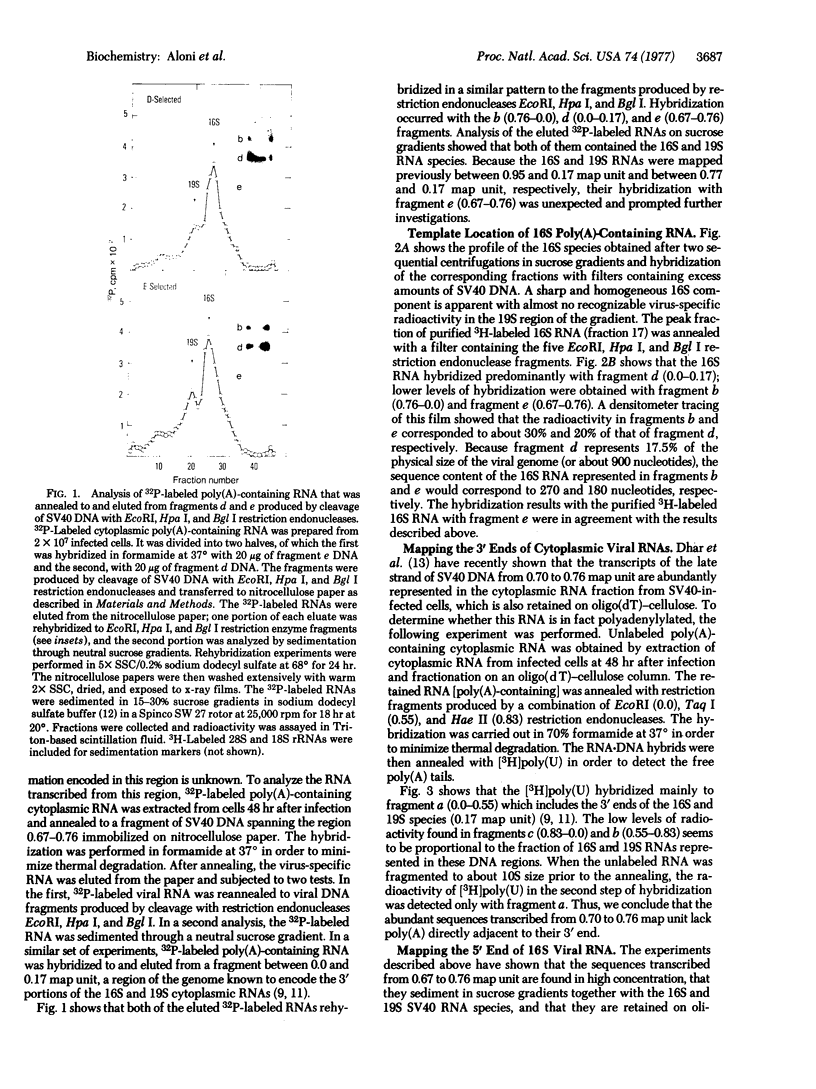
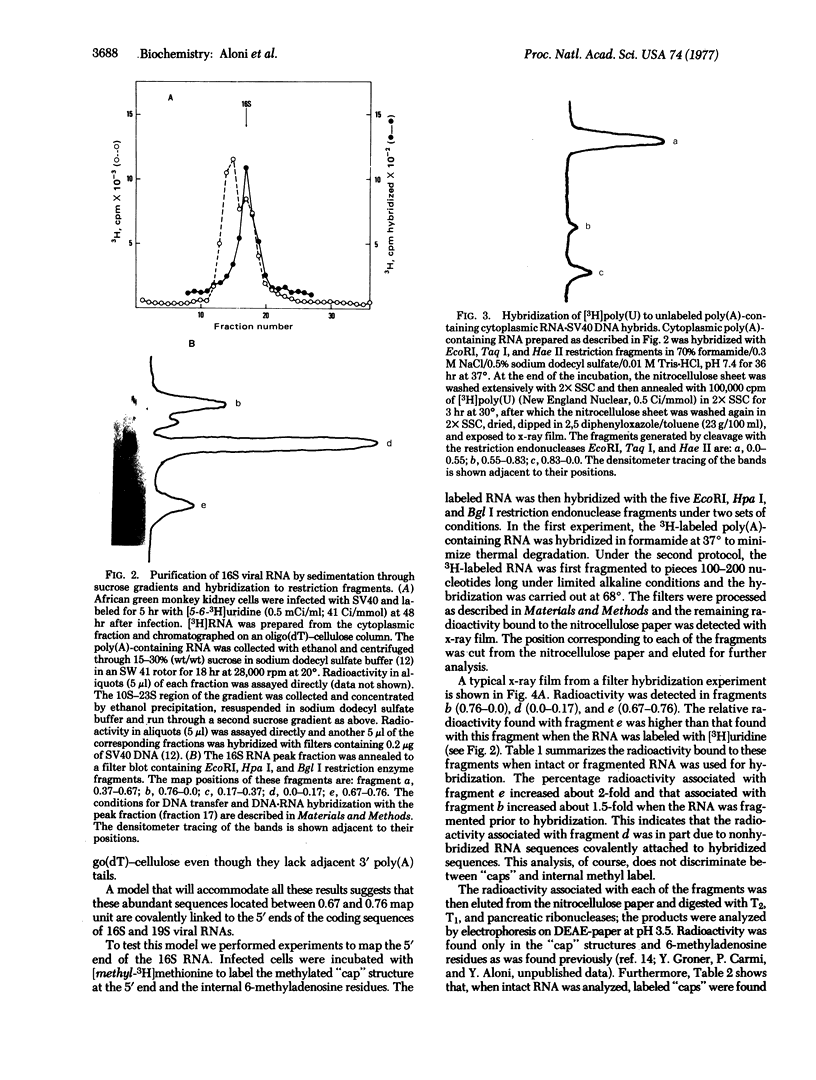
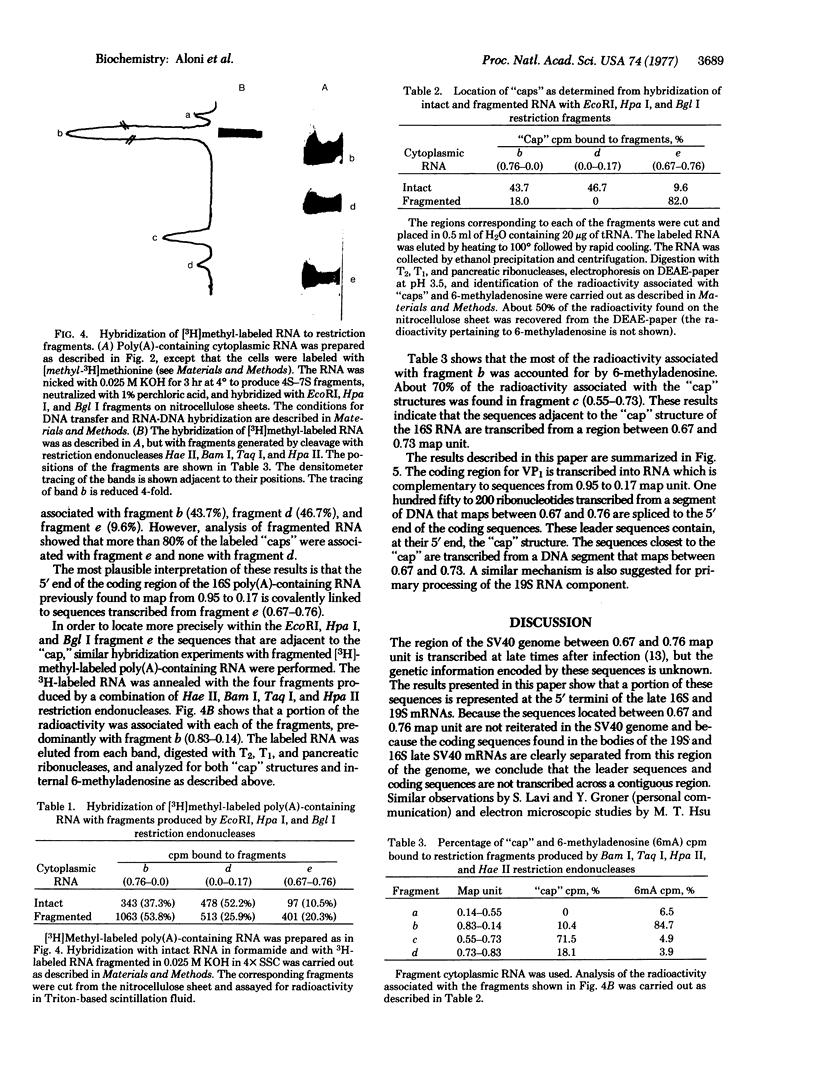
Abstract
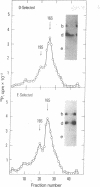
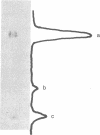
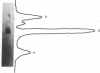
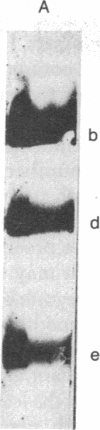
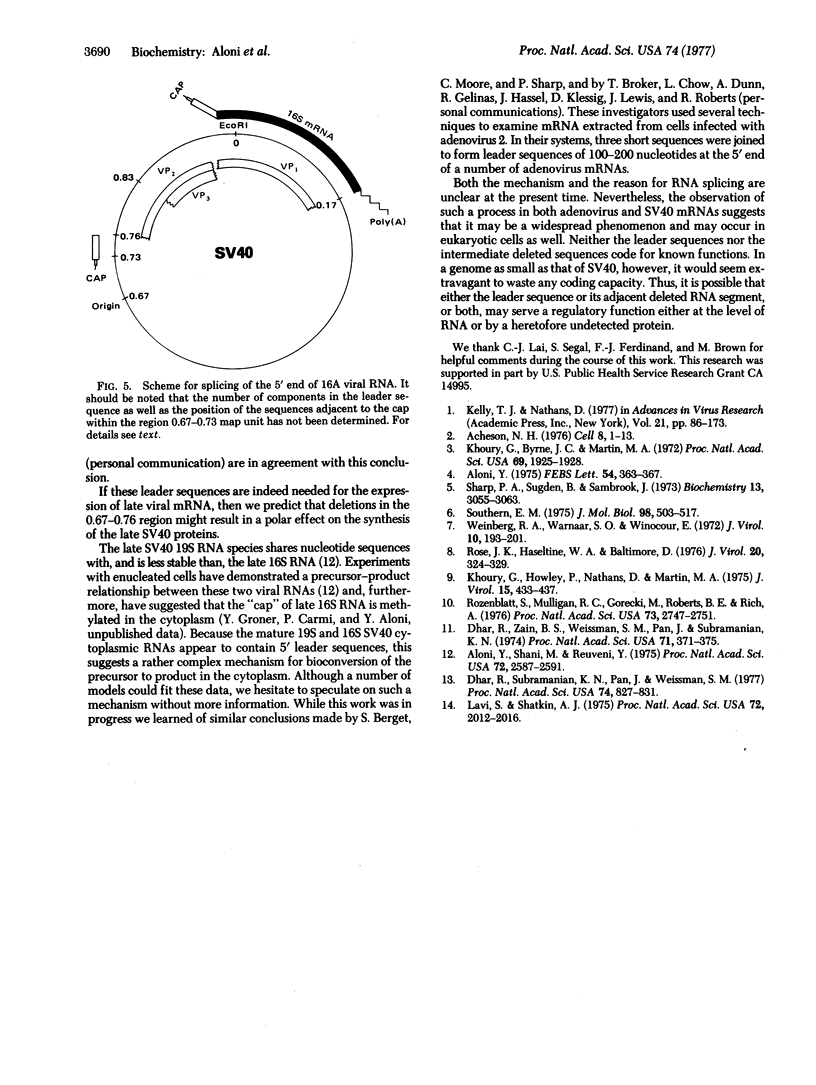
The 5'-terminal 100-200 ribonucleotides of late simian virus 40 (SV40) mRNAs are not transcribed immediately adjacent to their coding sequences. This conclusion is based on the following observations. The major late SV40 cytoplasmic RNA species, 16S and 19S, were purified from poly(A)-containing cytoplasmic RNA by hybridization to and elution from an SV40 DNA fragment that maps between 0.67 and 0.76. This fragment is remote from the DNA fragments that include the coding sequences. The RNA transcripts from the fragment located between 0.67 and 0.76 were found in abundance. Even though selected on oligo(dT)-cellulose columns, the 5'-terminal sequences did not contain poly(A) tails directly adjacent to their 3' termini. The 5' terminus of the 16S mRNA, as monitored by hybridization of the sequences adjacent to the "cap" structure, was found adjacent to the coding sequences when intact [3H]methyl-labeled RNA was hybridized with restriction fragments. However, after fragmentation, the methyl label of this same RNA hybridized with a fragment that is remote from the coding sequences and maps between 0.67 and 0.73. These results imply a novel mechanism for biosynthesis of SV40 mRNA.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H. Transcription during productive infection with polyoma virus and Simian virus 40. Cell. 1976 May;8(1):1–12. doi: 10.1016/0092-8674(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Aloni Y. Methylated SV40 mRNAs. FEBS Lett. 1975 Jul 1;54(3):363–367. doi: 10.1016/0014-5793(75)80940-9. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Shani M., Reuveni Y. RNAs of simian virus 40 in productively infected monkey cells: kinetics of formation and decay in enucleate cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2587–2591. doi: 10.1073/pnas.72.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Structure of a large segment of the genome of simian virus 40 that does not encode known proteins. Proc Natl Acad Sci U S A. 1977 Mar;74(3):827–831. doi: 10.1073/pnas.74.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Zain S., Weissman S. M., Pan J., Subramanian K. Nucleotide sequences of RNA transcribed in infected cells and by Escherichia coli RNA polymerase from a segment of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):371–375. doi: 10.1073/pnas.71.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Nathans D. The genome of simian virus 40. Adv Virus Res. 1977;21:85–173. doi: 10.1016/s0065-3527(08)60762-9. [DOI] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Howley P., Nathans D., Martin M. Posttranscriptional selection of simian virus 40-specific RNA. J Virol. 1975 Feb;15(2):433–437. doi: 10.1128/jvi.15.2.433-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Shatkin A. J. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Haseltine W. A., Baltimore D. 5'-terminus of Moloney murine leukemia virus 35s RNA is m7G5' ppp5' GmpCp. J Virol. 1976 Oct;20(1):324–329. doi: 10.1128/jvi.20.1.324-329.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Mulligan R. C., Gorecki M., Roberts B. E., Rich A. Direct biochemical mapping of eukaryotic viral DNA by means of a linked transcription-translation cell-free system. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2747–2751. doi: 10.1073/pnas.73.8.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]