Abstract
Objective
To investigate the cost effectiveness of ticagrelor versus clopidogrel in patients with acute coronary syndromes (ACS) in the Platelet Inhibition and Patient Outcomes (PLATO) study who were scheduled for non-invasive management.
Methods
A previously developed cost effectiveness model was used to estimate long-term costs and outcomes for patients scheduled for non-invasive management. Healthcare costs, event rates and health-related quality of life under treatment with either ticagrelor or clopidogrel over 12 months were estimated from the PLATO study. Long-term costs and health outcomes were estimated based on data from PLATO and published literature sources. To investigate the importance of different healthcare cost structures and life expectancy for the results, the analysis was carried out from the perspectives of the Swedish, UK, German and Brazilian public healthcare systems.
Results
Ticagrelor was associated with lifetime quality-adjusted life-year (QALY) gains of 0.17 in Sweden, 0.16 in the UK, 0.17 in Germany and 0.13 in Brazil compared with generic clopidogrel, with increased healthcare costs of €467, €551, €739 and €574, respectively. The cost per QALY gained with ticagrelor was €2747, €3395, €4419 and €4471 from a Swedish, UK, German and Brazilian public healthcare system perspective, respectively. Probabilistic sensitivity analyses indicated that the cost per QALY gained with ticagrelor was below conventional threshold values of cost effectiveness with a high probability.
Conclusions
Treatment of patients with ACS scheduled for 12 months’ non-invasive management with ticagrelor is associated with a cost per QALY gained below conventional threshold values of cost effectiveness compared with generic clopidogrel.
Trial registration number
NCT000391872.
Keywords: QUALITY OF CARE AND OUTCOMES
Introduction
Worldwide, 40–60% of patients with non-ST-elevation acute coronary syndromes (ACS) are medically managed without revascularisation. These patients have an increased risk of mortality, and receive antiplatelet therapy after discharge less frequently than patients who undergo revascularisation.1 2 Current guidelines recommend an early invasive approach in high-risk patients with non-ST-elevation ACS,3 4 but whether this consistently reduces mortality remains unclear.
Dual antiplatelet therapy with aspirin and a P2Y12 inhibitor is recommended for 12 months in patients with ACS not undergoing invasive therapy.3 In the Platelet Inhibition and Patient Outcomes (PLATO) trial in 18 624 patients with ACS,5 a prespecified analysis of 5216 patients who were planned at randomisation for non-invasive management demonstrated that, compared with clopidogrel, ticagrelor significantly reduced the rate of ischaemic events including the primary composite end point of myocardial infarction, stroke or death from vascular causes.6 Cardiovascular and all-cause mortality were also significantly reduced with ticagrelor.6 The incidence of total major bleeding and non-coronary artery bypass grafting-related major bleeding was numerically higher with ticagrelor than with clopidogrel,6 but these failed to reach statistical significance. These findings were consistent with the overall PLATO results.
In addition to clinical benefit, healthcare decision makers need to consider costs in order to prioritise treatments. The cost effectiveness of ticagrelor over generic clopidogrel in the overall PLATO population has been shown from an European healthcare perspective.7 The economic implications of using ticagrelor in the subgroup of patients with ACS intended for non-invasive management were not analysed in the previous study and the aim of this work was therefore to investigate the cost effectiveness of ticagrelor with generic clopidogrel in this subgroup. In order to investigate the impact of ticagrelor in a range of countries with different healthcare systems and life expectancy, the cost effectiveness analysis was conducted from the perspectives of four different public systems: Sweden, the UK, Germany and Brazil.
Methods
Overview
The PLATO trial (NCT00391872) enrolled 18 624 patients from 43 countries between October 2006 and July 2008; full details of the study design, inclusion criteria and results have already been published.5 6 Briefly, patients with ST-segment elevation ACS scheduled for primary percutaneous coronary intervention or non-ST segment elevation ACS, with onset of symptoms during the previous 24 h, were enrolled.
At randomisation, physicians initially allocated patients to intended invasive or conservative non-invasive management via an interactive voice randomisation system. These initial decisions were non-binding but were required to create statistically valid prospectively identified therapy groups; after randomisation, physicians were free to manage patients according to their clinical judgement. Patients were randomised to receive either ticagrelor 90 mg twice daily after a loading dose of 180 mg, or clopidogrel 300 mg loading dose (if required) with an additional 300 mg load allowed for patients undergoing percutaneous coronary intervention (PCI) , followed by 75 mg daily. All patients also received aspirin if tolerated; randomised treatment continued for 6–12 months. At the time of randomisation, 5216 (28%) patients were allocated initially to non-invasive management.6 Of these, 2601 and 2615 were randomised to ticagrelor and clopidogrel, respectively. To handle administrative censoring when analysing resource use, only patients eligible for 12 months’ follow-up were included in the 12-month cost analysis (1499 ticagrelor, 1516 clopidogrel).
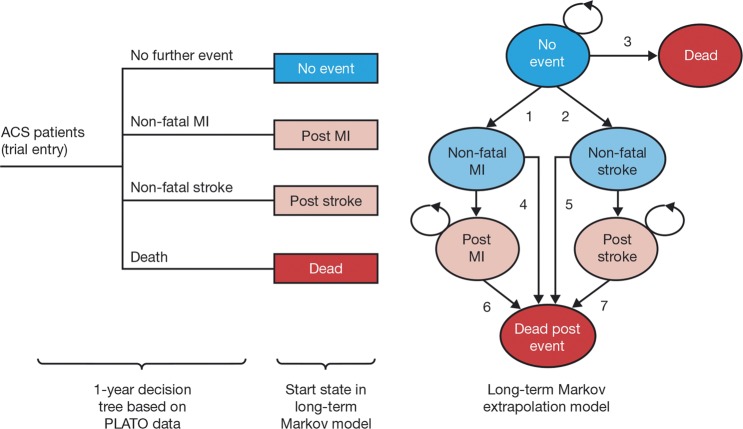
Lifetime costs and outcomes were estimated using the same two-part decision-analytical model used in the previous PLATO cost effectiveness analysis.7 Individual patient data from PLATO were used to determine rates of cardiovascular events, resource use and health-related quality of life for 12 months of therapy. Beyond the 1st year, a Markov structure was used for long-term extrapolation in order to estimate quality-adjusted survival conditional on whether a non-fatal stroke or a non-fatal myocardial infarction (MI) had occurred (figure 1). Full details of the model and its application have been published previously.7 To investigate the impact of different healthcare systems and life expectancy, the cost-effectiveness analysis was conducted from the perspectives of four different public systems: those of Sweden, the UK, Germany and Brazil. All costs were expressed in Euros (€) using 2010 prices and the average exchange rates for that year (€1=Kr9.54, €1=£0.86 and €1=R$2.33).8 9 Costs and health outcomes were discounted according to guidelines: 3%, 3.5% and 5% per annum in the Swedish, German, UK and Brazilian analyses, respectively.10 11 Results are presented as incremental cost effectiveness ratios showing the additional cost per unit of health outcome of treating patients with ticagrelor compared with clopidogrel.
Figure 1.
Decision tree and Markov model.7 Markov model transitions shown in this figure: (1) risk of non-fatal myocardial infarction (MI) for patients with no MI or stroke in the PLATO study. (2) Risk of non-fatal stroke for patients with no MI or stroke in the PLATO study. (3) Mortality risk for patients with no MI or stroke in the PLATO study. (4) Mortality risk at the 1st year after a non-fatal MI. (5) Mortality risk at the 1st year after a non-fatal stroke. (6) Mortality risk at second and subsequent years after a non-fatal MI. (7) Mortality risk at second and subsequent years after a non-fatal stroke. This model structure was developed by Nikolic et al,7 and is used with the permission of the European Heart Journal. ACS, acute coronary syndrome; PLATO, Platelet Inhibition and Patient Outcomes.
Data
The event risks for 12 months treatment with ticagrelor and clopidogrel were estimated for different clinical pathways using a parametric survival model and are reported in table 1 for the following clinical pathways: non-fatal MI occurring before a potential non-fatal stroke with no subsequent fatal event; a non-fatal stroke occurring before any potential non-fatal MI with no subsequent fatal event; death occurring at any point in the study follow-up; no further event. Following the approach in Nikolic et al,7 and in line with the clinical findings in PLATO,6 the risks for clopidogrel-treated patients were estimated using the sample of non-invasive patients whereas the overall treatment effects observed in the full PLATO sample were applied to derive the risks for ticagrelor-treated patients. In an alternative scenario the treatment effect observed in the non-invasive cohort was applied.
Table 1.
Event risks, costs and quality of life during 12 months of therapy
| Parameter | Sweden | UK | Germany | Brazil | ||||
|---|---|---|---|---|---|---|---|---|
| Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | |
| Probability non-fatal MI clinical pathway | 0.0535 | 0.0619 | 0.0535 | 0.0619 | 0.0535 | 0.0619 | 0.0535 | 0.0619 |
| Probability non-fatal stroke clinical pathway | 0.0119 | 0.0110 | 0.0119 | 0.0110 | 0.0119 | 0.0110 | 0.0119 | 0.0110 |
| Probability death clinical pathway | 0.0642 | 0.0811 | 0.0642 | 0.0811 | 0.0642 | 0.0811 | 0.0642 | 0.0811 |
| Probability no MI or stroke clinical pathway | 0.8704 | 0.8460 | 0.8704 | 0.8460 | 0.8704 | 0.8460 | 0.8704 | 0.8460 |
| Healthcare cost of non-fatal MI clinical pathway (€)* | 23 653 | 23 994 | 18 365 | 18 606 | 14 777 | 14 964 | 4401 | 4482 |
| Healthcare cost of non-fatal stroke clinical pathway (€)* | 22 925 | 23 266 | 16 731 | 16 972 | 13 193 | 13 380 | 2865 | 2946 |
| Healthcare cost of death clinical pathway (€)* | 17 227 | 17 568 | 12 267 | 12 508 | 9 921 | 10 108 | 2496 | 2577 |
| Healthcare cost of no MI or stroke clinical pathway (€)* | 10 294 | 10 635 | 8193 | 8434 | 6681 | 6856 | 1998 | 2079 |
| Daily cost of study drug (€)† | 2.21 | 0.06 | 2.27 | 0.07 | 2.91 | 0.35 | 2.34 | 0.52 |
| QALY non-fatal MI clinical pathway | 0.7667 | 0.7697 | 0.7667 | 0.7697 | 0.7667 | 0.7697 | 0.7667 | 0.7697 |
| QALY non-fatal stroke clinical pathway | 0.7388 | 0.7418 | 0.7388 | 0.7418 | 0.7388 | 0.7418 | 0.7388 | 0.7418 |
| QALY death clinical pathway | 0.2414 | 0.2445 | 0.2414 | 0.2445 | 0.2414 | 0.2445 | 0.2414 | 0.2445 |
| QALY no MI or stroke clinical pathway | 0.8422 | 0.8452 | 0.8422 | 0.8452 | 0.8422 | 0.8452 | 0.8422 | 0.8452 |
*Healthcare costs excluding drug costs, study drug costs are entered as separate parameters.
†2012 prices.
MI, myocardial infarction; QALY, quality-adjusted life-year.
Cost estimates per clinical pathway were based on resource use data collected in PLATO, as previously described.7 Brazilian, UK, German and Swedish unit costs were derived from national public databases for each country,12–15 and were multiplied with observed resource use to arrive at a total healthcare cost per patient in the study. Expert opinion was used for those German unit costs that were not feasible to derive from another source. Resource use, unit cost data and within-trial cost estimates are supplied in an online appendix to this article (see online supplementary table S1). The estimated costs per clinical pathway derived from the cost analysis and applied in the model are provided in table 1.
Daily drug costs for ticagrelor and generic clopidogrel were applied during the 12 months of therapy as long as patients were alive. This is a conservative assumption as it disfavours the ticagrelor strategy and follows Nikolic et al.7 The drug cost for each country is reported in table 1.
Quality-adjusted life-year (QALY) estimates for the clinical pathways were based on EuroQol 5 dimensions (EQ-5D)16 data collected prospectively in PLATO. The widely used UK tariff was applied to derive QALY-weights from the answers of the EQ-5D instrument.7 A 12-month QALY estimate was calculated for every patient with planned 12-month follow-up: for all patients still alive after all three measurements (index, 6 months and 12 months), the area under the curve was estimated assuming a linear relationship between all time points. The last available estimates were carried forward until the date of death for patients who died. The estimated mean per patient 12-month QALY estimates per clinical pathway are reported in table 1.
Parameters used for long-term extrapolation (beyond 12 months) are listed in table 2. Annual risks of non-fatal MI and non-fatal stroke (transitions 1 and 2 in figure 1) were estimated by extrapolating observed hazard functions from the clopidogrel arm in PLATO beyond 1 year's follow-up. Long-term survival was based on country-specific life tables with application of HRs to account for increase in risk due to further events (transitions 3–7 in figure 1), while costs and QALY weights for each health state in the Markov model were based on PLATO data and published literature (see Nikolic et al7).
Table 2.
Parameters for long-term extrapolation
| Parameter | Mean value |
|---|---|
| Annual risk of MI in the no event state | 0.019 |
| Annual risk of stroke in the no event state | 0.003 |
| Risk of death in the no event state* | 2.00 |
| Risk of death in the non-fatal MI state* | 6.00 |
| Risk of death in the post-MI state* | 3.00 |
| Risk of death in the non-fatal stroke state* | 7.43 |
| Risk of death in the poststroke state* | 3.00 |
| Cost in the non-fatal MI state (€)† | 15 656 (5836, 9558, 2971) |
| Cost in the post-MI state (€)† | 4172 (332, 3421, 792) |
| Cost in the non-fatal stroke state (€)† | 12 977 (15 262, 14 925, 1527) |
| Cost in the poststroke state (€)† | 3506 (4237, 4336, 413) |
| Cost in the no-event state (€)† | 1376 (253, 719, 243) |
| QALY weight in the non-fatal state age<69 years | 0.8737 |
| QALY weight in the non-fatal state age 70–79 years | 0.8130 |
| QALY weight in the non-fatal state age >79 years | 0.7537 |
| QALY decrement non-fatal MI state | 0.0755 |
| QALY decrement post-MI state | 0.0755 |
| QALY decrement non-fatal stroke state | 0.1034 |
| QALY decrement post-stroke state | 0.1034 |
*HR versus standard mortality.
†Values for the UK, Germany and Brazil shown in parentheses.
MI, myocardial infarction; QALY, quality-adjusted life-year.
Analysis
The base-case analysis was based on the full population of patients with ACS scheduled non-invasive management. An alternative scenario included only patients with NSTE-ACS who did not undergo any revascularisation procedure (PCI or coronary artery bypass grafting (CABG)) with or without angiography during the first 10 days.17
Uncertainty in the estimated incremental cost effectiveness ratios due to sampling uncertainty in the estimated input parameter values was evaluated by employing probabilistic sensitivity analysis in which simulation was used to propagate uncertainty in individual model inputs through the model in order to assess the uncertainty in the outcome of interest, incremental cost effectiveness. Furthermore, scenarios were also explored to investigate uncertainty related to model assumptions and data inputs not associated with sampling uncertainty.
All statistical analyses were performed using Stata V.7 (Stata Statistical Software: Release 7.0. College Station, Texas, USA: Stata Corporation). The decision-analytical model was programmed and analysed in Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA).
Results
Base-case analysis
In the base-case analysis of patients with ACS scheduled for non-invasive management, ticagrelor was associated with lifetime QALY gains of 0.17, 0.16, 0.17 and 0.13 in Sweden, the UK, Germany and Brazil, respectively, compared with generic clopidogrel (table 3). The QALY gains were primarily driven by the lower rate of mortality seen with ticagrelor treatment during the 12 months on therapy. Shorter life expectancy and a higher discount rate of future benefits in Brazil versus Sweden, the UK and Germany resulted in a smaller QALY gain for ticagrelor in the Brazilian analysis. Compared with clopidogrel, treatment with ticagrelor was associated with increased healthcare costs of €467, €545, €739 and €563 in Sweden, the UK, Germany and Brazil, respectively. The cost per QALY gained with ticagrelor was €2747, €3395, €4419 and €4471 from Swedish, UK, German and Brazilian perspectives, respectively (table 3).
Table 3.
Long-term cost-effectiveness results (€)
| Ticagrelor | Clopidogrel | Incremental | Cost per QALY | |
|---|---|---|---|---|
| Swedish healthcare perspective | ||||
| Healthcare costs | 35 910 | 35 443 | 467 | |
| QALYs | 9.10 | 8.93 | 0.17 | €2747 (SEK26 206)* |
| UK healthcare perspective | ||||
| Healthcare costs | 15 628 | 15 084 | 545 | |
| QALYs | 8.60 | 8.44 | 0.16 | €3395 (£2920)* |
| German healthcare perspective | ||||
| Healthcare costs | 24 186 | 23 448 | 739 | |
| QALYs | 8.93 | 8.76 | 0.17 | €4419 |
| Brazilian public healthcare perspective | ||||
| Healthcare costs | 5855 | 5292 | 563 | |
| QALYs | 6.77 | 6.64 | 0.13 | €4471 (BRL10417)* |
*Cost per QALY in local currency.
The results in bold are based on the probabilistic simulation which does not provide meaningful levels of significant of this ratio statistic. See for example the original publication.7
QALY, quality-adjusted life-year.
The results of the probabilistic sensitivity analyses (figures 2A, 3A, 4A and 5A) show the uncertainty around the cost effectiveness results. The majority of simulations are in the north-east quadrant for all four countries, indicating that ticagrelor is associated with a QALY gain and an incremental cost compared with generic clopidogrel.
Figure 2.
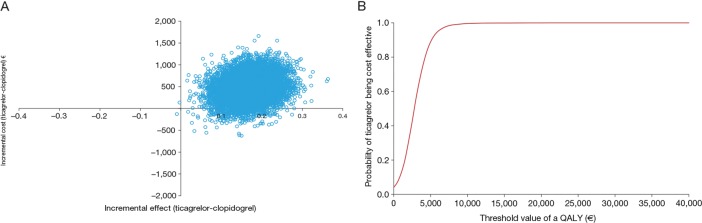
(A) Cost effectiveness plane and (B) cost effectiveness acceptability curve, from a Swedish perspective. QALY, quality-adjusted life-year.
Figure 3.
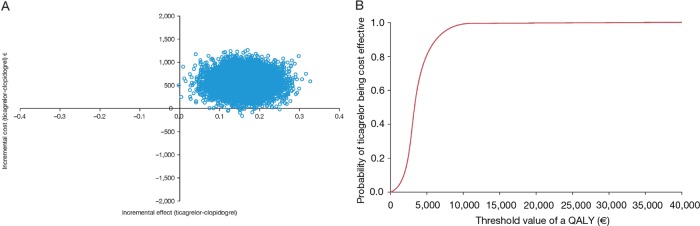
(A) Cost effectiveness plane and (B) cost effectiveness acceptability curve, from a UK perspective. QALY, quality-adjusted life-year.
Figure 4.
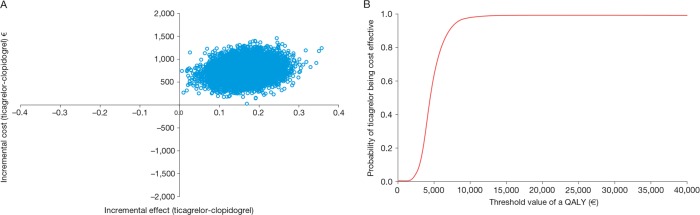
(A) Cost effectiveness plane and (B) cost effectiveness acceptability curve, from a German perspective. QALY, quality-adjusted life-year.
Figure 5.
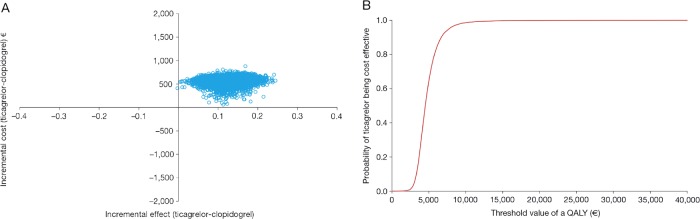
(A) Cost effectiveness plane and (B) cost effectiveness acceptability curve, from a Brazilian perspective. QALY, quality-adjusted life-year.
The cost effectiveness acceptability curves show the probability of ticagrelor being cost effective compared with generic clopidogrel at different threshold values of cost effectiveness in the Swedish (figure 2B), UK (figure 3B), German (figure 4B) and Brazilian (figure 5B) analyses. The curves indicate a high probability that the incremental cost effectiveness ratio of ticagrelor is below conventional willingness-to-pay thresholds in Sweden, the UK, Germany and Brazil.
Sensitivity analyses
The alternative scenarios showed that altering the value of input parameters not associated with sampling uncertainty (and hence not varied in the probabilistic sensitivity analysis) had minor impact on the cost effectiveness results. Applying a clopidogrel cost of €0 per day yielded a cost per QALY gained with ticagrelor of €5252 and €5917 from German and Brazilian healthcare systems, respectively. From a Swedish and UK perspective this analysis was not performed as the daily cost of clopidogrel is close to zero in the base-case analysis. In accordance with the results reported by Nikolic et al, there were small differences in the cost effectiveness results when analysing men and women separately, and at different ages. An alternative scenario based on the recently published PLATO subpopulation with non-ST-elevation ACS who had no revascularisation during the first 10 days17 shows similar results to the base-case analysis. The cost per QALY gained with ticagrelor was €2920 (incremental costs €503 and incremental QALY 0.1722), €3483 (incremental costs €566 and incremental QALY 0.1625), €4503 (incremental costs €762 and incremental QALY 0.1692) and €4489 (incremental costs €565 and incremental QALY 0.1258) from Swedish, UK, German and Brazilian healthcare system perspectives, respectively. Finally, applying the treatment effect observed in the non-invasive patients rather than the overall treatment effect from the PLATO study, yielded a cost effectiveness ratio of €3041, €3132, €3987 and €3958 for Sweden, the UK, Germany and Brazil, respectively.
Discussion
The results show that treatment with ticagrelor is associated with a cost per QALY of €2747, €3395, €4419 and €4471 from the perspectives of the Swedish, UK, German and Brazilian healthcare systems, respectively. Findings appeared robust in the alternative scenarios and the probabilistic sensitivity analyses indicate a high probability that the cost effectiveness ratio of ticagrelor is below conventional threshold values for cost effectiveness in patients with ACS scheduled for non-invasive management. Given previously reported results of cost effectiveness of ticagrelor this finding is not particularly surprising as we found small differences in clinical event rates, costs and quality of life in the cohort analysed in this study compared with previously reported subgroups.7 In particular, the difference in mortality rates between ticagrelor and clopidogrel after 12 months of therapy account for most of the gain in QALYs.
Although early invasive management is generally recommended for moderate-to-high-risk patients,3 4 many patients with NSTE-ACS continue to be managed conservatively due to unavailability of early invasive procedures in many countries. The experience of use of ticagrelor in PLATO indicates the clinical benefits of evolving medical therapy in this setting.6 In PLATO, 28% of all patients and 37% of those with non-ST-elevation ACS were intended for non-invasive management. These patients had higher long-term event rates than those scheduled for invasive management,18 although this was probably related to their increased risk profile at baseline.6 Patients scheduled for non-invasive management were older, more often women, and more often had cardiovascular risk factors such as diabetes, previous myocardial infarction, heart failure, stroke, and renal and peripheral artery disease compared with patients which were scheduled for invasive management.6 The absolute risk reduction in all-cause mortality in the group scheduled for non-invasive management was 1.7% compared with 1.3% in the overall PLATO sample.7
Interestingly, the present analysis reveals that the group scheduled for non-invasive management appears to have a relatively severe disease burden. In the clopidogrel group, the estimated 12-month mortality rate was 8.1%, substantially higher than in the overall PLATO sample (5.9%). The present analysis shows that this translates into a quality-adjusted life-expectancy of 8.93 for clopidogrel-treated patients in the non-invasive cohort compared with 9.63 in the full PLATO sample, corresponding to a difference of 0.7 years in full health. This is an interesting finding in itself and suggests that, based on disease burden, this group of patients with ACS should (at least) be given the same attention as other groups of patients with ACS. The severity of disease also implies that there may be more to gain from effective treatment as indicated by the 0.17 gain in QALYs with ticagrelor in non-invasive patients from a Swedish perspective; a comparable result for the full PLATO sample was 0.13.
A secondary aim of the present study was to investigate how differences in healthcare systems and life expectancy may influence the cost effectiveness of ticagrelor. To achieve this we used input data from Sweden, the UK, Germany and Brazil in the present study. All four countries have fully developed and universal public healthcare systems funded and administered by central and state/provincial authorities. However, there are also substantial differences between the countries as noted by WHO.19 The gross national income per capita (2012 figures) is almost three to four times larger in Sweden ($43 980), the UK ($37 340) and Germany ($42 230), compared with Brazil ($11 530) and the government expenditures on health per capita were substantially higher in Sweden ($4158), the UK ($3495) and Germany ($4617) than in Brazil ($1109) according to WHO 2012 figures. There were also large differences in life expectancies between the countries. At birth, inhabitants of Sweden, the UK and Germany can expect to live on average 9 years longer in 2011 compared with Brazil (82 years vs 73 years).20
The present analyses also support the conclusion in the overall PLATO population7 that the cost effectiveness of ticagrelor appears robust in different subgroups. Notably, although the study examined patients with planned non-invasive management, this population did in fact include patients who subsequently underwent coronary intervention. The clinical results from PLATO in patients undergoing non-invasive management remained in favour of ticagrelor regardless of whether revascularisation took place post randomisation.6 Furthermore, similar findings have recently been reported specifically in the PLATO subpopulation with non-ST-elevation ACS, with benefit of ticagrelor over clopidogrel remaining independent of actually performed revascularisation during the first 10 days.17 A sensitivity analysis of the subpopulation with non-ST-elevation ACS who had no revascularisation during the first 10 days showed similar cost effectiveness results as the base-case analysis in this study. In fact, this finding only reiterates the previous conclusions regarding the cost effectiveness of ticagrelor that the main driver is the clinical results during the 12 months on dual antiplatelet therapy.
Although many patients with non-ST-elevation ACS are managed non-invasively,2 21–23 much of the existing health-economic literature focuses on patients undergoing coronary intervention, and there is a paucity of data with which to compare and contrast the present findings. One study of interest is TRILOGY-ACS,24 which showed no superiority of prasugrel over clopidogrel in patients aged <75 years (n=7243) and patients aged ≥75 years (n=2083), with non-ST-elevation ACS treated for up to 30 months. Most of these patients were maintained on a non-invasive strategy after randomisation (only 571 aged <75 years subsequently underwent coronary intervention). The economic implications of these findings were not evaluated, however.
Limitations
The current analysis took a public healthcare perspective. However, in Brazil about 25% of the population (reaching 40% in certain regions of the country) have an additional private healthcare insurance plan.25 The reason for applying only the public healthcare perspective and not the private one was the lack of uniformity among the private unit costs. The unit costs from the public insurance system are invariably lower than in the private sector. However, since ticagrelor is associated with lower healthcare costs (excluding drug) from a public perspective (see online supplementary table S3), the analysis from a private perspective would likely improve the result.
Conclusions
Treatment of patients with ACS scheduled for 12 months’ non-invasive management with ticagrelor is associated with a cost per QALY gained below conventional threshold values of cost effectiveness compared with generic clopidogrel. This finding appears to be generalisable across different healthcare settings and countries with different life expectancies, and is primarily driven by reduced mortality with ticagrelor over clopidogrel.
Key messages.
What is known on this subject?
Dual antiplatelet therapy with aspirin and a P2Y12 inhibitor is recommended for 12 months in patients with non-ST-elevation acute coronary syndromes (ACS) not undergoing invasive management. Ticagrelor has been shown to reduce the incidence of ischaemic events relative to clopidogrel in these patients.
What might this study add?
In the subpopulation of patients with ACS who were scheduled for non-invasive management, the cost per quality-adjusted life-year gained with ticagrelor compared with generic clopidogrel was €2747, €3395, €4419 and €4471 from a Swedish, UK, German and Brazilian public healthcare system perspective, respectively. These values are below conventional thresholds for cost effectiveness.
How might this impact on clinical practice?
Twelve months treatment with ticagrelor should be considered in patients with ACS scheduled for non-invasive management as improved health outcomes can be achieved at a cost below conventional thresholds of cost effectiveness.
Supplementary Material
Acknowledgments
The authors thank Dr Tony Piha (AstraZeneca Brazil) for his contribution of Brazilian input data. The authors also thank David Evans (Gardiner-Caldwell Communications) who provided medical writing support funded by AstraZeneca.
Footnotes
Contributors: MJ: contributed to study design, recruitment, data collection, data analysis, interpretation of results, and drafting, review and final approval of the manuscript. He is also responsible for the accuracy of data and integrity of the study. SJ, CPC, RFS, JCN and LW: contributed to study design, recruitment, data collection, review and final approval of the manuscript.CM: contributed to data analysis, interpretation of results, and drafting, review and final approval of the manuscript.MH: contributed to study design, data analysis, interpretation of results, and drafting, review and final approval of the manuscript.
Funding: The PLATO study was funded by AstraZeneca.
Competing interests: MJ: Lecture fees from AstraZeneca, Pfizer; advisory board fee from AstraZeneca. SJ: receives institutional research grant from AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Terumo Inc, Medtronic and Vascular Solutions; Honoraria from The Medicines Company, AstraZeneca, Eli Lilly, Bristol-Myers Squibb, and IROKO; and consultant/advisory board fees from AstraZeneca, Eli Lilly, Merck, Medtronic and Sanofi. CPC: research grants/support from Accumetrics, AstraZeneca, CSL Behring, Essentialis, GlaxoSmithKline, Merck, Regeneron, Sanofi, Boehringer-Ingelheim and Takeda; on advisory boards for Alnylam, Bristol-Myers Squibb, Lipimedix and Pfizer (funds donated to charity); and holds equity in Automedics Medical Systems. RFS: research grants from AstraZeneca and Merck; research support from Accumetrics; honoraria from AstraZeneca, Merck, Accumetrics, and Medscape; consultancy fees from AstraZeneca, Merck, Accumetrics, Sanofi-Aventis, Regeneron, Roche, PlaqueTec, Correvio and Daiichi Sankyo. CM: Employee of AstraZeneca. JCN: is a consultant for AstraZeneca, Sanofi and Bayer; has received grants from Sanofi, GlaxoSmithKline, Bayer and Novartis; and has received lecture fees from Sanofi, Daiichi-Sankyo, AstraZeneca, Bayer and BMS. LW: research grants from AstraZeneca, Merck, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline; consultant for Abbott, Merck, Regado Biosciences, Athera Biotechnologies, Boehringer-Ingelheim, AstraZeneca, GlaxoSmithKline and Bristol-Myers Squibb/Pfizer; lecture fees from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline; honoraria from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline; travel support from AstraZeneca, Bristol-Myers Squibb/Pfizer and GlaxoSmithKline. MH: Employee of AstraZeneca.
Patient consent: Obtained.
Ethics approval: The study design was approved by the appropriate national and institutional regulatory authorities and ethics committees.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.The ACCESS Investigators. Management of acute coronary syndromes in developing countries: acute coronary events—a multinational Survey of current management Strategies. Am Heart J 2011;162:852–9. [DOI] [PubMed] [Google Scholar]
- 2.Chan MY, Mahaffey KW, Sun LJ, et al. Prevalence, predictors, and impact of conservative medical management for patients with non-ST-segment elevation acute coronary syndromes who have angiographically documented significant coronary disease. JACC Cardiovasc Interv 2008;1:369–78. [DOI] [PubMed] [Google Scholar]
- 3.Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e663–828. [DOI] [PubMed] [Google Scholar]
- 5.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- 6.James SK, Roe MT, Cannon CP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ 2011;342:d3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolic E, Janzon M, Hauch O, et al. Cost-effectiveness of treating acute coronary syndrome patients with ticagrelor for 12 months: results from the PLATO study. Eur Heart J 2013;34:220–8. [DOI] [PubMed] [Google Scholar]
- 8.European Central Bank. Statistical Data Warehouse: exchange rates. http://sdw.ecb.europa.eu (accessed Oct 2013).
- 9. Brazilian Central Bank: exchange rates. http://www4.bcb.gov.br/pec/taxas/port/ptaxnpesq.asp?id=txcotacao.
- 10.The Dental and Pharmaceutical Benefits Agency (TLV). General guidelines for economic evaluations from the Pharmaceutical Benefits Board (LFNAR 2003:2). http://www.tlv.se
- 11.International Society for Pharmacoeconomics and Outcomes Research [Internet]. Pharmacoeconomics Guidelines Around the World: Brazil. http://www.ispor.org/PEguidelines/countrydet.asp?c=32&t=1 (accessed Oct 2013).
- 12.Departamento de Informática do SUS. DATASUS por Dentro 2.0. http://www2.datasusNCT00391872.br/DATASUS/index.php (accessed Oct 2013).
- 13.Swedish unit costs. Linköping University hospital, 2010. http://www.lio.se/pages/16047/Prislista2010totalrev1.pdf (accessed Oct 2013).
- 14.National Institute for Health and Care Excellence. Ticagrelor for the treatment of acute coronary syndromes (TA236). Manufacturer's submission. http://guidance.nice.org.uk/TAG/219/Consultation/EvaluationReport/ManufacturerSubmissions
- 15. Institut für das Entgeltsystem im Krankenhaus (2008) DRG browser. http://www.g-drg.de.
- 16.Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108. [DOI] [PubMed] [Google Scholar]
- 17.Lindholm D, Varenhorst C, Cannon C, et al. Ticagrelor versus clopidogrel in patients with non-ST-elevation acute coronary syndrome: results from the PLATO trial. Eur Heart J 2014;35:2083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon CP, Harrington RA, James S, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 2010;375:283–93. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization Country Statistics. http://www.who.int/countries/en/ (accessed Jun 2014).
- 20.World Bank: average life expectancy. http://data.worldbank.org/country/ (accessed Oct 2013).
- 21.Goto K, Lansky AJ, Fahy M, et al. Predictors of outcomes in medically treated patients with acute coronary syndromes after angiographic triage: an Acute Catheterization And Urgent Intervention Triage Strategy (ACUITY) substudy. Circulation 2010;121:853–62. [DOI] [PubMed] [Google Scholar]
- 22.Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010;56:254–63. [DOI] [PubMed] [Google Scholar]
- 23.Roe MT, White JA, Kaul P, et al. Regional patterns of use of a medical management strategy for patients with non-ST-segment elevation acute coronary syndromes: insights from the EARLY ACS Trial. Circ Cardiovasc Qual Outcomes 2012;5:205–13. [DOI] [PubMed] [Google Scholar]
- 24.Roe MT, Armstrong PW, Fox KAA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–309. [DOI] [PubMed] [Google Scholar]
- 25.Nicolau JC, Corbalan R, Diaz R, et al. Cardiovascular clinical research in South America. Am Heart J 2013;165:848–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.