Abstract
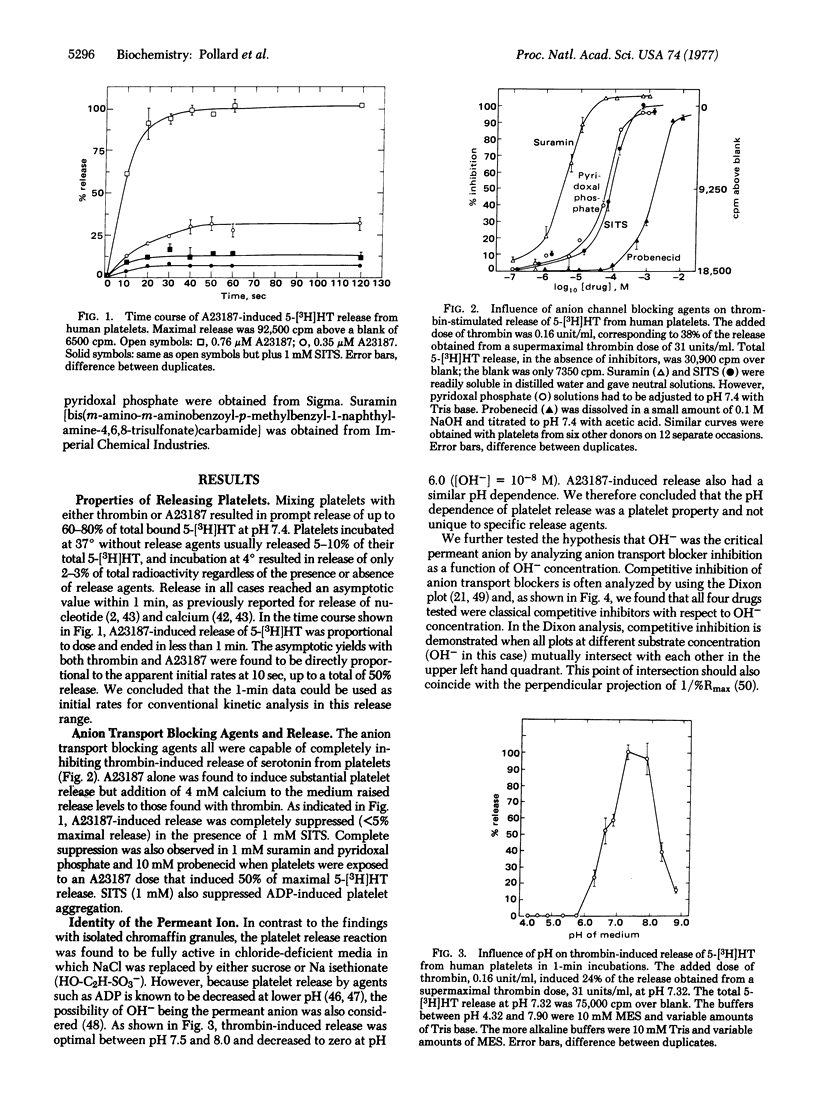
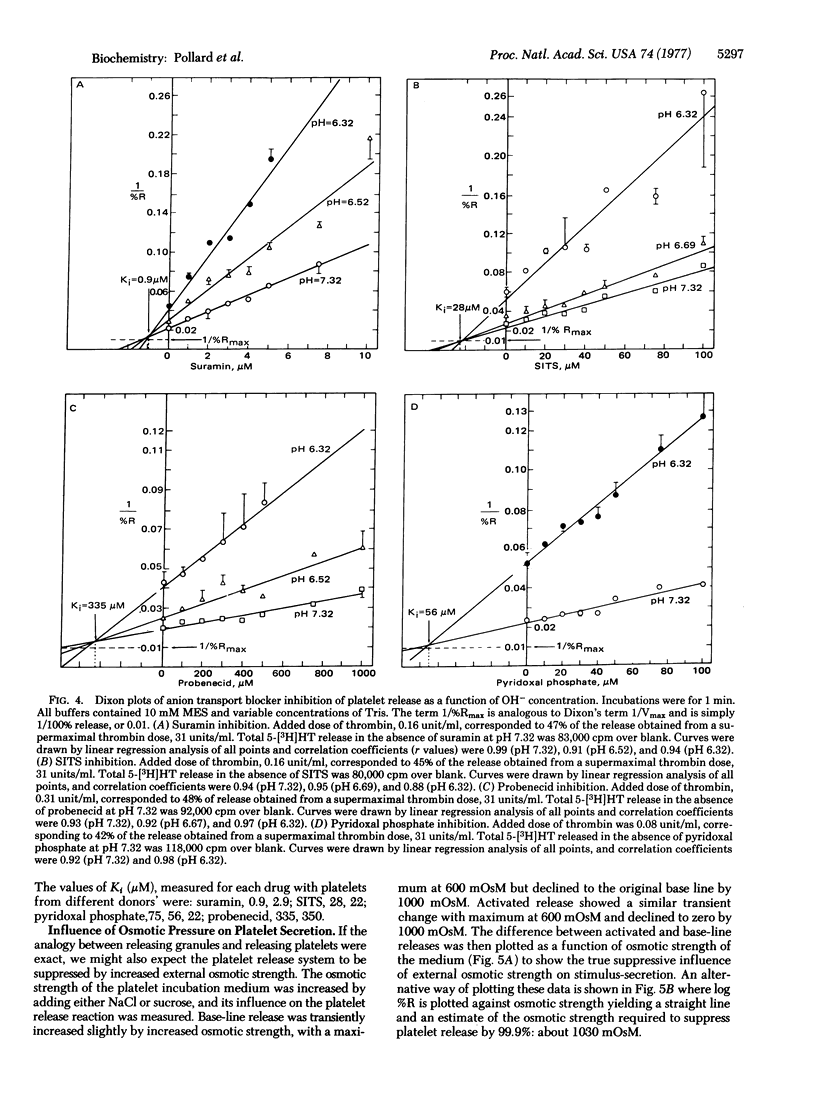
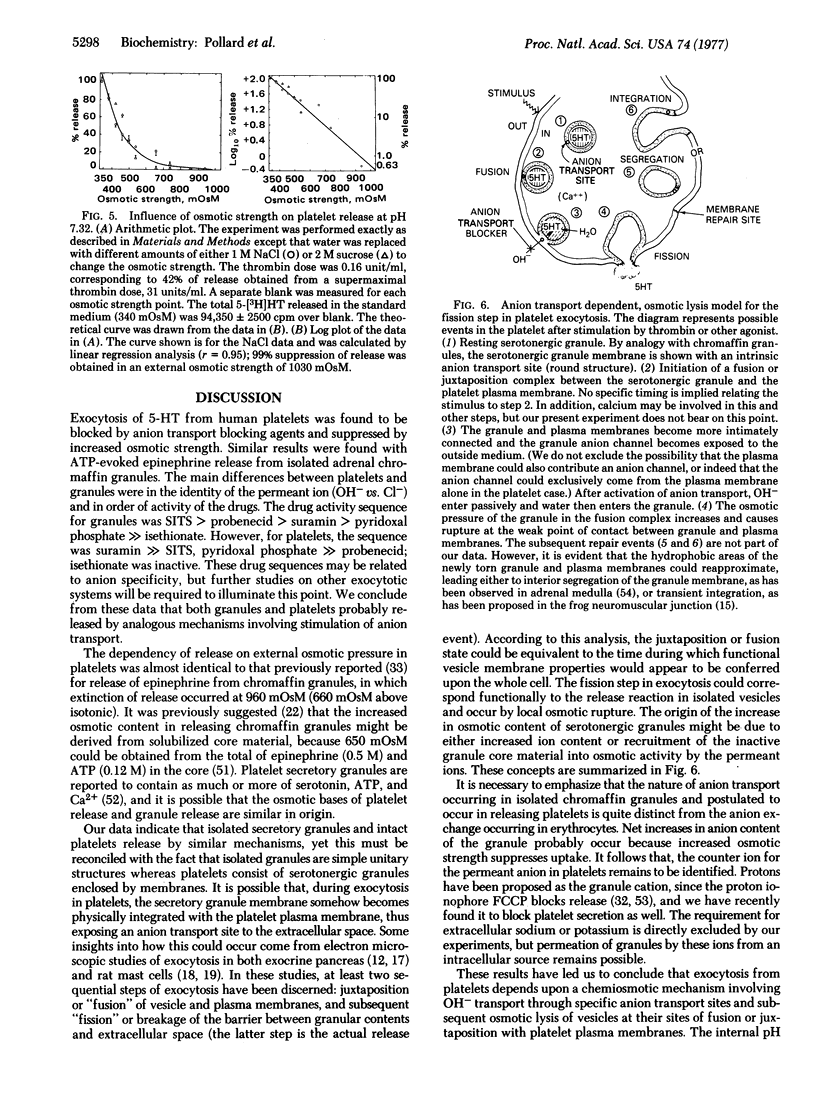
Serotonin secretion from human platelets, stimulated either by thrombin or the calcium ionophore A23187, was found to be inhibited by anion transport blocking drugs such as 4-acetamido-4'-isothiocyanostilbene-2,2'-disulfonic acid (SITS), pyridoxal phosphate, probenecid, and suramin. These drugs have previously been shown to inhibit ATP-evoked release of epinephrine from isolated chromaffin granules by blocking chloride uptake and subsequent osmotic lysis. However, in contrast to granule release, platelet secretion was insensitive to chloride and, instead, was dependent on OH-. Platelet release was suppressed by low pH, and inhibition by the transport blocking drugs was competitive only with respect to OH-. Serotonin release from platelets was also suppressed by increasing extracellular osmotic strength, and the relationship between suppression and external osmotic strength was quantitatively similar to that observed in the case of chromaffin granules. We conclude that platelet exocytosis could occur when serotonergic granules are closely juxtaposed to the plasma membrane, thus exposing the granule anion transport site to the more alkaline medium. Secretion of serotonin could occur as a consequence of OH- transport and osmotic lysis of the granule-plasma membrane complex, analogous to the chemiosmotic mechanism of chloride-dependent epinephrine release from isolated chromaffin granules.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Ohad I., Schramm M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969 Jun;41(3):753–773. doi: 10.1083/jcb.41.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. R., Foulks J. G. Competitive inhibition by lithium and hydrogen ions of the effect of calcium on the aggregation of rabbit platelets. Thromb Haemost. 1976 Nov 30;36(2):343–359. [PubMed] [Google Scholar]
- BRECHER G., CRONKITE E. P. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950 Dec;3(6):365–377. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- Berneis K. H., Da Prada M., Pletscher A. Micelle formation between 5-hydroxytryptamine and adenosine triphosphate in platelet storage organelles. Science. 1969 Aug 29;165(3896):913–914. doi: 10.1126/science.165.3896.913. [DOI] [PubMed] [Google Scholar]
- Cabantchik I. Z., Balshin M., Breuer W., Rothstein A. Pyridoxal phosphate. An anionic probe for protein amino groups exposed on the outer and inner surfaces of intact human red blood cells. J Biol Chem. 1975 Jul 10;250(13):5130–5136. [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. II. Effects of proteolytic enzymes on disulfonic stilbene sites of surface proteins. J Membr Biol. 1974;15(3):227–248. doi: 10.1007/BF01870089. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Njus D., Radda G. K., Sehr P. A. Adenosine triphosphate-evoked catecholamine release in chromatin granules. Osmotic lysis as a consequence of proton translocation. Biochem J. 1976 Sep 15;158(3):583–588. doi: 10.1042/bj1580583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D., Koehler J. K. Freeze-fracture study of mast cell secretion. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2823–2827. doi: 10.1073/pnas.73.8.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. L., Detwiler T. C., Feinman R. D., Murphy D. L., Patlak C. S., Pettigrew K. D. Quantitative evaluation of the loss of human platelet dense bodies following stimulation by thrombin or A23187. J Physiol. 1977 Jan;264(1):297–306. doi: 10.1113/jphysiol.1977.sp011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. L., Murphy D. L., Kafka M. S. Demonstration and evaluation of apparent cytoplasmic and vesicular serotonin compartments in human platelets. Biochem Pharmacol. 1977 Mar 15;26(6):517–521. doi: 10.1016/0006-2952(77)90327-6. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler T. C., Feinman R. D. Kinetics of the thrombin-induced release of adenosine triphosphate by platelets. Comparison with release of calcium. Biochemistry. 1973 Jun 19;12(13):2462–2468. doi: 10.1021/bi00737a015. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M., Rubin R. P. Efflux of adenine nucleotides from perfused adrenal glands exposed to nicotine and other chromaffin cell stimulants. J Physiol. 1965 Jul;179(1):130–137. doi: 10.1113/jphysiol.1965.sp007652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman R. D., Detwiler T. C. Platelet secretion induced by divalent cation ionophores. Nature. 1974 May 10;249(453):172–173. doi: 10.1038/249172a0. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Fraser C. Human platelet secretion and aggregation induced by calcium ionophores. Inhibition by PGE1 and dibutyryl cyclic AMP. J Gen Physiol. 1975 Nov;66(5):561–581. doi: 10.1085/jgp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes P. A., Ellory J. C., Lew V. L. Suramin: a potent ATPase inhibitor which acts on the inside surface of the sodium pump. Biochim Biophys Acta. 1973 Aug 22;318(2):262–272. doi: 10.1016/0005-2736(73)90119-3. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., White J. G., Rao G. H. Effects of the lonophore A23187 on the blood platelets II. Influence on ultrastructure. Am J Pathol. 1974 Nov;77(2):151–166. [PMC free article] [PubMed] [Google Scholar]
- Grynszpan-Winograd O. Morphological aspects of exocytosin in the adrenal medulla. Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):291–292. doi: 10.1098/rstb.1971.0058. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. K., Guidotti G. A membrane protein from human erythrocytes involved in anion exchange. J Biol Chem. 1975 Jan 25;250(2):675–683. [PubMed] [Google Scholar]
- Hoffman P. G., Zinder O., Bonner W. M., Pollard H. B. Role of ATP and beta-gamma-iminoadenosinetriphosphate in the stimulation of epinephrine and protein release from isolated adrenal secretory vesicles. Arch Biochem Biophys. 1976 Sep;176(1):375–388. doi: 10.1016/0003-9861(76)90177-6. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Stormorken H. The blood platelet release reaction. Scand J Haematol Suppl. 1969;8:3–26. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J Cell Biol. 1971 Jul;50(1):135–158. doi: 10.1083/jcb.50.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshner N., Sage H. J., Smith W. J., Kirshner A. G. Release of catecholamines and specific protein from adrenal glands. Science. 1966 Oct 28;154(3748):529–531. doi: 10.1126/science.154.3748.529. [DOI] [PubMed] [Google Scholar]
- Kirshner N., Sage H. J., Smith W. J. Mechanism of secretion from the adrenal medulla. II. Release of catecholamines and storage vesicle protein in response to chemical stimulation. Mol Pharmacol. 1967 May;3(3):254–265. [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. Some effects of ionophores for divalent cations on blood platelets. Comparison with the effects of thrombin. Biochim Biophys Acta. 1974 Nov 4;372(1):109–121. doi: 10.1016/0304-4165(74)90077-4. [DOI] [PubMed] [Google Scholar]
- Motais R., Cousin J. L. The inhibitor effect of probencid and structural analogues on organic anions and chloride permeabilities in ox erythrocytes. Biochim Biophys Acta. 1976 Jan 21;419(2):309–313. doi: 10.1016/0005-2736(76)90356-4. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. Thrombin-induced release of calcium from blood platelets. Science. 1969 Oct 31;166(3905):623–623. doi: 10.1126/science.166.3905.623. [DOI] [PubMed] [Google Scholar]
- Nahas G. G., Zagury D., Milhaud A., Manger W. M., Pappas G. D. Acidemia and catecholamine output of the isolated canine adrenal gland. Am J Physiol. 1967 Nov;213(5):1186–1192. doi: 10.1152/ajplegacy.1967.213.5.1186. [DOI] [PubMed] [Google Scholar]
- Orci L., Amherdt M., Malaisse-Lagae F., Rouiller C., Renold A. E. Insulin release by emiocytosis: demonstration with freeze-etching technique. Science. 1973 Jan 5;179(4068):82–84. doi: 10.1126/science.179.4068.82. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pletscher A., Da Prada M., Berneis K. H., Steffen H., Lütold B., Weder H. G. Molecular organization of amine storage organelles of blood platelets and adrenal medulla. Adv Cytopharmacol. 1974;2:257–264. [PubMed] [Google Scholar]
- Pollard H. B., Zinder O., Hoffman P. G., Nikodejevic O. Regulation of the transmembrane potential of isolated chromaffin granules by ATP, ATP analogs, and external pH. J Biol Chem. 1976 Aug 10;251(15):4544–4550. [PubMed] [Google Scholar]
- Rossi E. C. The effect of albumin upon the loss of enzymes from washed platelets. J Lab Clin Med. 1972 Feb;79(2):240–246. [PubMed] [Google Scholar]
- Satir B., Schooley C., Satir P. Membrane fusion in a model system. Mucocyst secretion in Tetrahymena. J Cell Biol. 1973 Jan;56(1):153–176. doi: 10.1083/jcb.56.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F. H., Smith A. D., Winkler H. Secretion from the adrenal medulla: biochemical evidence for exocytosis. Br J Pharmacol Chemother. 1967 Sep;31(1):94–104. doi: 10.1111/j.1476-5381.1967.tb01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoza L., Zucker M. B., Jerushalmy Z., Grant R. Kinetic studies of platelet aggregation induced by adenosine diphosphate and its inhibition by chelating agents, guanidino compounds, and adenosine. Thromb Diath Haemorrh. 1967 Dec 31;18(3-4):713–725. [PubMed] [Google Scholar]
- Smith U., Smith D. S., Winkler H., Ryan J. W. Exocytosis in the adrenal medulla demonstrated by freeze-etching. Science. 1973 Jan 5;179(4068):79–82. doi: 10.1126/science.179.4068.79. [DOI] [PubMed] [Google Scholar]
- Villereal M. L., Levinson C. Inhibition of sulfate transport in Ehrlich ascites tumor cells by 4-acetamido-4'-isothiocyano-stilbene-2,2'-disulfonic acid(SITS). J Cell Physiol. 1976 Oct;89(2):303–311. doi: 10.1002/jcp.1040890213. [DOI] [PubMed] [Google Scholar]
- WILSON E. J., WORMALL A. Studies on suramin; further observations on the combination of the drug with proteins. Biochem J. 1949;45(2):224–231. [PubMed] [Google Scholar]
- White J. G., Rao G. H., Gerrard J. M. Effects of the lonophore A23187 on blood platelets I. Influence on aggregation and secretion. Am J Pathol. 1974 Nov;77(2):135–149. [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Inhibition of platelet energy production and release reaction by PGE1, theophylline and cAMP. Biochem Biophys Res Commun. 1970 Oct 9;41(1):128–134. doi: 10.1016/0006-291x(70)90478-x. [DOI] [PubMed] [Google Scholar]
- Wörner P., Brossmer R. Platelet aggregation and the release induced by inophores for divalent cations. Thromb Res. 1975 Apr;6(4):295–305. doi: 10.1016/0049-3848(75)90079-1. [DOI] [PubMed] [Google Scholar]


