Abstract
Background:
Intrauterine growth restriction (IUGR) is a major cause of fetal morbidity and mortality during pregnancy. The role of mutation in the factor V gene, prothrombin gene, MTHFR gene, as risk factors for intrauterine growth restriction during pregnancy, is not very well known so far.
Materials and methods:
This is a retrospective study of 151 pregnant women with a history of complicated pregnancy: intrauterine growth restriction, preeclampsia, recurrent pregnancy loss or maternal venous thromboembolism, who were admitted in Bucharest Emergency University Hospital, during the period January 2010 to July 2014. Genetic testing was performed for all the cases to detect: factor V Leiden mutation, G20210A mutation in the prothrombin gene, C677T mutation and A1298C mutation in methylenetetrahydrofolate reductase (MTHFR) gene. Blood samples were obtained as soon as the diagnosis of intrauterine growth restriction was established with ultrasonography.
Results:
The following gene mutations were associated with increased risk of IUGR: G20210A prothrombin gene mutation (OR 4.81, 95% CI 1.05 - 2.22, p= 0.043), G1691A factor V gene mutation (factor V Leiden) (OR 1.58, 95% CI 0.61 - 4.080, p= 0.347), C677T MTHFR gene mutation (OR 1.61, 95% CI 0.79 to 3.26, p= 0.186), compound heterozygous MTHFR C677T and A1298C (OR 1.66, 95% CI 0.81- 3.42, p= 0.169). Particularly, for G20210A prothrombin gene mutation we found statistically significant risk (p≤0.05) of IUGR.
Keywords: intrauterine growth restriction, pregnancy, hereditary thrombophilia
INTRODUCTION
Intrauterine growth restriction (IUGR) is defined as estimated fetal weight below gestational age, according to ultrasound data, and birth weight below the 10th percentile of the birth weight for gestational age reference curve (1).
The most serious complication that can occur during this pathology, is intrauterine fetal death (2), and the risk rises when difference between the clinical gestational age and ultrasound estimated age has a higher value. Although there are many studies in the literature (3-6), no reliable data regarding hereditary thrombophilia involvement in IUGR (7) exists. Results vary from one study to another depending on the number of patients included and selection criteria used. Weak evidence of association between IUGR and inherited thrombophilia was emphasized in a meta-analysis (8) evaluating the relationship between IUGR and homozygous or heterozygous for prothrombin 20210 gene mutation, homozygous or heterozygous for factor V Leiden, homozygous C677T in the MTHFR gene.
The purpose of our study is the quest for finding an association between IUGR and hereditary thrombophilia: G20210A prothrombin gene mutation, G1691A mutation in factor V (factor V Leiden), C677T / A1298C mutations in the MTHFR gene. This association is possible based on the hypothesis that IUGR is a placental-mediated complication due to thrombosis in the maternal-placental vascular territories with decreased blood flow. ❑
MATERIAL AND METHODS
We studied 151 pregnant women with a history of complications as: IUGR, preeclampsia, recurrent miscarriages or maternal venous thromboembolism, admitted in Bucharest Emergency University Hospital, Department of Obstetrics, between January 2010 and July 2014. IUGR was diagnosed by fetal ultrasound which revealed abnormal growth velocity, amniotic fluid volume, and Doppler velocimetry. Blood samples were sent to the Fundeni Institute for genetic analysis and coagulation tests.
Laboratory tests
All pregnant women had negative microbiological tests and normal laboratory assessment: complete blood count (CBC), urea, creatinine, glucose, normal coagulation. We performed: genetic tests (BITEST, amniocentesis with karyotyping), infections tests (TORCH complex) and tests for exclusion of other chronic maternal diseases, in order to exclude other causes of IUGR.
Molecular analysis
All the tests were conducted in the Laboratory of Molecular Biology Hematology, Center of Hematology and Bone Marrow Transplantation, Fundeni Institute.
2 ml of venous blood in EDTA were collected from patients who met the study inclusion criteria. Equipment used for molecular diagnosis was: Real Time PCR Platform Light Cycler 480, Termocycler Gene Amp PCR System 9700 Classic, Nano Drop ND-1000 Spectrophotometer, Electrophoresis Systems. After PCR amplification, mutation identification was performed by Restriction Fragment Length Polymorphism (RFLP), Real Time PCR, and in some cases direct sequencing (Sanger's method). ❑
STATISTICAL ANALYSIS
This is a population-based retrospective cohort study. Data obtained from patients were introduced in a database in Excel, and statistically analyzed using STATA software / SE11. Absolute frequency (number of occurrences) was calculated for qualitative measurements - ordinal and nominal - and the prevalence was expressed as a percentage. The Chi square test was used for comparison of qualitative data. All variables collected were statistically analyzed and presented in a descriptive manner. Inferential statistical analysis aimed to investigate the relationship between risk factors and IUGR. The investigated risk factors were: G20210A factor II mutation genotype, Factor V Leiden mutation genotype, C677T MTHFR mutation genotype, A1298C MTHFR mutation genotype, compound heterozygous MTHFR C677T and A1298C genotype. Association between risk factors and IUGR was assessed by Chi-square test and the actual statistical association was evaluated by calculating the odds ratio (OR) and 95% confidence interval [CI] using univariate logistic regression. All tests used were bilateral. Threshold of statistical significance was p ≤0.05.
This study was conducted according to local and national ethical regulations. ❑
RESULTS
In our group of 151 pregnant women we looked for association between intrauterine growth restriction (IUGR) and presence of hereditary thrombophilia: 20210 prothrombin gene mutation, factor V Leiden mutation, C677T and A1298C mutations in the MTHFR gene and compound heterozygous A1298C and C677T in MTHFR gene.
94 pregnant women (62.3 %) were diagnosed with IUGR by fetal Doppler ultrasound, with mean birth weight 1655 grams +/- 530. Parturients with IUGR, delivered at a mean gestational age of 33.2 ± 3.1 weeks by Caesarean section.
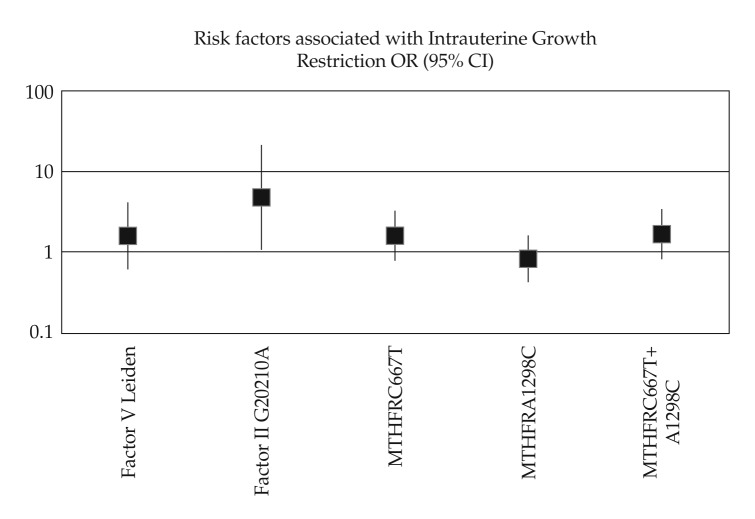
We found a strong, statistically significant association (p = 0.043), between IUGR in current pregnancy and 20210 prothrombin gene mutation (OR 4.81, 95% CI 1.05 - 22.02) (Table 1) (Figure 1).
Table 1.
IUGR and G20210A prothrombin gene mutation.
| Factor II G20210A associated with IUGR | ||||||
|---|---|---|---|---|---|---|
| IUGR in pregnancy | ||||||
| Absent | Present | Total | ||||
| Factor II G20210A | N | % | N | % | N | % |
| Negative | 55 | 36.4 | 80 | 53.0 | 135 | 89.4 |
| Heterozygote | 2 | 1.3 | 14 | 9.3 | 16 | 10.6 |
| Total | 57 | 37.8 | 94 | 62.3 | 151 | 100 |
| Chi square test – p = 0.028 | ||||||
| Logistic regression | OR | 95%CI | p | |||
| 4.81 | 1.05-22.02 | 0.043 | ||||
IUGR - Intrauterine growth restriction
Figure 1. Risk factors for Intrauterine Growth Restriction.
We also found positive associations between FV Leiden mutation (heterozygous or homozygous), respectively C677T MTHFR gene mutation and IUGR, although not statistically significant (p >0.05). Risk of IUGR in pregnant patients with factor V Leiden or C677T MTHFR gene mutation is 1.58 fold higher (OR 1.58, 95% CI 0.61-4.080, p = 0.347) (Table 2), respectively 1.61-fold higher (95% CI 0.79 to 3.26, OR1.61, p = 0.186) (Table 3) than those who do not have these mutations. Another positive association was observed between IUGR and heterozygous compound C677T and A1298C in MTHFR gene (OR 1.66 95% CI 0.81-3.42 and p = 0.169) (Table 5). Risk of IUGR in these compound heterozygotes is 1.66-fold higher than those negative for this mutation (Table 5). Although this is not statistically significant (p >0.05), the results indicate a trend towards statistical association, because the OR is greater than 1 (Figure 1).
Table 2.
IUGR and FV Leiden mutation.
| FV Leiden associated with IUGR | ||||||
|---|---|---|---|---|---|---|
| IUGR in pregnancy | ||||||
| Absent | Present | Total | ||||
| Factor V Leiden | N | % | N | % | N | % |
| Negative | 50 | 33.1 | 77 | 51.0 | 127 | 84.1 |
| Heterozygote | 6 | 4.0 | 16 | 10.6 | 22 | 14.6 |
| Homozygote | 1 | 0.7 | 1 | 0.7 | 2 | 1.3 |
| Total | 57 | 37.8 | 94 | 62.3 | 151 | 100.0 |
| Chi square test – p = 0.523 | ||||||
| Logistic regression | OR | 95%CI | p | |||
| 1.58 | 0.61-4.08 | 0.347 | ||||
IUGR - Intrauterine growth restriction
Table 3.
IUGR and C677T MTHFR gene mutation.
| MTHFR C677T associated with IUGR | ||||||
|---|---|---|---|---|---|---|
| IUGR in pregnancy | ||||||
| Absent | Present | Total | ||||
| MTHFR C667T | N | % | N | % | N | % |
| Negative | 21 | 13.9 | 25 | 16.6 | 46 | 30.5 |
| Heterozygote | 27 | 17.9 | 52 | 34.4 | 79 | 52.3 |
| Homozygote | 9 | 6.0 | 17 | 11.3 | 26 | 17.2 |
| Total | 57 | 37.8 | 94 | 62.3 | 151 | 100 |
| Chi square test – p = 0.415 | ||||||
| Logistic regression | OR | 95%CI | p | |||
| 1.61 | 0.79-3.26 | 0.186 | ||||
IUGR - Intrauterine growth restriction
Table 5.
IUGR and compound heterozygous C6777T and A1298C in MTHFR gene.
| Compound heterozygous MTHFR C667T + MTHFR A1298C associated with IUGR | ||||||
|---|---|---|---|---|---|---|
| IUGR in pregnancy | ||||||
| Absent | Present | Total | ||||
| MTHFR C667T + MTHFR A1298C Compound heterozygous | N | % | N | % | N | % |
| Negative | 42 | 27.8 | 59 | 39.1 | 101 | 66.9 |
| Heterozygote | 15 | 9.9 | 35 | 23.2 | 50 | 33.1 |
| Total | 57 | 37.8 | 94 | 62.3 | 151 | 100 |
| Chi square test – P = 0.167 | ||||||
| Logistic regression | OR | 95%CI | p | |||
| 1.66 | 0.81-3.42 | 0.169 | ||||
IUGR - Intrauterine growth restriction
No association was found between IUGR and A1298C MTHFR mutation (OR 0.83, 95% CI 0.43-1.61 and p = 0.574) (Table 4). ❑
Table 4.
IUGR and A1298C MTHFR gene mutation.
| MTHFR A1298C associated with IUGR | ||||||
|---|---|---|---|---|---|---|
| IUGR in pregnancy | ||||||
| Absent | Present | Total | ||||
| MTHFR A1298C | N | % | N | % | N | % |
| Negative | 24 | 15.9 | 44 | 29.1 | 68 | 45.0 |
| Heterozygote | 25 | 16.6 | 44 | 29.1 | 69 | 45.7 |
| Homozygote | 8 | 5.3 | 6 | 4.0 | 14 | 9.3 |
| Total | 57 | 37.8 | 94 | 62.3 | 151 | 100 |
| Chi square test – p = 0.289 | ||||||
| Logistic regression | OR | 95%CI | p | |||
| 0.83 | 0.43-1.61 | 0.574 | ||||
IUGR - Intrauterine growth restriction
DISCUSSION
There are few data in the literature concerning the association between IUGR and thrombophilic mutations (8-12) and they show conflicting results. In a case-control study conducted by Kupferminc et al., with 26 pregnant women who developed severe IUGR (birth weight <3rd percentile and associated with oligohydramions) in the second trimester of pregnancy and a control group of 56 pregnant women, they found an incidence of 69% for factor V Leiden mutation, G20210A prothrombin gene mutation, and protein S congenital deficiency, compared to 14% in the control group (OR 4.5, 95% CI 2.3-9, p <0.001) (13).
In another study by Martinelli et al., 63 women who developed IUGR, compared with 93 women with normal pregnancies, were investigated for G20210 prothrombin gene mutation and factor V Leiden mutation. 13% of pregnant women with IUGR were positive for factor V Leiden mutation compared with 2.2% in controls (OR 6.9, 95% CI 1.4-33.5) and G20210A prothrombin gene mutation was positive in 12% of pregnant women in the group with IUGR, compared with 2.2% observed in controls (OR 5.9, 95% CI 29.4-1.2) (14).
In the meta-analysis we mentioned in the introduction8, ten case-control studies and two cohort studies were evaluated for the relationship between C6777T homozygous mutation in the gene MTHFR and IUGR and no association was found (OR 1.01, 95% CI 0.88-1.17). In this meta-analysis factor V Leiden mutation was studied in twelve case-control studies and four cohort studies and a statistically significant association was found only for factor V Leiden and IUGR (OR 1.23, 95% CI 1.04 -1.44, p = 0.06) (8). There are no significant studies in the literature on compound heterozygous C677T and A1298C MTHFR status (15).
Rodger et all. has published a meta-analysis on the association between IUGR and thrombophilic mutations, which found no statistical relationship between factor V Leiden (OR = 1.0, 95% CI 0.80-1.25), respectively G20210A prothrombin gene mutation (OR 1.25, 95% CI 0.92-1.70) and IUGR (12).
Our work has several limitations. This study is limited by its small sample size and study design (retrospective cohort study). We did not intend a case-control study because of the difficulty of obtaining a large control group and due to the molecular testing cost.
In our study, similar to the studies discussed above, we observed an increased risk of IUGR in women carrying G20210A prothrombin gene mutation or factor V Leiden mutation. Heterozygous G20210A prothrombin gene mutation determines a 4.81-fold statistically significant (p ≤0.05; OR >1) higher risk to develop IUGR in carriers than in non-carriers. The risk of IUGR in pregnant patients with factor V Leiden is 1.58-fold higher (OR 1.58 95% CI 0.61-4.080) than the risk for women who do not have this mutation, but this is not statistically significant (p >0.05).
While the above mentioned studies did not identify any association between C677T MTHFR gene mutation and IUGR, our study revealed a 1.61 fold higher risk for IUGR in pregnant carriers of this mutation, although this is not statistically significant (p >0.05).
Although there are no published data in the literature about the compound heterozygous A1298C and C677T in the MTHFR gene, we observed in our study a statistical trend towards increased risk of IUGR (OR 1.66 95% CI 0.81-3.42) in patients with this status. Similar to published studies, we did not find any relationship between IUGR and A1298C mutation in the MTHFR gene (Figure 1).
The presence of factor V Leiden mutation, C677T MTHFR gene mutation, and compound heterozygous A1298C and C677T in the MTHFR gene did not significantly increase the risk of IUGR (p >0.05), but using logistic regression analysis of these data however, we found a possible association with IUGR (Figure 1).
Due to the assumed thrombotic placental pathology, early detection of inherited thrombophilia in pregnancies complicated with IUGR, could result in better fetal monitoring until delivery (weekly ultrasound examination until 36 weeks) (1,16). The results of molecular tests for thrombophilia mutations can help the obstetrician to take the optimal therapeutic decision in some particular clinical situations.
In conclusion, inherited thrombophilia: G20210A prothrombin gene mutation, factor V Leiden mutation, C677T MTHFR gene mutation, compound heterozygous A1298C and C677T in MTHFR gene may cause intrauterine growth restriction in pregnant women. Only G20210A prothrombin gene mutation has a statistically significant risk of IUGR.
The results of this work could be a good reason to start an extended study that would bring more powerful evidence for these findings.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
The corresponding author of the article, on behalf of all authors of this work, declare that this article was supported by the grant PN II 42-099/2008 from the Romanian Ministry of Research and Technology.
References
- 1.American College of Obstetricians and Gynecologists. Intrauterine Growth Restriction. ACOG Practice Bulletin 12. Reaffirmed 2008. Washington (DC): ACOG; 2000
- 2.Gardosi J, Madurasinghe V, Williams M, et al. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108–f108. doi: 10.1136/bmj.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dizon-Townson D, Miller C, Sibai B, et al. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005:106–517. doi: 10.1097/01.AOG.0000173986.32528.ca. [DOI] [PubMed] [Google Scholar]
- 4.Said JM, Higgins JR, Moses EK, et al. Inherited thrombophilia polymorphisms and pregnancy outcomes in nulliparous women. Obstet Gynecol. 2010;115:5–5. doi: 10.1097/AOG.0b013e3181c68907. [DOI] [PubMed] [Google Scholar]
- 5.Clark P, Walker ID, Govan L, et al. The GOAL study: a prospective examination of the impact of factor V Leiden and ABO(H) blood groups on hemorrhagic and thrombotic pregnancy outcomes. Br J Haematol. 2008;140:236–40. doi: 10.1111/j.1365-2141.2007.06902.x. [DOI] [PubMed] [Google Scholar]
- 6.Silver RM, Zhao Y, Spong CY, et al. Prothrombin gene mutation and obstetric complications. Obstet Gynecol. 2010;115:14–20. doi: 10.1097/AOG.0b013e3181c88918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockwood CJ, Bauer KA. Inherited thrombophilias in pregnancy. UpToDate. 2014:e1–e22. [Google Scholar]
- 8.Facco F, You W, Grobman W. Genetic thrombophilias and intrauterine growth restriction: a meta-analysis. Obstet Gynecol. 2009;113:1206–16. doi: 10.1097/AOG.0b013e3181a6e96a. [DOI] [PubMed] [Google Scholar]
- 9.Rodger MA, Paidas M, McLintock C, et al. Inherited thrombophilia and pregnancy complications revisited. Obstet Gynecol. 2008;112:320–4. doi: 10.1097/AOG.0b013e31817e8acc. [DOI] [PubMed] [Google Scholar]
- 10.Kjellberg U, van Rooijen M, Bremme K, et al. Factor V Leiden mutation and pregnancy-related complications. Am J Obstet Gynecol. 2010;203:469e1–8. doi: 10.1016/j.ajog.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132:171–96. doi: 10.1111/j.1365-2141.2005.05847.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodger MA, Betancourt MT, Clark P, et al. The association of factor V Leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: a systematic review and meta-analysis of prospective cohort studies. PLoS Med. 2010;7:e1000292–e1000292. doi: 10.1371/journal.pmed.1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kupferminc MJ, Many A, Bar-Am A, et al. Mid-Trimester Severe intrauterine growth restriction is associated with a high prevalence of thrombophilia. BJOG. 2002;109:1373–1376. doi: 10.1046/j.1471-0528.2002.02194.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinelli P, Grandone E, Colaizzo D, et al. Familial thrombophilia and the occurrence of fetal growth restriction. Haematologica. 2001;86:428–431. [PubMed] [Google Scholar]
- 15.Kupferminc MJ. Thrombophilia and pregnancy Reproductive Biology and. Endocrinology. 2003;1:111–111. doi: 10.1186/1477-7827-1-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Divon Y et al. Fetal growth restriction: Diagnosis. UpToDate. 2014:e1–e26. [Google Scholar]