Abstract
Introduction:
Low vitamin D (VD) is associated with secondary hyperparathyroidism and both contribute to deleterious consequences (reduced bone mineral density (BMD), risk of fractures and falls).
Objective:
To study the VD status and biological correlates in a group of postmenopausal women.
Material and methods:
We studied 123 postmenopausal women evaluated in the C.I.Parhon National Institute of Endocrinology, the Pituitary and Neuroendocrine Diseases department. All cases had been reffered for the evaluation of BMD by the general practitioner. The evaluation included serum measurements of total and ionised calcium, phosphorus, alkaline phosphatase (ALP), 25 hydroxi vitaminD (25OHD), parathyroid hormone (PTH), osteocalcin, betacrosslaps. Central DXA osteodensitometry was performed.
Results:
91.9% of cases had 25OHD serum levels below 30 ng/ml (74.8% had VD deficiency, 17.1% VD insufficiency). Only 8.1% had sufficient VD levels. A history of fragility fractures was present in 45.83% of the osteoporotic patients, 27.27% of the osteopenic ones and 15.15% of the women with normal BMD.
32 women (26%) were on VD supplementation at the time of evaluation. Among these subjects, the 25OHD level was significantly higher in those with prior fragility fractures (p=0.018) and osteoporosis (p=0.008).
25OHD concentration negatively correlated with PTH, alkaline phosphatase (ALP) and osteocalcin. The bone markers evaluated had a significant inverse correlation with the radius BMD, T and Z scores (p=0.004).
27.17% of the cases with VD deficiency had secondary hyperparathyroidism. The 25OHD concentration was significantly lower in these cases (p=0.000).
Conclusions:
VD insufficiency is widely prevalent but still under-recognized and under-treated, possibly leading to secondary hyperparathyroidism. The compliance to VD supplementation is lower in subjects without osteoporosis or fragility fractures. Primary prevention measures should be more actively implemented.
Keywords: vitamin D, deficiency, postmenopausal, secondary hyperparathyroidism
INTRODUCTION
Vitamin D (VD) is essential for the optimal functioning of the musculoskeletal system as it stimulates dietary calcium absorption, the mineralization of the osteoid and has a major regulating role in bone turnover and muscle function (1).
VD deficiency is extremely prevalent in the general population worldwide (2). Very high prevalence has also been demonstrated in a large cohort of Romanian postmenopausal women with osteoporosis (3).
In adults, VD deficiency becomes manifest as muscle weakness, bone mineralization defect (leading to osteomalacia), muscle pain (2), reduced bone mineral density (4) and higher incidence of fractures (5) and falls (6). Biochemically, low vitamin D status is associated with an increase in serum parathyroid hormone (PTH) concentration which contributes to some of the deleterious effects of VD deficiency (7).
The optimal marker for the assessment of the VD status of the organism is the serum concentration of 25hydroxivitamin D (25OHD) (8). The US Endocrine Society defines VD deficiency as 25OHD serum levels below 20 ng/ml, VD inadequacy at values between 20-30 ng/ml and VD sufficiency at concentrations above 30 ng/ml (9). These are the criteria that we will use in this paper although the debate about the optimal cutoffs persists and other authorities use lower values (10).
As the data in the Romanian population are scarce, in the present study we aim to describe the VD status in a group of postmenopausal women as well as the correlations between VD deficiency, secondary hyperparathyroidism, bone markers and osteodensitometric parameters. ❑
MATERIAL AND METHODS
Subjects
We studied 123 postmenopausal women evaluated in the "C.I. Parhon" National Institute of Endocrinology, the Department of Pituitary and Neuroendocrine Diseases between 1.09.2013-1.06.2014. All patients had been reffered for the evaluation of the bone mineral density by the general practitioner. 20 of them were under antiosteoporotic treatment for a minimum of 1 year at the evaluation. The rest of the cases were evaluated for the first time; the reasons for referral were: age >65 years, or postmenopausal status and other risk factors for osteoporosis.
We excluded patients with primary hyperparathyroidism or hypercalcemia of other causes, end-stage kidney failure, active malignancy, inflammatory or metabolic bone disease. ❑
METHODS
In all cases the evaluation included total and ionised calcium, phosphorus, 25OHD, PTH serum measurements; in many of them the bone markers (osteocalcin, βcrosslaps) and calciuria were also evaluated. Serum concentrations of 25OHD, PTH, β crosslaps and osteocalcin were measured by electrochemiluminiscence Elecsys 2010. The normal ranges of the commercial kits used are showed in Table 1.
Table 1.
Demographic and laboratory data in the study group
| Normal range | Measured range | Mean | SD (Std. deviation) | |
|---|---|---|---|---|
| General data | ||||
| Age (years) | 42-79 | 57.97 | 8.03 | |
| BMI (kg/m2) | 18-24.9 | 18-43 | 28.68 | 5.68 |
| postmenopausal years | 1-33 | 10.93 | 7.83 | |
| Laboratory data | ||||
| 25 OH D (ng/mL) | 30-100 | 3-46 | 15.89 | 8.19 |
| Ca (mg/dL) | 8.4-10.3 | 8.8-10.2 | 9.23 | 0.48 |
| Ionised Ca (mg/dL) | 3.6-5.2 | 2.8-4.5 | 3.92 | 0.29 |
| Alkaline phosphatase (ALP) (IU/L) | 18-63 | 4-136 | 74.83 | 20.68 |
| P (mg/dL) | 2.5-4.5 | 1.9-5.5 | 3.46 | 0.59 |
| PTH (pg/mL) | 15-65 | 18-114 | 52.39 | 21.82 |
| Osteocalcin (ng/mL) | 15-46 | 0-80 | 23.28 | 12.14 |
| Betacrosslaps (ng/ml) | 0.22-1.008 | 0.1-2.6 | 0.5 | 0.42 |
All patients were also evaluated by central DXA osteodensitometry (lumbar spine L1-L4, hip, ultradistal radius in some cases) using a GE Healthcare Lunar Prodigy machine.
The history of previous fragility fractures was carefully documented in all cases (based on clinical and radiological grounds). Vertebral fractures were diagnosed on plain radiographs recommended in selected cases based on the clinical suspicion (pain, kyphosis, height loss).
Statistical analysis. The statistical analysis was done using the SPSS programme, version 17.0. ❑
RESULTS
The mean age of the 123 women included in the study was 57.9 years and the mean postmenopausal interval 10 years (see table). 85% of the cases (105 women) were non-smokers, 11.4 (14 cases) active smokers and 3.3% (4 cases) were past smokers.
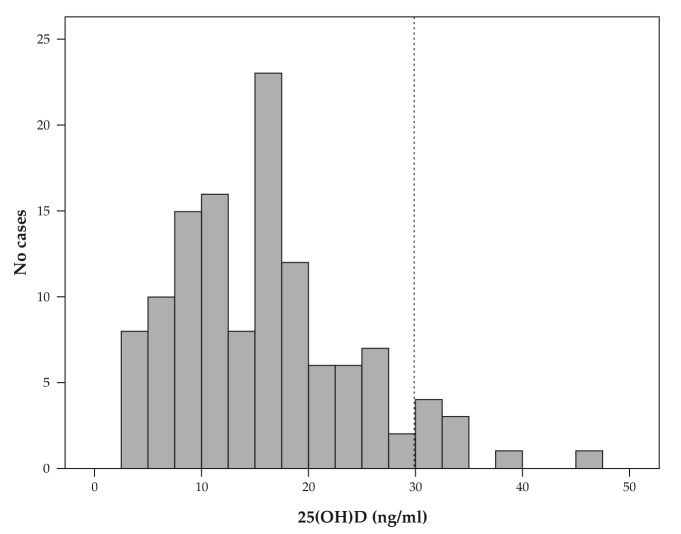
The vitamin D status in the whole group was skewed toward lower values so that the mean serum concentration was well below the cut-off defining VD deficiency (15.89 ng/mL – see Table 1). Calcium and phosphorus serum concentrations were normal in all subjects but the alkaline phosphatase (ALP) and PTH concentrations were raised in some cases – see Table 1. As shown in Figure 1, the prevalence of insufficient levels of VD was very high in the study group: 91.9% of cases (114 women) had 25OHD serum levels below 30 ng/ml. 74.8% of the cases had VD deficiency (92 cases) and 17.1% of cases had inadequate VD levels (21 cases) and only 8.1% % (10 cases) had sufficient VD levels.
Figure 1. Histogram of the 25OHD serum concentrations in the study group showing a high prevalence of VD deficiency and insufficiency.
After the osteodensitometric evaluation 66 cases were found to have normal BMD, 33 had DXA criteria diagnostic of osteopenia (T-score between -1.5 and -2.5 SD) and 24 cases had osteoporosis according to the DXA results (T score below -2.5 SD).
93 (75.6%) of the patients had no history of fragility fractures, 24 (19.5%) had one fragility fracture while 6 (4.9) of them had a history of 2 or more fragility fractures. The fragility fractures did not occur solely in the osteoporotic group (according to the DXA criteria). 45.83% of the osteoporotic patients had a history of fragility fractures but also 27.27% of the osteopenic ones as well as 15.15% of the women with normal BMD on DXA examination. Even cases with multiple fragility fractures were found among the subgroups with normal DXA or osteopenia: the total number of the previous fragility fractures was 13 in the normal DXA subgroup, 12 in the osteopenic subgroup and 15 in the osteoporotic subgroup.
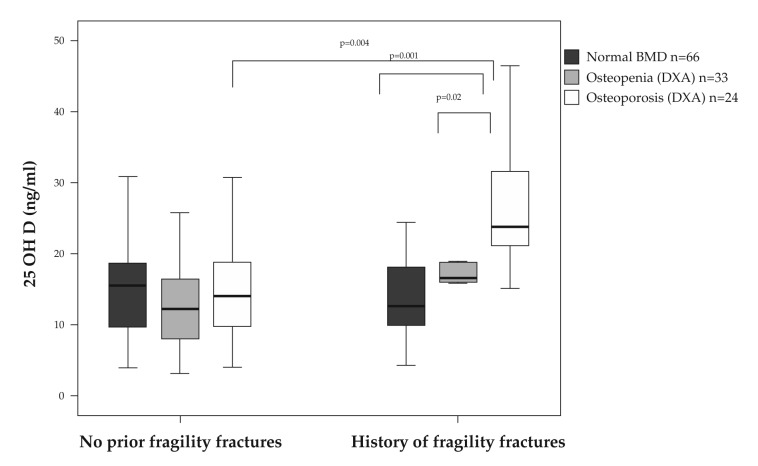
The mean 25OHD serum concentration was, however, significanly higher in osteoporotic patients with a history of fractures (26.56 ± 8.86 ng/dl) compared both with the ones without fragility fractures (15.58± 8.14 ng/dl) and with the osteopenic and normal BMD subjects that have suffered a fragility fracture (having mean 25OHD concentrations of 17.45 ± 9.45 ng/ml and 13.42 ± 6.53 ng/ml, respectively) (Figure 2).
Figure 2. Serum concentration of 25OHD according to the bone mass and fracture history status.
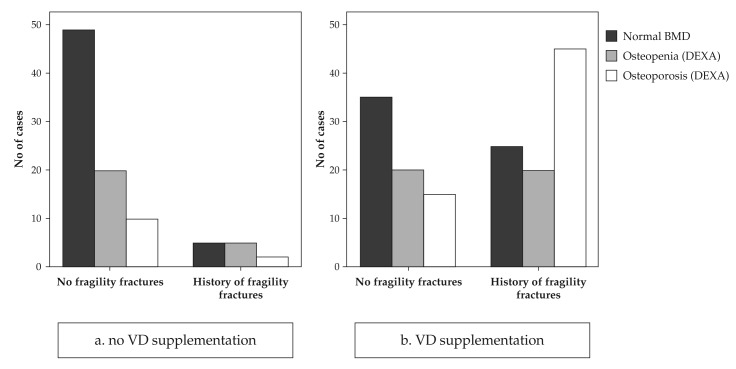
In order to explain this, we analysed next the results taking into account the current treatment with VD. Only 32 women (26% of the study group) were on VD supplementation at the time of the evaluation. VD supplementation had been offered either as adjunctive treatment to antiresorbtive medication for already diagnosed osteoporosis or as preventive measure against postmenopausal osteoporosis and consisted of 1000 UI native VD daily for 8-16 months before our evaluation. As seen in Figure 3, most cases receiving current VD supplementation were among the osteopenic/osteoporotic or with a positive history of fragility fractures.
Figure 3. BMD categories and fragility fractures history in cases without VD supplementation (panel a) and with VD supplementation (panel b) showing that most cases receiving supplementation are the ones with low bone mass and/or pathological fractures.
It is of note that only 18 out of the 30 patients with positive history for fragility fractures (60%) were on current VD supplementation, while 12 were not (40%). The corresponding percentages for different bone mass categories are irrelevant because many patients with low bone mass have been diagnosed at the time of the evaluation in our clinic.
If we analysed separately the subjects with current VD supplementation, the 25OHD serum concentration was significantly higher in those with prior fragility fractures (23.63 ±10.48 versus 17.10 ±6.84 ng/dl in those without fractures; p=0.018) and osteoporosis (26.19 ± 9.65 versus 17.52 ± 8.01 in those without osteoporosis, p=0.008).
The Pearson bivariate correlations in the whole sample showed that 25OHD negatively correlated with PTH, ALP and osteocalcin and was positively correlated with the calciuria, but not the serum calcium level – see Table 2.
Table 2.
Correlations between the 25OHD serum concentration, bone markers and osteodensitometric parameters.
| PTH (ng/dL) | P (mg/dL) | ALP | Osteocalcin (ng/dL) | Betacross-laps | Calciuria (mg/24h) | |
|---|---|---|---|---|---|---|
| 25OHD (ng/mL) | r= -0.185 p=0.042 |
r= 0.192 p=0.036 |
r= -0.226 p=0.012 |
r= -0.185 p=0.042 |
ns | r= 0.361 p=0.004 |
| Age (years) | ns | ns | ns | r= 0.216 p=0.022 |
ns | ns |
| BMI (kg/m2) | ns | ns | ns | r= -0.380 p=0.000 |
ns | ns |
| BMD radius (g/cm2) | ns | ns | ns | r= -0.560 p=0.000 |
r= -0.534 p=0.000 |
ns |
| T score radius (SD) | ns | ns | ns | r= -0.546 p=0.002 |
r= -0.541 p=0.002 |
ns |
| Z score radius (SD) | ns | ns | ns | r= -0.515 p=0.004 |
r= -0.687 p=0.000 |
ns |
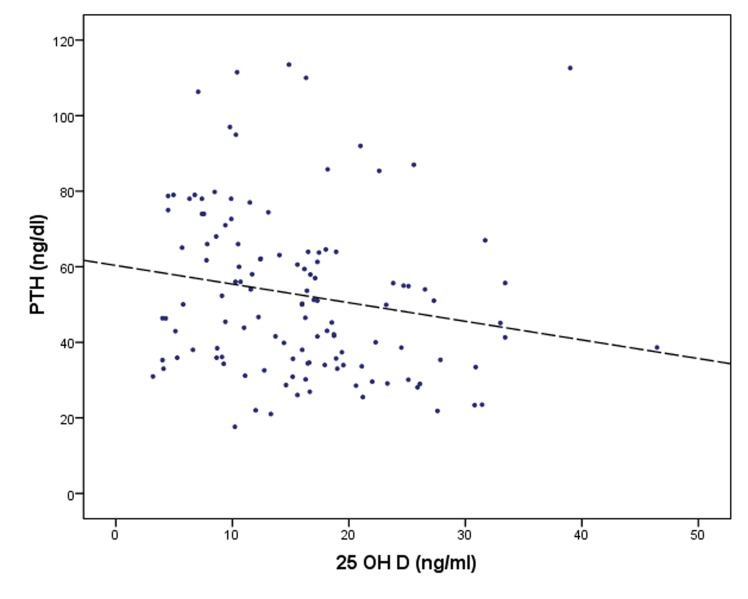
The negative correlation between the 25OHD and PTH concentrations is also illustrated in Figure 4.
Figure 4. Negative correlation between the 25OHD and the PTH serum concentrations.
The bone markers evaluated had a significant inverse correlation with the radius BMD, T and Z scores, but not with the osteodensitometric parameters at other sites. Osteocalcin alone was significantly correlated with the age and BMI of the subjects (Table 2).
No significant difference between the smokers and non- or ex-smokers was identified for the study variables- data not shown.
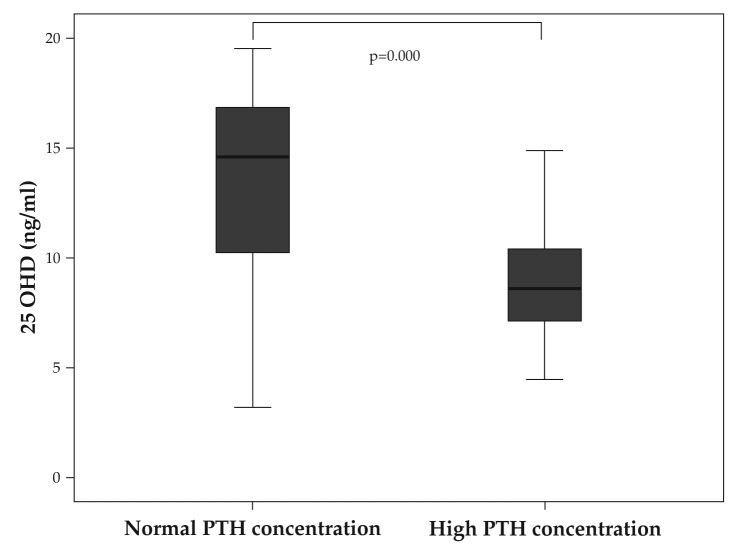
Not all cases with 25OHD <20 ng/ml have secondary hyperparathyroidism: only 25 out of 92 cases (27.17%), while the rest (67 cases, 72.82%) have values of PTH below the upper limit of normal (65 pg/ml). There was no statistically significant difference between the BMD, T or Z scores at all levels (lumbar spine, hip or radius), calcium concentration in the serum or urine or bone markers between the VD deficient cases with and without increased PTH levels. However, the 25OHD concentration, although low in both these groups, was significantly lower in VD deficient cases with secondary hyperparathyroidism: 9.25 ±3.5 ng/dl versus 13.29 ±4.63 ng/dl (Figure 5). ❑
Figure 5. VD deficient cases with and without secondary hyperparathyroidism have significantly different 25OHD levels.
DISCUSSION
Vitamin D deficiency has been recently described as a pandemic condition with significant consequences for the individual and public health, related not only to the musculoskeletal health but also to a wide range of different chronic diseases (2).
The studies adressing the prevalence of this condition in Romania are scarce but they have all demonstrated high prevalence of VD deficiency in various groups of subjects and a very low mean 25OHD concentration in these subjects (3, 11-13). Our results are in line with the previous reports and demonstrate a very high prevalence of low VD status in a group of postmenopausal women. Although a selection bias that could overestimate the results is probably involved (all cases had been reffered by the general practitioner with a suspicion of low bone mass or for the follow-up of already diagnosed low bone mass), the prevalence in the general postmenopausal population can be inferred to be significant. The possible coexistence of a subclinical malabsorbtive state in some cases should also be borne in mind, given the high prevalence of an underdiagnosed gastrointestinal etiology for the low vitamin D status (14).
Low VD status is associated with low bone mass and fractures. These effects are, at least in part, mediated by the secondary hyperparathyroidism which leads to osteoclast activation and bone resorption by enhancing the expression of RANKL (15). The secondary increase in PTH serum levels does not occur in all cases with VD deficiency (16-18) – this was present in only 27.17% of VD deficient cases in our group, those with most severely decreased 25OHD levels. This subgroup of patients with VD deficiency is at particularly increased risk for bone loss (17) so they should receive proper VD replacement which has the potential to decrease the PTH concentration (19), to improve the BMD and decrease the incidence of fractures (20).
In our group, low VD status was inversely associated with increased PTH, ALP and osteocalcin levels – results which are in line with previous reports on very large cohorts of postmenopausal women (21). These correlation underlines the association between low vitamin D status and increased bone turnover rate leading to accelerated bone mass loss and low BMD. The bone markers evaluated (osteocalcin and betacrosslaps) were both associated with lower BMD, T and Z scores at the ultradistal radius, but not at other sites. This last result was slightly different from others (22) who found a correlation between high osteocalcin and both ultradistal radius and lumbar spine bone mass.
The target 25OHD concentration in VD-deficient patients is at least 30 ng/ml. Concentrations below 30 ng/ml have been associated with bone biopsies with incipient aspects suggestive of osteomalacia (increased osteoid volume) (23). On the other hand, 25OHD concentrations above 30 ng/ml are associated with low fracture risk (24) and recommended both by the International Osteoporosis Foundation (IOF) guide (24) and the Task Force for Vitamin D of the US Endocrine Society (9). The daily dose of D3 to be taken in order to achieve this concentration is about 800-1000 UI (25).
In our study, patients with a history of pathological fractures or previously diagnosed osteoporosis had significantly higher 25OHD levels than those without these features. This was surprising at first, because it has been shown that VD deficiency is more common in patients with fragility fractures than in age-matched control subjects (17). However, in our group these cases were also the ones for whom VD supplementation was most frequently recommended and this could account for this finding.
There is also the possibility that the compliance to VD supplementation differs between different subgroups of patients. Although we did not try to objectively assess the compliance to tretament in our study, the fact that the 25OHD level did not attain the sufficiency level in the vast majority of cases receiving supplementation according to current guidelines suggest that compliance might be suboptimal. This appears to be particularly true in subjects without a diagnosis of osteoporosis or history of fragility fractures, who had significantly lower 25OHD concentration than the other VD-replaced subjects. In our group the VD supplementation was homogeneous and consisted of 800-1000 UI vitamin D3 daily. The most reasonable explanation we have for the above results is that the compliance to the daily administration of VD was better in those already exposed to the diagnosis of osteoporosis or, even more, to its more serious complication, the fragility fracture. This suggestion contradicts the findings of a Spanish study that revealed that patients with fractures are less likely to persist in longterm supplementation (26). It must also be acknowledged that the measurement uncertainty associated with the 25OHD measurement is still high (27) and for the electrochemiluminiscence assay used in our clinic specificity can be an issue especially in relation to the proportion of 25OHD2 (28).
The compliance to VD supplementation and the factors affecting that in the Romanian population are presently unknown and should be the subject of future research.
In our study, the fragility fractures were not found solely in the osteoporotic group (according to the DXA criteria), a result in line with that of previous studies (29). Overall, the number of fragility fractures was lower in the osteoporotic group than in the other two subgroups together, a finding also acknowledged by others (13), explained by the significantly larger number of subjects within normal DXA or osteopenic categories than within the osteoporosis category. Patients with a history of fragility fractures should universally benefit from the recommendation of VD supplementation but in our study, only 60% of them had been recommended VD. Taken together, our results suggest that a more active policy recommending VD supplementation at least in osteoporotic patients and especially those that already suffered a fracture is likely to be highly beneficial. ❑
CONCLUSIONS
The VD deficiency, although widely prevalent, is still under-treated. Low vitamin D status is highly prevalent in postmenopausal women. Vitamin D supplementation is indicated in postmenopausal women, especially in those at high risk of fracture, but it is still not recommended in all cases. Even when VD supplementation is offered, the compliance is low, possibly even lower in subjects without osteoporosis or fragility fractures. Both primary and secondary prevention measures should be more actively implemented in the Romanian population.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
This paper is supported by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/137390/ to Cristina Capatina.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 3.Grigorie D, Sucaliuc A, Ivan M, et al. High prevalence of vitamin D deficiency in 1048 Romanian postmenopausal women with osteoporosis. Acta Endocrinologica (Buc). 2008;IV:33–45. [Google Scholar]
- 4.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 7.Ebeling PR. Vitamin D and bone health: Epidemiologic studies. Bonekey Rep. 2014;3:511–511. doi: 10.1038/bonekey.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lips P. Relative value of 25(OH) D and 1,25(OH) 2D measurements. J Bone Miner Res. 2007;22:1668–1671. doi: 10.1359/jbmr.070716. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 10.Francis R. Vitamin D and Bone health: A Practical Clinical Guideline for Patient Management 2013 [Google Scholar]
- 11.Mocanu V. Vitamin D deficiency and metabolic syndrome among nursing home residents. Acta Endocrinologica (Buc). 2013;9:53–61. [Google Scholar]
- 12.Branisteanu DD, Botterman P, Zbranca E, et al. Sunlight exposure and vitamin D supplementation at the institutionalized elderly - effects on calcium and bone metabolism. Acta Endocrinologica (Buc). 2007;3:169–178. [Google Scholar]
- 13.Carsote M, Ene C, Radoi V, et al. An analysis on bone turnover markers and calcaneal ultrasonometry in patients with postmenopausal osteoporosis. Revista Romana de Reumatologie. 2012;XXI:38–42. [Google Scholar]
- 14.Bikle DD. Vitamin D insufficiency/deficiency in gastrointestinal disorders. J Bone Miner Res. 2007;22(Suppl2):V50–V54. doi: 10.1359/jbmr.07s208. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibli-Rahhal A, Paturi B. Variations in parathyroid hormone concentration in patients with low 25 hydroxyvitamin D. Osteoporos Int. 2014;25:1931–1936. doi: 10.1007/s00198-014-2687-4. [DOI] [PubMed] [Google Scholar]
- 17.Sahota O, Gaynor K, Harwood RH, et al. Hypovitaminosis D and 'functional hypoparathyroidism'-the NoNoF (Nottingham Neck of Femur) study. Age Ageing. 2001;30:467–472. doi: 10.1093/ageing/30.6.467. [DOI] [PubMed] [Google Scholar]
- 18.Sahota O, Mundey MK, San P, et al. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004;35:312–319. doi: 10.1016/j.bone.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki R, Sugimoto T, Kaji H, et al. Vitamin D insufficiency defined by serum 25-hydroxyvitamin D and parathyroid hormone before and after oral vitamin D(3) load in Japanese subjects. J Bone Miner Metab. 2011;29:103–110. doi: 10.1007/s00774-010-0200-5. [DOI] [PubMed] [Google Scholar]
- 20.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 21.Lips P, Duong T, Oleksik A, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 22.Minisola S, Rosso R, Romagnoli E, et al. Serum osteocalcin and bone mineral density at various skeletal sites: a study performed with three different assays. J Lab Clin Med. 1997;129:422–429. doi: 10.1016/s0022-2143(97)90075-5. [DOI] [PubMed] [Google Scholar]
- 23.Priemel M, von DC, Klatte TO, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 24.Dawson-Hughes B. IOF position statement: vitamin D recommendations for older adults, 2010. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 25.Dawson-Hughes B. Estimates of optimal vitamin D status, 2005. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 26.Castelo-Branco C, Cortes X, Ferrer M. Treatment persistence and compliance with a combination of calcium and vitamin D. Climacteric. 2010;13:578–584. doi: 10.3109/13697130903452804. [DOI] [PubMed] [Google Scholar]
- 27.Cavalier E, Rozet E, Gadisseur R, et al. Measurement uncertainty of 25-OH vitamin D determination with different commercially available kits: impact on the clinical cut offs. Osteoporos Int. 2010;21:1047–1051. doi: 10.1007/s00198-009-1052-5. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Wareth L, Haq A, Turner A, et al. Total vitamin D assay comparison of the Roche Diagnostics "Vitamin D total" electrochemiluminescence protein binding assay with the Chromsystems HPLC method in a population with both D2 and D3 forms of vitamin D. Nutrients. 2013;5:971–980. doi: 10.3390/nu5030971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posen J, Beaton DE, Sale J, et al. Bone mineral density testing after fragility fracture: Informative test results likely. Can Fam Physician. 2013;59:e564–e571. [PMC free article] [PubMed] [Google Scholar]