45.Reich T, Rice J, Cloninger CR, et al. The use of multiple thresholds and segregation analysis in analyzing the phenotypic heterogeneity of multifactorial traits. Ann Hum Genet 1979;42:371–90. [DOI] [PubMed] [Google Scholar]
- 46.Wray NR, Gottesman II. Using summary data from the danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet 2012;3:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet 2008;9:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cea Soriano L, Rothenbacher D, Choi HK, et al. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther 2011;13:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikuls TR, Farrar JT, Bilker WB, et al. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005;64:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Souza A, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol 2005;32:2186–8. [PubMed] [Google Scholar]
- 51.Adamopoulos D, Vlassopoulos C, Seitanides B, et al. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol (Copenh) 1977;85:198–208. [DOI] [PubMed] [Google Scholar]
- 52.Hak AE, Curhan GC, Grodstein F, et al. Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis 2010;69:1305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin YC, Yen LL, Chen SY, et al. Prevalence of overweight and obesity and its associated factors: findings from National Nutrition and Health Survey in Taiwan, 1993–1996. Prev Med 2003;37:233–41. [DOI] [PubMed] [Google Scholar]
- 54.Wu SJ, Pan WH, Yeh NH, et al. Trends in nutrient and dietary intake among adults and the elderly: from NAHSIT 1993–1996 to 2005–2008. Asia Pac J Clin Nutr 2011;20:251–65. [PubMed] [Google Scholar]
- 55.Rice TK. Familial resemblance and heritability. Adv Genet 2008;60:35–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.