Abstract
Sepsis is a serious condition characterized by an infectious process that induces a severe systemic inflammatory response. In this study, the effects of gemfibrozil (GFZ) on the inflammatory response associated with abdominal sepsis were investigated using a rat model of cecal-ligation and puncture (CLP). Male Wistar rats were randomly divided into three groups: Sham-operated group (sham), where laparotomy was performed, the intestines were manipulated, and the cecum was ligated but not punctured; control group, subjected to CLP; and GFZ group, which received GFZ prior to undergoing CLP. The groups were then subdivided into three different time-points: 2, 4 and 24 h, indicating the time at which blood samples were obtained for analysis. Serum concentrations of tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), malondialdehyde (MDA), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) were determined. The LDH, AST and ALT values were significantly elevated following CLP compared with those in the sham group, and GFZ treatment was able to reduce these elevations. GFZ also reduced the sepsis-induced elevations of TNF-α and IL-1. In conclusion, GFZ treatment was able to attenuate the inflammatory response associated with CLP-induced sepsis, by diminishing the release of inflammatory cytokines, thereby reducing tissue injury and oxidative stress.
Keywords: sepsis, gemfibrozil, fibrates, inflammation, rat
Introduction
Sepsis is a serious condition associated with great mortality, characterized by an infectious process that induces a severe systemic inflammatory response. The physiopathology of sepsis is well understood, and mediators such as pro-inflammatory cytokines, reactive oxygen species, nitric oxide, toll-like receptors and transcription factors such as nuclear factor κB (NF-κB) play central roles in regulating the immune response responsible for tissue injury and circulatory collapse (1,2). These events lead to hypotension, increased capillary permeability, multiple organ failure and mortality.
Although various therapeutic strategies have been implemented with the aim of reducing sepsis-associated morbidity and mortality, the cornerstone of treatment remains prompt antibiotic use and hemodynamic management (3). The use of immunomodulators, such as activated protein C, has been evaluated and early results were promising (4). However, a recent trial did not find any clinical benefit with the use of this drug (5), thus questioning the use of drugs that attempt to modulate the inflammatory response. Various drugs with pleiotropic immunomodulatory properties are currently being studied, and this remains an active field of research (6).
Fibrates such as gemfibrozil (GFZ) and fenofibrate are drugs commonly used in the management of dyslipidemia. They are ligands for peroxisome proliferator-activated receptor α (PPAR-α), receptors that belong to the steroid nuclear receptor family and that participate in lipid peroxidation, the cell cycle and fatty acid synthesis. Potent immunomodulatory effects have been associated with fibrates (7). Fibrates have shown the ability to reduce the expression of inflammatory genes in endothelial cells, the release of pro-inflammatory cytokine such as interleukins (ILs) and tumor-necrosis factor-α (TNF-α), and the transcription of NF-κB in experimental studies (8,9). Fibrates have also been shown to reduce IL-1 and C-reactive protein in humans (10). Fibrates also possess antioxidant effects, and regulate the activation and function of inflammatory cells (11,12). These effects are independent of the lipid-lowering properties of fibrates.
The anti-inflammatory effects of fibrates have been tested in various experimental models of tissue and organ injury, showing beneficial results (reduced injury and inflammation) in models of autoimmune encephalomyelitis (13), ischemia-reperfusion injury (14) and alcohol-induced hepatotoxicity (15), among others. Considering the wide ranging immunomodulatory effects of fibrates, some of which overlap with the physiopathology of sepsis, the use of fibrates emerges as an interesting option.
Materials and methods
Animals
Animal procedures were performed in accordance with the proper use and care of laboratory animals, approved by the ethics committee of the University Hospital ‘José Eleuterio González’ (Monterrey, Mexico). Experiments were performed on 45 male Wistar rats weighing 200–250 g (Vivarium of the Department of Physiology, Autonomous University of Nuevo León, Monterrey, Mexico). Animals were maintained under standard conditions, including a stable room temperature (24±3°C), a 12 h light/12 h dark cycle, and had access to commercial rat pellets and water ad libitum.
Cecal ligation and puncture model (CLP) (16)
After fasting for 12 h, the rats underwent ketamine/xylazine anesthesia (Anesket; Pfizer Inc., Mexico City, Mexico) at a dose of 50/10 mg/kg, intraperitoneally (i.p). Animals were placed under a heating lamp in order to preserve a core body temperature of 37°C. A midline incision exposed the intestines and the cecum was ligated (using 2-0 silk sutures) immediately proximal to the ileocecal valve causing a 50% obstruction and allowing for permeability. Two through-and-through punctures using a 18-gauge needle were performed at the anti-mesenteric side of the cecum. Light pressure was applied to confirm that fecal matter could emerge into the peritoneal cavity. The cecum was returned to its site, the wound was closed and 3 ml/100 g saline was administered subcutaneously.
The rats were randomly divided into three groups (n=15 per group): i) Sham-operated group (sham), where laparotomy was performed, the intestines were only manipulated and the cecum was ligated but not punctured. ii) Control group, subjected to CLP as described above. iii) GFZ group that received GFZ (Pfizer Inc.) prior to undergoing CLP. A dose of 100 mg/kg GFZ was chosen based on dose-response experiments and was administered 24 h and immediately prior to surgery. This dose has been shown to decrease oxidative stress in rodent models (11). The groups were then subdivided into three different time-points: 2, 4 and 24 h. These time-points indicated the time after CLP when samples were obtained for analysis.
Serum analysis
Blood samples obtained following CLP were used to determine the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) by standard biochemical automated methods (Vitros Chemical Products; Johnson & Johnson, New Brunswick, NJ, USA), using commercially available kits and DT6011 and DTSC11 analyzers (Vitros Chemical System; Johnson & Johnson). The serum concentrations of TNF-α and IL-1 were determined using a rat ELISA kit (PeproTech, Mexico City, Mexico). Lipid peroxidation, expressed as the malondialdehyde (MDA) level, was assessed by the thiobarbituric acid reactive substances (TBARS) method using a TBARS colorimetric assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). The level of LDH was only evaluated at the 24-h time-point after CLP.
Survival
To assess survival, additional groups of rats (n=10 per group) underwent the same study protocol as those in the control and GFZ groups, and the rats were left to recover following the surgery. The animals were observed for a period of five days and the mortality rate was recorded.
Statistical analysis
SPSS statistical software, version 11.0 (SPSS Inc., Chicago, IL, USA) was used to analyze data using one-way analysis of variance (ANOVA) and with LSD post-hoc test (when data were found to be normally distributed) and Kruskal-Wallis test (when data were not normally distributed) so as to evaluate comparisons between groups, and differences between groups, respectively. The survival curves were determined using the Kaplan-Meier method and the log-rank test was used to compare the curves. All values are expressed as mean ± standard deviation (SD) and P<0.05 was considered to indicate a statistically significant result.
Results
ALT and AST
AST and ALT values were significantly elevated following CLP compared with those in the sham group, peaking at 4 h for AST and 24 h for ALT (Table I). GFZ treatment was able to reduce the elevations in AST and ALT, with its effects reaching statistical significance at 2 and 4 h in the case of AST and at 4 h in the case of ALT. At 24 h after CLP, LDH levels were significantly elevated, and GFZ treatment was also able to attenuate this increase.
Table I.
Serum levels of ALT, AST and LDH.
| Group | AST (UI) | ALT (UI) | LDH (UI) |
|---|---|---|---|
| Sham | 109.0±10.6 | 15.8±5.2 | 537.0±141.4 |
| CLP 2 h | 271.4±58.7a | 27.4±10.2 | |
| CLP 4 h | 293.8±113.9a | 39.8±16.3 | |
| CLP 24 h | 200.8±16.6a | 44.0±2.7a | 1983.4±530.7a |
| GFZ 2 h | 175.8±69.9b | 19.0±11.6 | |
| GFZ 4 h | 204.6±28.8b | 19.4±5.1b | |
| GFZ 24 h | 172.2±33.9 | 37.4±8.3 | 1057.4±403.2b |
Values are expressed as mean ± standard deviation. ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase;
P<0.05 vs. Sham;
P<0.05 vs. CLP.
GFZ, gemfibrozil; CLP, cecal ligation and puncture.
Serum cytokine levels
Levels of TNF-α were significantly elevated in all time-points in the CLP group as compared with those in the sham controls (Fig. 1). TNF-α levels peaked at 4 h and remained elevated even at 24 h. GFZ treatment resulted in significantly lower levels of TNF-α at 4 and 24 h after CLP. In the case of IL-1, there was no difference between the groups at 2 h, where levels were undetectable by the assay. At 4 and 24 h, the IL-1 levels were elevated; however, statistical significance was reached only at 24 h. At this time-point, GFZ reduced IL-1 levels significantly (Fig. 1).
Figure 1.
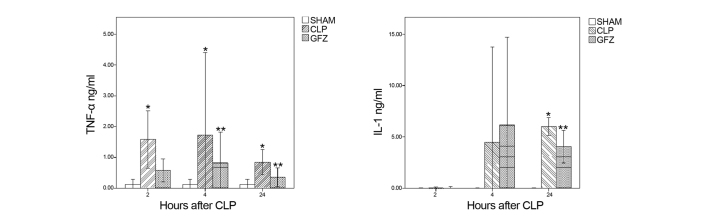
Levels of inflammatory cytokines. *P<0.05 vs. SHAM; **P<0.05 vs. CLP. TNF, tumor necrosis factor; IL-1, interleukin-1; GFZ, gemfibrozil; CLP, cecal ligation and puncture.
MDA
There were two identifiable time-points of increased MDA levels in the CLP group, at 2 and 24 h. However, no difference was found between the CLP and GFZ groups (data not shown).
Survival
By 48 h, five rats in the CLP group and three in the GFZ group had died. At 5 days, seven rats in the CLP group and five rats in the GFZ group had died. The difference in survival between groups did not reach statistical significance (P=0.1).
Discussion
PPARs have been studied in models of sepsis, and PPAR-γ agonists have been found to be effective in reducing the severity of endotoxic shock (17). A recent study showed that PPAR-α expression was decreased in patients with septic shock and that the values correlated with the severity of disease; in addition, in the CLP model, knockout mice lacking PPAR-α had decreased survival rates compared with wild-type animals (18). Fenofibrate treatment has been shown to reduce endothelial dysfunction and endothelial cell injury in a rabbit model of endotoxemia by lipopolysaccharide (LPS) injection (19). Improved endothelial-dependent relaxation and decreased monocyte tissue factor expression were observed. Another model of LPS-induced endotoxemia in rats also demonstrated reductions in myocardial contractility depression and in TNF-α levels after fenofibrate treatment (20). These results are in contrast to those in another study where dietary fenofibrate caused increased levels of TNF-α and mortality in endotoxemic wild-type mice, while showing lower TNF-α levels in PPAR-α knockout mice (21). Using a physiologically relevant model of abdominal sepsis, in the present study it was demonstrated that GFZ pretreatment reduced the inflammatory response associated with abdominal sepsis. GFZ had immunomodulatory effects, reducing the circulating levels of TNF-α, IL-1 and MDA, as well as markers of tissue injury such as AST and ALT. Differences between the LPS injection and CLP models could be relevant in explaining these inconsistent results, although the present study would support those showing a beneficial effect of PPAR-α ligands such as fibrates, despite only a trend towards a survival benefit being observed.
Inflammatory cytokines play an important but complex role in sepsis (1,2,22). Some of the mechanisms by which fibrates might suppress inflammatory cytokines are beginning to be uncovered. In a study with LPS-activated cells, PPAR-α ligands, including fibrates, were able to inhibit NF-κB DNA binding activity in astrocytes, leading to a modulation of the expression of inflammatory genes responsible for the production of cytokines such as IL-1 and TNF-α (23). Similar results were obtained in LPS-stimulated cardiac myocytes (24), and in injured endothelial cells (9). Clinical studies have shown that GFZ reduces TNF-α production in human peripheral blood mononuclear cells (25), and fibrates reduce IL-1 in human whole blood stimulated by endotoxin (26). Fibrates dose-dependently inhibit cytokine production (ILs, TNF-α and interferons) in activated T cells, probably via the inhibition of transcription factors associated with the inflammatory response, such as activator protein-1, c-Jun NH2-terminal protein kinase and P38 mitogen-activated protein kinase (27). The expression of IL-1 receptor antagonist, which is acutely stimulated by LPS treatment in the liver, can also be induced by PPARα (28). The switching of T cells from Th1 to Th2 profiles by GFZ is responsible for the tissue protection afforded against experimental encephalomyelitis in rats (12). Interestingly, the majority of studies use LPS, a key mediator of the sepsis inflammatory cascade, as a stimulant for cytokine production. In one study, using LPS-stimulated airway inflammation, PPAR-α−/− mice exhibited increased neutrophil infiltration and TNF-α production compared with that in PPAR-α+/+ mice, and fenofibrate was able to reduce TNF-α production in wild-type mice (8).
NF-κB is a key mediator of the sepsis inflammatory cascade. NF-κB activity is markedly increased in every organ studied, both in human and experimental models of septic shock, and greater levels of NF-κB activity are associated with a higher rate of mortality and worse clinical outcome in septic patients (29). The inhibition of NF-κB, and of the subsequent overproduction of inflammatory cytokines such as TNF-α, appears to be a crucial step in the immunomodulatory effects of PPAR-α activators. Cultured injured endothelial cells show activation of NF-κB that can be inhibited by incubation with fenofibrate (9). Fibrates were able to induce the expression of the inhibitory protein IκBα in human aortic smooth muscle cells as well as in primary human hepatocytes, providing a possible mechanism for NF-κB inhibition (30). Another study has also linked TLR to PPAR-α signaling. The PPARα agonist fenofibrate was found to reduce inflammatory cytokines and inflammation by antagonizing LPS-mediated inflammatory responses in vascular smooth muscle cells through a mechanism involving TLR-4 (31).
Marked oxidative stress results from the initiation of the inflammatory response in sepsis, and it initiates changes in mitochondrial function that may result in organ damage (32). Sepsis suppresses free fatty acid oxidation with the result of increased circulating fatty acids, through LPS-induced suppression of PPAR-α (33). Additionally, LPS induces the production of MDA and depletes catalase and superoxide dismutase in inflammatory cells (34). A previous study found elevations in MDA and glutathione depletion following CLP in rats (35). Fibrates are known to diminish MDA production in diabetic rats (36), and following severe liver ischemia-reperfusion injury in rats, fibrate pretreatment is able to reduce elevations in MDA and depletion of endogenous antioxidants (37). Elevations in MDA after CLP were observed in the present study, but only in untreated animals. No difference in MDA levels was identified between the CLP and GFZ group.
Although the inhibition of single cytokines or the addition of antioxidants has not shown value in the treatment of sepsis (38,39), the use of immunomodulators with pleiotropic effects has been continuously studied (6). Lipid-lowering drugs such as statins are currently thought to be prime candidates as adjunct treatments in sepsis (40), and clinical trials are underway. In this study it was found that another class of lipid-lowering drugs, fibrates, also have immunomodulating properties and could also be of value. The use of these drugs, alone or in combination, warrants further study.
Acknowledgements
The authors would like to thank the staff at their laboratories for invaluable assistance.
References
- 1.Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin. 2009;25:677–702. doi: 10.1016/j.ccc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37:291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neyrinck AP, Liu KD, Howard JP, Matthay MA. Protective mechanisms of activated protein C in severe inflammatory disorders. Br J Pharmacol. 2009;158:1034–1047. doi: 10.1111/j.1476-5381.2009.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranieri VM, Thompson BT, Barie PS, et al. PROWESS-SHOCK Study Group: Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 6.Parrish WR, Gallowitsch-Puerta M, Czura CJ, Tracey KJ. Experimental therapeutic strategies for severe sepsis: mediators and mechanisms. Ann NY Acad Sci. 2008;1144:210–236. doi: 10.1196/annals.1418.011. [DOI] [PubMed] [Google Scholar]
- 7.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N, Pons F. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir Res. 2005;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang TL, Chen MF, Luo BL, Xie QY, Jiang JL, Li YJ. Fenofibrate decreases asymmetric dimethylarginine level in cultured endothelial cells by inhibiting NF-kappaB activity. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:401–407. doi: 10.1007/s00210-005-1060-8. [DOI] [PubMed] [Google Scholar]
- 10.Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-alpha activators: clinical and experimental evidence. Arterioscler Thromb Vasc Biol. 2006;26:977–986. doi: 10.1161/01.ATV.0000204327.96431.9a. [DOI] [PubMed] [Google Scholar]
- 11.Ozansoy G, Akin B, Aktan F, Karasu C. Short-term gemfibrozil treatment reverses lipid profile and peroxidation but does not alter blood glucose and tissue antioxidant enzymes in chronically diabetic rats. Mol Cell Biochem. 2001;216:59–63. doi: 10.1023/A:1011000327529. [DOI] [PubMed] [Google Scholar]
- 12.Roy A, Pahan K. Gemfibrozil, stretching arms beyond lipid lowering. Immunopharmacol Immunotoxicol. 2009;31:339–351. doi: 10.1080/08923970902785253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol. 2007;72:934–946. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]
- 14.Sivarajah A, Chatterjee PK, Hattori Y, et al. Agonists of peroxisome-proliferator activated receptor-alpha (clofibrate and WY14643) reduce renal ischemia/reperfusion injury in the rat. Med Sci Monit. 2002;8:BR532–BR539. [PubMed] [Google Scholar]
- 15.Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther. 2004;310:417–424. doi: 10.1124/jpet.103.064717. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, III, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 17.Wu WT, Lee CC, Lee CJ, Subeq YM, Lee RP, Hsu BG. Rosiglitazone ameliorates endotoxin-induced organ damage in conscious rats. Biol Res Nurs. 2011;13:38–43. doi: 10.1177/1099800409353358. [DOI] [PubMed] [Google Scholar]
- 18.Standage SW, Caldwell CC, Zingarelli B, Wong HR. Reduced peroxisome proliferator-activated receptor α expression is associated with decreased survival and increased tissue bacterial load in sepsis. Shock. 2012;37:164–169. doi: 10.1097/SHK.0b013e31823f1a00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiel E, Lebuffe G, Robin E, et al. Pretreatment with peroxysome proliferator-activated receptor alpha agonist fenofibrate protects endothelium in rabbit Escherichia coli endotoxin-induced shock. Intensive Care Med. 2005;31:1269–1279. doi: 10.1007/s00134-005-2730-1. [DOI] [PubMed] [Google Scholar]
- 20.Jozefowicz E, Brisson H, Rozenberg S, et al. Activation of peroxisome proliferator-activated receptor-alpha by fenofibrate prevents myocardial dysfunction during endotoxemia in rats. Crit Care Med. 2007;35:856–863. doi: 10.1097/01.CCM.0000256843.75446.A0. [DOI] [PubMed] [Google Scholar]
- 21.Hill MR, Clarke S, Rodgers K, Thornhill B, Peters JM, Gonzalez FJ, Gimble JM. Effect of peroxisome proliferator-activated receptor alpha activators on tumor necrosis factor expression in mice during endotoxemia. Infect Immun. 1999;67:3488–3493. doi: 10.1128/iai.67.7.3488-3493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13:413–426. doi: 10.1016/S0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J Neuroimmunol. 2006;176:95–105. doi: 10.1016/j.jneuroim.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Takano H, Nagai T, Asakawa M, et al. Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes. Circ Res. 2000;87:596–602. doi: 10.1161/01.RES.87.7.596. [DOI] [PubMed] [Google Scholar]
- 25.Rosenson RS. Effect of fenofibrate on adiponectin and inflammatory biomarkers in metabolic syndrome patients. Obesity (Silver Spring) 2009;17:504–509. doi: 10.1038/oby.2008.530. [DOI] [PubMed] [Google Scholar]
- 26.Zhao SP, Ye HJ, Zhou HN, Nie S, Li QZ. Gemfibrozil reduces release of tumor necrosis factor-alpha in peripheral blood mononuclear cells from healthy subjects and patients with coronary heart disease. Clin Chim Acta. 2003;332:61–67. doi: 10.1016/S0009-8981(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 27.Cheng SM, Chu KM, Lai JH. The modulatory mechanisms of fenofibrate on human primary T cells. Eur J Pharm Sci. 2010;40:316–324. doi: 10.1016/j.ejps.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Stienstra R, Mandard S, Tan NS, et al. The interleukin-1 receptor antagonist is a direct target gene of PPARalpha in liver. J Hepatol. 2007;46:869–877. doi: 10.1016/j.jhep.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Liu SF, Malik AB. NF-kappaB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 30.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IkappaB alpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 31.Ji Y, Wang Z, Li Z, Liu J. Modulation of LPS-mediated inflammation by fenofibrate via the TRIF-dependent TLR4 signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem. 2010;25:631–640. doi: 10.1159/000315082. [DOI] [PubMed] [Google Scholar]
- 32.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 33.Maitra U, Chang S, Singh N, Li L. Molecular mechanism underlying the suppression of lipid oxidation during endotoxemia. Mol Immunol. 2009;47:420–425. doi: 10.1016/j.molimm.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victor VM, De la Fuente M. Immune cells redox state from mice with endotoxin-induced oxidative stress. Involvement of NF-kappaB. Free Radic Res. 2003;37:19–27. doi: 10.1080/1071576021000038522. [DOI] [PubMed] [Google Scholar]
- 35.Koksal GM, Sayilgan C, Aydin S, Oz H, Uzun H. Correlation of plasma and tissue oxidative stresses in intra-abdominal sepsis. J Surg Res. 2004;122:180–183. doi: 10.1016/j.jss.2004.07.246. [DOI] [PubMed] [Google Scholar]
- 36.Olukman M, Sezer ED, Ulker S, Sözmen EY, Cınar GM. Fenofibrate treatment enhances antioxidant status and attenuates endothelial dysfunction in streptozotocin-induced diabetic rats. Exp Diabetes Res. 2010;2010:828531. doi: 10.1155/2010/828531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boshra V, Moustafa AM. Effect of preischemic treatment with fenofibrate, a peroxisome proliferator-activated receptor-α ligand, on hepatic ischemia-reperfusion injury in rats. J Mol Histol. 2011;42:113–122. doi: 10.1007/s10735-011-9313-y. [DOI] [PubMed] [Google Scholar]
- 38.Lorente JA, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24(Suppl 1):107–119. doi: 10.1097/01.shk.0000191343.21228.78. [DOI] [PubMed] [Google Scholar]
- 39.Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012;9:CD006616. doi: 10.1002/14651858.CD006616.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mermis JD, Simpson SQ. HMG-CoA reductase inhibitors for prevention and treatment of severe sepsis. Curr Infect Dis Rep. 2012;14:484–492. doi: 10.1007/s11908-012-0277-1. [DOI] [PubMed] [Google Scholar]


