Abstract
Endocarditis is an important, although less common, cause of cerebral embolism. All forms of endocarditis share an initial common pathophysiologic pathway, best illustrated by the non-bacterial thrombotic form, but also a final potential for embolization. Stroke associated with endocarditis has signifficant mortality and morbidity rates, especially due to the frequent concomitant multiple sites of brain embolization.
In this article we aim to briefly review endocarditis with a focus on stroke as a complication, while also presenting case correlates from our department.
Keywords: endocarditis, stroke
PREAMBLE
Embolism from any source (cardiac or arterial) accounts for 15-70% of ischemic strokes. While not as prevalent as atrial fibrillation, endocarditis is an important source of cardiac embolism, as it carries a high embolic risk, it is associated with high morbidity and mortality, and its diagnosis is, unfortunately, too often delayed (1).
Three main forms of endocarditis have been described: infective endocarditis, nonbacterial thrombotic endocarditis (NBTE – most commonly associated with malignancy), and Libman-Sacks endocarditis (LSE – in systemic lupus erithematosus and antiphospholipid syndrome).
An abnormality of the endocardium or endothelial lesion is needed as an initial step in thrombus formation, as it fosters the interaction between the subendothelial connective tissue and the circulating platelets and fibrin. As a continuation of the pathogenic process, an episode of superimposed bacteremia leads to the formation of a vegetation, a variably sized amorphous mass of platelets and fibrin with enmeshed microorganisms and inflammatory cells (2-5).
The diagnostic of infective endocarditis is based on the modified Duke criteria, published in 2000, but theese criteria do not cover NBTE or LSE. The clinician requires a high index of suspicion to direct diagnostic procedures towards endocarditis in the setting of an ischemic stroke. Clinical signs and symptoms, echocardiographic findings, laboratory workup and microbiological data, and possibly other imaging techniques such as cerebral MRI need to be employed in determining endocarditis as a cause of stroke. ❑
STROKE AS A COMPLICATION OF ENDOCARDITIS
The brain is one of the most frequent sites of embolization in both left-sided IE and in NBTE. Clinically apparent acute brain embolization is estimated to occur in 10-35% of patients with left-sided IE. The majority of the cerebral emboli in IE patients are clinically silent, as shown by recent MR imaging studies, where up to 80% of IE patients had imagistic signs of cerebrovascular involvement; the use of MRI is further warranted in identifying patients at risk for hemorrhagic transformation by showing microbleeds suggestive of subacute microvascular inflammation associated with mycotic aneurysms on T2*-weighted sequences. Stroke and transient ischemic attacks through occlusion of cerebral arteries account for 40-50% of neurologic complications in IE, surpassing others such as meningitis or meningeal reaction (2-20%), brain abscess (12%), mycotic aneurysm (9%) and intracranial hemorrhage (5%). Septic embolization can also result in hemorrhagic stroke (see also Case report 1). Three possible mechanisms are involved in the pathogenesis of hemorrhagic stroke: bleeding into infarcts, rupture of a vessel wall affected by septic arteritis, or rupture of a mycotic aneurysm (6-20). ❑
CASE REPORT 1
A 75 year-old female presented with diffuse headache and two episodes of vomiting. She had a history of hypertension, diabetes, and chronic kidney disease, for which she underwent hemodialysis. Upon examination, she had normal blood pressure, sinus rhythm, body temperature of 37°C, coarse crackles upon auscultation, and no pathological neurological signs. A CT-scan was performed, showing cortical atrophy. A chest X-ray was also performed, raising the suspicion of a right basal pneumonia, with reactive pleural effusion. Given the negative CT scan, the headache and the possible infection, a lumbar puncture was performed, which showed a clear cerebrospinal fluid, with normal WBC, RBC, glucose and protein count. The patient was referred to an Infectious diseases department.
She returned the following day with altered general status, left hemiparesis, nuchal rigidity, intense headache, and fever. A new CT-scan revealed multiple hemorrhagic lesions of the same age, and of different sizes, findings which were subsequently confirmed by MRI (Figure 1). Repeated blood cultures were negative, while there was an elevated WBC count, as well as high ESR and fibrinogen. Transthoracic ecocardiography showed a small mass attached to the floor of the left atrium in the proximity of anterior cuspid of the mitral valve, described as a possible atrial myxoma.
Figure 1. Imagistic aspects of Case report 1. A: Cerebral CT scan: multiple hemorrhages (white areas), of different sizes, same age, in both hemispheres. B: Brain MRI, FLAIR sequences, multiple hemorrhages (hypointense areas) with surrounding edema (hyperintense areas).
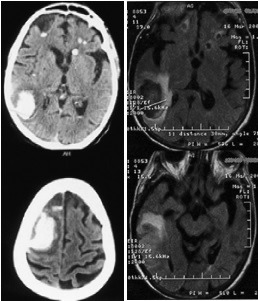
The patient's condition progressively worsened and she subsequently died. Necropsy revealed hemorrhagic abscesses with presence of blood in the subarachnoid space, infective endocarditis with vegetations, and bronchopneumonia.
An apparently higher overall rate of embolization is seen in NBTE (up to 91% of patients), with stroke being the most common clinical manifestation of NBTE. The high degree of correlation between malignancy, NBTE and stroke has been shown in several studies which establish NBTE as the leading cause of symptomatic ischemic stroke in cancer patients (see also Case report 2) (2,21,22).
The risk of embolization is difficult to ascertain in LSE, as the neuropsychiatric syndromes of SLE occur in about 5-18% of patients, with ischemic stroke accounting for about 80% of cases. The mechanisms leading to cerebral ischemia are diverse, given the broad spectrum of changes associated with SLE and APS, and while valvular abnormalities, and even vegetations, can be found in such patients with stroke, it is difficult to attribute the cerebrovascular event to embolism (23-25). ❑
CASE REPORT 2
A 65-year old woman was admitted to our clinic following the sudden onset of speech impairment. Upon presentation, the patient had motor aphasia, but also mild ataxia of the right limbs. The patient had been recently diagnosed with deep venous thrombosis of her left inferior limb, for which she was prescribed low molecular weight heparin (LMWH), antibiotics, and pain relievers. Also, an abdomen and pelvis CT-scan had been performed, which showed a 30 mm mass in the cervix, with low contrast uptake.
Admission cerebral CT-scan showed a right cerebellar hipodensity. Duplex ultrasonography was suggestive of thrombosis of the right vertebral artery, a finding supported by the consequent cerebral contrast CT-scan.
Transthoracic, followed by transoesophageal cardiac ecocardiography showed a 4/4mm mass of the atrial side of the mitral valve. Three sets of blood cultures were performed, although the patient never presented fever and didn't have leukocytosis or positive inflammation markers in the serum, all of which were negative.
Biopsy of the cervix mass showed endocervical mucinous adenocarcinoma. Taking into consideration the patient's history a chest X-ray was also performed, and it showed multiple bilateral disseminated lesions, suggestive of metastases.
Before being able to perform additional tests, the patient asked to be discharged. For the duration of the stay in our clinic, she received LMWH and empirical antibiotic therapy with gentamycin and vancomycin.
The patient returned 2 days after discharge for right hemiparesis. A repeated cerebral CT scan showed acute MCA stroke. A repeated transthoracic echocardiography showed no more vegetations, and blood cultures were consistently negative. Aspirin was added to LMWH, but had to be stopped due to persistent metroragia. The right hemiparesis and the aphasia gradually improved and the patient was discharged being directed to a local oncologic service. ❑
MANAGEMENT OF ENDOCARDITIS IN THE SETTING OF STROKE
Once endocarditis has been diagnosed in acute stroke patients, efforts should be directed towards minimizing the risk of subsequent cerebral embolization and, if possible, curing the pathology underlying the embolic source.
Overall embolic risk in IE is considered to be between 10-50%. Initiation of targeted antibiotic therapy has shown to lower this risk to 6-21%, with most events (65%) occuring in the first two weeks of treatment (see also Case report 3). Several predictors of embolic risk have been identified in various studies, involving vegetation characteristics: Staphylococcus spp. infection, high mobility, size of >10 mm (measured by transoesophageal echocardiography), which doesn't decrease under appropriate antibiotic treatment, mitral valve (or multivalvular) position, and associated embolic events; furthermore, elevated C reactive protein levels have been linked to an increased risk of embolism from IE (9,26,27). ❑
CASE REPORT 3
A 51-year old woman, with no prior medical history, presented to our hospital's Emergency Department with motor aphasia, right central facial palsy, right-sided predominantly brachial hemiparesis, and decresed muscular tonus in her right limbs. Admission cerebral CT-scan showed no recent vascular lesions. Duplex ultrasonography revealed only minor cerebrovascular atheromatosis, and Holter monitoring disclosed sinus rhythm throughout the 24-hour recording period.
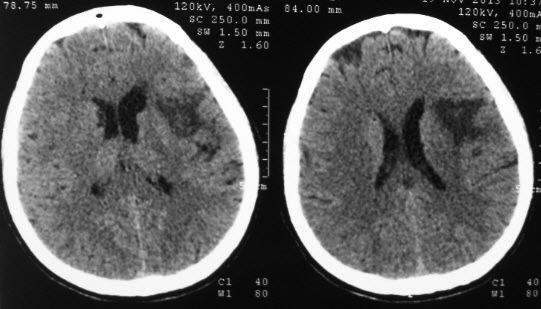
The patient received antiplatelet therapy and statin. In the following week her speech improved and she gradually regained strength in her right limbs. However, on the seventh day, prior to the scheduled transthoracic cardiac ultrasound, the neurologic deficits worsened and she developed a 38°C fever. A new cerebral CT-scan was performed which showed a subacute hipodensity in the right frontal and temporal lobes and in the right insula (below).
Emergent cardiac ultrasonography showed a mass on the ventricular side of the mitral valve. Blood cultures were taken and the patient received empirical antibiotic therapy with ampicillin and vancomycin (she could not receive gentamycin due to impaired renal function). Blood cultures came back positive for MRSA+ Staphylococcus aureus and the patient was subsequently transfered to an infectious diseases department, with progressive resolution of the infection and improvement in neurological status.
While the role in embolic risk reduction has been demonstrated for prompt and targeted antibiotic therapy, the benefit of anticoagulation therapy is debatable. IE is not an indication for anticoagulation per se, but this therapeutic approach may be necessary when taking into consideration special situations such as the presence of mechanical prosthetic valves, atrial fibrillation or hypercoagulability disorders; furthermore, ongoing previous anticoagulation has been suggested, albeit by limited data, to be associated with a reduction in vegetation size and lower cerebrovascular complications. A special consideration must be given to patients with prosthetic valve endocarditis who develop intracranial hemorrhage, as they require ongoing anticoagulation, and should be switched to intravenous heparin at a lower anticoagulation intensity than non-IE patients (27-30).
Figure 2.
Platelets are a constituent of the NBT preceding the formation of the IE vegetation. Therefore antiplatelet treatment could be viewed as beneficial in altering part of the pathogenic mechanism of IE. However, its potential benefits in IE remain unproven, despite data suggesting a lower risk of embolic events, decreased rate of acute valve replacement surgery, or mortality, for patients taking chronic aspirin therapy prior to developing endocarditis (31-34).
While surgery has certain indications in IE, concomitant cerebrovascular pathology forces reassessment of the benefit-to-risk ratio when undertaking this approach, with the possible necessity of postponement for up to 4 weeks in the event of large cerebral infarction or intracerebral hemorrhage, in order to prevent neurological deterioration. Furthermore, the risks of cardiopulmonary bypass have to be taken into consideration when deciding whether to perform cardiac surgery in IE patients with stroke. Nevertheless, early surgery in selected patients has been proven effective, with up to 70% of survivors achieving full neurological recovery after cardiac surgery (17,35).
IE is a contraindication for thrombolysis in acute ischemic stroke due to increased hemorrhagic risk. However, the suspicion of IE is only rarely raised in the acute setting and thrombolysis is often initiated if the patient presents within the required therapeutic window, leading either to thrombus resolution or to multifocal hemorrhage (36,37).
Patients with mycotic aneurysms may benefit from a surgical or endovascular approach. Open neurosurgery with proximal ligation rather than clipping, due to technical difficulties, may be employed for ruptured aneurysms; an endovascular approach is to be considered for patients deemed unfit due to underlying cardiac disease. For unruptured aneurysms, depending on size, antibiotics alone, for small ones, or combined with endovascular treatment, for larger ones, can be used (30,38,39).
There are currently no guidelines for the treatment of NBTE. Nevertheless efforts should be made in order to decrease embolisation risk through anticoagulation with unfractioned heparin or low mollecular weight heparin, and also to treat the underlying cause of the disease (2,4).
Different views exist when addressing the management of LSE patients. There is no strong evidence that antiplatelet or anticoagulant medication stops the progression of valve lesions. The role of corticosteroid or immunosuppressive therapy is unclear, as it may prevent the development of cardiac lesions in the long-term, but may also induce fibrotic changes on its own, potentially leading to valvular dysfunction (23,40,41).
Given the sparse data, cardiac surgery in the setting of NBTE or LSE should be considered on a case-by-case basis. ❑
DISCUSSION
Endocarditis is still an underdiagnosed pathology in relation to stroke. A prompt diagnosis should be established and an appropriate treatment initiated, but the lack of pathognomonic signs and symptoms delay both. In general, endocarditis is taken into consideration as a possible cause for stroke after other, more frequent, causes are ruled out, if the patient presents with fever, sepsis, or in the setting of recurrent embolic events. There is no test that can readily and reliably prove endocarditis, except perhaps for echocardiography, which is however a user-dependent examination and thus susceptible to human error.
Endocarditis has a high potential to embolize, with stroke being one of the major complications. Cerebrovascular lesions are often bilateral, and can be either ischemic or hemorrhagic. Aside from supportive stroke care, efforts should be directed towards minimizing the embolic risk. While antibiotic therapy is the mainstay in IE, less clear indications have been defined for NBTE and LSE, although in these cases anticoagulation is the main option for secondary prevention of thrombotic events; surgery is also indicated, in selected cases.
We presented three cases from our clinic aiming to highlight the variable clinical presentation and course of cerebral vascular lesions caused by endocarditis, as well as the possible difficulties in establishing this diagnosis.
The first patient presented with signs of pulmonary infection and non-specific symptoms (headache, vomiting), with a causative CNS infection being ruled out by the lumbar puncture. The subsequent worsening in the patient's neurologic status, the hemorrhagic lesions showed by the repeated CT scan and the inflammatory syndrome raised the suspicion of an infective endocarditis. The blood cultures were negative and the cardiac ultrasonography inconclusive, the diagnosis being established by necropsy. Given the patient's medical history, preexisting diffuse endothelial damage might have promoted infective thrombus formation in the setting of the concurrent pulmonary infection, which afterwards embolized causing mycotic aneurysms which ruptured.
The second patient had a previously undiagnosed mucin producing cervical cancer and had already had a thrombotic episode upon admission to our clinic. This fact, as well as the consecutive cerebrovascular events involving different vascular territories, the transient mitral valve vegetation (possibly favoured by the known friability of NBTE thrombi), the negative blood cultures and the lack of inflammation, supported the diagnosis of paraneoplastic NBTE.
The third patient was a more classical case of bacterial IE associated stroke, with cardiac ultrasonography, blood cultures and clinical presentation being supportive for the diagnosis, which was however delayed by the lack of initial signs of infection. This led to an atherothrombotic stroke management approach, and the patient initially received no antibiotic therapy which could have prevented the second embolic event.
Further research is needed to better diagnose endocarditis as a cause of stroke in order to offer these patients early adequate treatment. This is particularly the case for infective endocarditis, as NBTE and LSE are rare stroke etiologies and an even higher index of suspicion is required than for IE. The classic Duke criteria are somewhat restrictive. Firstly, specific organisms are listed as a major criterion, with culture-negative IE seeming a remote possibility; this can further hinder the diagnosis of IE as not all microbiology laboratories can perform tests for the more infrequent agents, which are increasingly described in relation to IE (such as Tropheryma whipplei). Secondly, cardiac ultrasonography, initially transthoracic followed by transesophageal if the first was negative, is an investigation susceptible to personal interpretation and can be unreliable in some cases (obese or poorly compliant patients). A fever of >38°C is again restrictive as not all infectious agents lead to a high grade fever, and immunologic phenomena (Osler nodes, Roth spots) and some of the vascular ones (particularly Janeway lesions) are infrequent (<10% of cases) and most clinicians are unfamiliar with them, thus not being commonly sought. Finally, embolic phenomena, particularly in the case of stroke, should probably have a more important role in the diagnosis of IE, as they may be of more signifficance to the outcome of the patient and warrant specific antibiotic treatment (42,43).
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Toni D, Sacco RL, Brainin M, et al. In: Mohr JP, Wolf PA, Grotta JC, et al. Stroke - Pathophysiology, Diagnosis, and Management. 5th ed. Elsevier Saunders; Philadelphia: 2011. Classification of Ischemic Stroke. pp. 293–306. [Google Scholar]
- 2.Asopa S, Patel A, Khan OA, et al. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007;32:696–701. doi: 10.1016/j.ejcts.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Lee JL, Naguwa SM, Cheema GS, et al. Revisiting Libman-Sacks Endocarditis: A Historical Review and Update. Clin Rev Allergy Immunol. 2009;36:126–130. doi: 10.1007/s12016-008-8113-y. [DOI] [PubMed] [Google Scholar]
- 4.el-Shami K, Griffiths E, Streiff M. Nonbacterial Thrombotic Endocarditis in Cancer Patients: Pathogenesis, Diagnosis, and Treatment. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 5.Keynan Y, Rubinstein E. Pathophysiology of Infective Endocarditis. Curr Infect Dis Rep. 2013;15:342–346. doi: 10.1007/s11908-013-0346-0. [DOI] [PubMed] [Google Scholar]
- 6.Fowler VG, Scheld WM, Bayer AS. In: Mandell GL, Bennett JE, Dolin R Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. Churchill Livingstone Elsevier; Philadelphia: 2009. Endocarditis and Intravascular Infections. pp. 1067–112. [Google Scholar]
- 7.Karchmer AW. In: Bonow RO, Mann DL, Zipes DP, Libby P Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Elsevier Saunders; Philadelphia: 2011. Infective Endocarditis. pp. 1540–1563. [Google Scholar]
- 8.Singhal AB, Topcuoglu MA, Buonanno FS. Acute Ischemic Stroke Patterns in Infective and Nonbacterial Thrombotic Endocarditis: A Diffusion-Weighted Magnetic Resonance Imaging Study. Stroke. 2002;33:1267–1273. doi: 10.1161/01.str.0000015029.91577.36. [DOI] [PubMed] [Google Scholar]
- 9.Habib G, Hoen B, Tornos P, et al. Guideline on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). Eur Heart J. 2009;30:2369–2431. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 10.Sonneville R, Mirabel M, Hajage D, et al. Neurologic Complications and Outcomes of Infective Endocarditis in Critically Ill Patients: The ENDOcardite en REAnimation Prospective Multicenter Study. Crit Care Med. 2011;39:1474–1481. doi: 10.1097/CCM.0b013e3182120b41. [DOI] [PubMed] [Google Scholar]
- 11.Entrikin DW, Gupta P, Kon ND, et al. Imaging of Infective Endocarditis with Cardiac CT Angiography. J Cardiovasc Comput Tomogr. 2012;6:399–405. doi: 10.1016/j.jcct.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Correia E, Almeida A, Madureira AJ, et al. Mycotic Aneurysm of the Left Ventricular Free Wall Complicating Aortic Valve Endocarditis. Rev Port Cardiol. 2012;31:31–34. doi: 10.1016/j.repc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Snygg-Martin U, Gustafsson L, Rosengren L, et al. Cerebrovascular Complications in Patients with Left-Sided Infective Endocarditis Are Common: a Prospective Study Using Magnetic Resonance Imaging and Neurochemical Brain Damage Markers. Clin Infect Dis. 2008;47:23–30. doi: 10.1086/588663. [DOI] [PubMed] [Google Scholar]
- 14.Sonneville R, Mourvilier B, Bouadma L, et al. Management of Neurological Complications of Infective Endocarditis in ICU Patients. Ann Intensive Care. 2011;1:10–10. doi: 10.1186/2110-5820-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess A, Klein I, Ioung B, et al. Brain MRI Findings in Neurologically Asymptomatic Patients with Infective Endocarditis. AJNR Am J Neuroradiol. 2013;34:1579–1584. doi: 10.3174/ajnr.A3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper HA, Thompson EC, Laureno R, et al. Subclinical Brain Embolization in Left-Sided Infective Endocarditis - Results From the Evaluation by MRI of the Brains of Patients with Left-Sided Intracardiac Solid Masses (EMBOLISM) Pilor Study. Circulation. 2009;120:585–591. doi: 10.1161/CIRCULATIONAHA.108.834432. [DOI] [PubMed] [Google Scholar]
- 17.Ruttmann E, Willeit J, Ulmer H, et al. Neurological Outcome of Septic Cardioembolic Stroke After Infective Endocarditis. Stroke. 2006;37:2094–2099. doi: 10.1161/01.STR.0000229894.28591.3f. [DOI] [PubMed] [Google Scholar]
- 18.Aziz F, Perwaiz S, Penupolu S, et al. Intracranial Hemorrhage in Infective Endocarditis: A Case Report. J Thorac Dis. 2011;3:134–137. doi: 10.3978/j.issn.2072-1439.2010.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okazaki S, Sakaguchi M, Hyun B, et al. Cerebral Microbleeds Predict Impending Intracranial Hemorrhage in Infective Endocarditis. Cerebrovasc Dis. 2011;32:483–488. doi: 10.1159/000331475. [DOI] [PubMed] [Google Scholar]
- 20.Molina CA, Selim MH. Anticoagulation in Patients With Stroke With Infective Endocarditis - The Sword of Damocles. Stroke. 2011;42:1799–1800. doi: 10.1161/STROKEAHA.111.622423. [DOI] [PubMed] [Google Scholar]
- 21.Lee V, Gilbert JD, Byard RW. Marantic endocarditis - A not so benign entity. J Forensic Leg Med. 2012;19:312–315. doi: 10.1016/j.jflm.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Martín-Martorell P, Insa-Molla A, Chirivella-González MI, et al. Nonbacterial thrombotic endocarditis associated with lung adenocarcinoma. Clin Transl Oncol. 2007;9:744–746. doi: 10.1007/s12094-007-0133-1. [DOI] [PubMed] [Google Scholar]
- 23.Malvar B, Mendonça Almeida F, Rebocho L, et al. Cerebral embolism from Libman-Sacks endocarditis. BMJ Case Rep. 2011 doi: 10.1136/bcr.04.2011.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West SG. In: Wallace DJ, Hahn BH Dubois's Lupus Erythematosus and Related Syndromes. 8th ed. Elsevier Saunders; Philadelphia: 2013. Clinical Aspects of the Nervous System. pp. 368–381. [Google Scholar]
- 25.Jennekens FGI, L K. The central nervous system in systemic lupus erythematosus. Part 2. Pathogenetic mechanisms of clinical syndromes: a literature investigation. Rheumatology (Oxford) 2002;41:619–630. doi: 10.1093/rheumatology/41.6.619. [DOI] [PubMed] [Google Scholar]
- 26.Di Salvo G, Habib G, Pergola V, et al. Echocardiography Predicts Embolic Events in Infective Endocarditis. Am J Coll Cardiol. 2001;37:1069–1076. doi: 10.1016/s0735-1097(00)01206-7. [DOI] [PubMed] [Google Scholar]
- 27.Vilacosta I, Graupner C, San Román JA, et al. Risk of Embolization After Institution of Antibiotic Therapy for Infective Endocarditis. Am J Coll Cardiol. 2002;39:1489–1495. doi: 10.1016/s0735-1097(02)01790-4. [DOI] [PubMed] [Google Scholar]
- 28.Snygg-Martin U, Rasmussen RV, Hassager C, et al. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis. 2011;30:151–157. doi: 10.1007/s10096-010-1063-3. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen RV. Anticoagulation in Patients With Stroke With Infective Endocarditis Is Safe. Stroke. 2011;42:1795–1796. doi: 10.1161/STROKEAHA.110.611681. [DOI] [PubMed] [Google Scholar]
- 30.Morris NA, Matiello M, Lyons JL, et al. Neurologic complications in infective endocarditis: identification, management, and impact on cardiac surgery. Neurohospitalist. 2014;4:213–222. doi: 10.1177/1941874414537077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habib A, Baddour LM, Sohail MR. Impact of Antiplatelet Therapy on Clinical Manifestations and Outcomes of Cardiovascular Infections. Curr Infect Dis Rep. 2013;15:347–352. doi: 10.1007/s11908-013-0347-z. [DOI] [PubMed] [Google Scholar]
- 32.Anavekar NS, Murphy JG. Is There a Role for Antiplatelet Therapy in Infective Endocarditis? A Review of Current Scientific Evidence. Curr Infect Dis Rep. 2010;12:253–256. doi: 10.1007/s11908-010-0115-2. [DOI] [PubMed] [Google Scholar]
- 33.Anavekar NS, Schultz JC, Baddour LM, et al. Modifiers of Symptomatic Embolic Risk in Infective Endocarditis. Mayo Clin Proc. 2011;86:1068–1074. doi: 10.4065/mcp.2011.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepin J, Tremblay V, Bechard D, et al. Chronic antiplatelet therapy and mortality among patients with infective endocarditis. Clin Microbiol Infect. 2009;15:193–199. doi: 10.1111/j.1469-0691.2008.02665.x. [DOI] [PubMed] [Google Scholar]
- 35.Derex L, Bonnefoy E, Delahaye F. Impact of stroke on therapeutic decision making in infective endocarditis. J Neurol. 2010;257:315–321. doi: 10.1007/s00415-009-5364-3. [DOI] [PubMed] [Google Scholar]
- 36.Ong E, Mechtouff L, Bernard E, et al. Thrombolysis for stroke caused by infective endocarditis: an illustrative case and review of the literature. J Neurol. 2013;260:1339–1342. doi: 10.1007/s00415-012-6802-1. [DOI] [PubMed] [Google Scholar]
- 37.Bhuva P, Kuo SH, Claude Hemphill J, et al. Intracranial Hemorrhage Following Thrombolytic Use for Stroke Caused by Infective Endocarditis. Neurocrit Care. 2010;12:79–82. doi: 10.1007/s12028-009-9253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dababneh H, Hedna VS, Ford J, et al. Endovascular intervention for acute stroke due to infective endocarditis. Neurosurg Focus. 2012;32:E1–E1. doi: 10.3171/2011.11.FOCUS11263. [DOI] [PubMed] [Google Scholar]
- 39.Kang G, Yang TK, Choi JH, et al. Effectiveness of Mechanical Embolectomy for Septic Embolus in the Cerebral Artery Complicated with Infective Endocarditis. J Korean Med Sci. 2013;28:1244–1247. doi: 10.3346/jkms.2013.28.8.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Irastoza G, Khamashta M. In: Wallace DJ, Hahn BH Dubois' Lupus Erythematosus and Related Syndromes. 8th ed. Elsevier Saunders; Philadelphia: 2013. Cardiopulmonary Disease in SLE. pp. 352–362. [Google Scholar]
- 41.Ferreira E, Bettencourt PM, Moura LM. Valvular lesions in patients with systemic lupus erythematosus and antiphospholipid syndrome: An old disease but a persistent challenge. Rev Port Cardiol. 2012;31:295–299. doi: 10.1016/j.repc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Fenollar F, Célard M, Lagier JC, et al. Tropheryma whipplei endocarditis. Emerg Infect Dis. 2013;19:1721–1730. doi: 10.3201/eid1911.121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanakunakom C, Burkert T. Infective endocarditis at a large community teaching hospital, 1980-1990. A review of 210 episodes. Medicine (Baltimore) 1993;72:90–102. doi: 10.1097/00005792-199303000-00003. [DOI] [PubMed] [Google Scholar]


