Abstract
Objectives
This study aimed to evaluate the persistence of nasal carriage of Staphylococcus aureus, methicillin-resistant S. aureus and multidrug-resistant S. aureus over 14 days of follow-up among industrial hog operation workers in North Carolina.
Methods
Workers anticipating at least 24 h away from work were enrolled June–August 2012. Participants self-collected a nasal swab and completed a study journal on the evening of day 1, and each morning and evening on days 2–7 and 14 of the study. S. aureus isolated from nasal swabs were assessed for antibiotic susceptibility, spa type and absence of the scn gene. Livestock association was defined by absence of scn.
Results
Twenty-two workers provided 327 samples. S. aureus carriage end points did not change with time away from work (mean 49 h; range >0–96 h). Ten workers were persistent and six were intermittent carriers of livestock-associated S. aureus. Six workers were persistent and three intermittent carriers of livestock-associated multidrug-resistant S. aureus. One worker persistently carried livestock-associated methicillin-resistant S. aureus. Six workers were non-carriers of livestock-associated S. aureus. Eighty-two per cent of livestock-associated S. aureus demonstrated resistance to tetracycline. A majority of livestock-associated S. aureus isolates (n=169) were CC398 (68%) while 31% were CC9. No CC398 and one CC9 isolate was detected among scn-positive isolates.
Conclusions
Nasal carriage of livestock-associated S. aureus, multidrug-resistant S. aureus and methicillin-resistant S. aureus can persist among industrial hog operation workers over a 14-day period, which included up to 96 h away from work.
What this paper adds.
While cross-sectional studies have examined prevalence of nasal carriage of antibiotic-resistant Staphylococcus aureus of livestock origin among industrial hog operation workers, no studies to date have assessed persistence of carriage of these bacteria among workers in the USA.
We found that nearly half (45.5%) of the 22 industrial hog operation workers who participated were persistent carriers of livestock-associated S. aureus over a 14-day period, which included up to 96 h away from work.
Persistent carriers of livestock-associated S. aureus included six persistent carriers of livestock-associated multidrug-resistant S. aureus and one persistent carrier of livestock-associated methicillin-resistant S. aureus.
Our findings in this occupational setting are of interest because persistent nasal carriage of S. aureus has previously been associated with increased risk of infection in clinical settings.
Introduction
The trend towards raising large numbers of animals in concentrated production settings has increased globally. In the USA in particular, the production of pork has intensified, with a shift towards fewer, larger operations.1 2 In addition, the US swine industry remains geographically concentrated. The highest density of swine production in the nation is in eastern North Carolina.3 4 The number of individuals working on hog production operations in North Carolina is difficult to determine, but census data from 2007 suggest that at least 6400 workers are employed at the 938 hog operations that report hired labour.4
Changes in swine production practices may affect the health of persons living near or working in production sites. Among these practices, the use of antibiotics to prevent disease and promote animal growth rather than to treat infections is extremely common,5 with the majority of antibiotics sold in the USA used non-therapeutically via supplementation of water and feed consumed by food animals.6 There is evidence that routine, non-therapeutic uses of these drugs increase the risk of development and propagation of antibiotic-resistant bacteria,7 and studies have shown that antibiotic-resistant bacteria can be transmitted to humans working in production sites8 and mobilised from these sites via multiple environmental pathways.9–11
Much of the literature regarding human exposure to antibiotic-resistant bacteria from food animal production sites has focused on Staphylococcus aureus, an opportunistic pathogen of humans and animals and an important cause of global morbidity and mortality.12 Strains of S. aureus adapted to colonise or infect livestock and poultry are referred to as livestock-associated. Livestock-associated S. aureus, including methicillin-resistant S. aureus (MRSA) and multidrug-resistant S. aureus (MDRSA), can be exchanged between humans and animals,5 13 and nasal carriage has been observed among individuals in contact with livestock and poultry throughout Europe, the USA and Canada.8 Human-to-human transmission of livestock-associated S. aureus may also occur,14 although livestock-associated strains appear to be transmitted less effectively than human-adapted strains.15 Persistent nasal colonisation with S. aureus is associated with an increased risk of infection in the clinical setting compared with intermittent or non-colonisation.16 Whether livestock-associated S. aureus can persistently colonise the noses of those occupationally exposed to livestock and poultry remains unclear.
Previous studies of persistence have not investigated methicillin-susceptible S. aureus (MSSA) or MDRSA, though carriage of these bacteria may have important implications for clinical care and public health.16 In addition, previous work has largely focused on the presence of non-specific indicators of livestock association (ie, strain type CC398, which circulates among both human and animal reservoirs),5 although genetic markers with improved sensitivity and specificity are now available.5 17–19 To date, no published work has examined the persistence of carriage of livestock-associated S. aureus, including MRSA and MDRSA, among livestock workers in the USA.
We conducted a 14-day study examining the temporal dynamics of nasal carriage of livestock-associated S. aureus, MRSA and MDRSA among 22 workers employed at industrial hog operations in North Carolina. We present information regarding (1) genetic and phenotypic characteristics of S. aureus detected; and (2) persistence of S. aureus nasal carriage before, during and after time away from work at an industrial hog operation.
Methods
Data were collected between June and August 2012 in North Carolina by researchers from the University of North Carolina at Chapel Hill (UNC) with community organisers from the Rural Empowerment Association for Community Help (REACH).
Data collection
Community organisers from REACH recruited volunteers who fit the following inclusion criteria: worked at an industrial hog operation; resided in North Carolina; were at least 18 years old; could speak and read English or Spanish; had access to a refrigerator and anticipated at least 24 h away from work during the first 7 days of the study. Participants were enrolled in four cycles. On enrolment, participants responded to a baseline questionnaire administered by a community organiser. The baseline questionnaire assessed demographic information, household member characteristics, pet ownership, work activities, medical history and risk factors for exposure to S. aureus, including MRSA. Because of concerns about privacy and confidentiality with respect to employment, no identifying information about livestock operations was collected.
On day 1 of the 14-day study, each cycle of participants attended a training session where study protocols were reviewed and participants received instruction about how to complete study activities. After receiving instruction from researchers, participants self-collected a baseline nasal swab during the training session. For the next 6 days of the study, participants self-collected a nasal swab in the morning (prior to going to work at an industrial hog operation) and in the evening, regardless of whether or not they worked at their job that day (see online supplementary figure S1). Swabs from days 1–7 were used to examine short-term changes in persistence of S. aureus nasal carriage, including after one or more days off work. Participants also self-collected a nasal swab in the morning and in the evening on day 14 of the study so that longer term persistence of S. aureus nasal carriage could be investigated. In total, up to 15 swabs were collected per participant. At the same time each swab was collected, participants recorded information about exposures, symptoms and work activities, including time away from work, in a study journal. Community organisers regularly checked in with participants during the 14-day period to answer questions and assist with data collection.
Identification and characterisation of S. aureus, MRSA and MDRSA
Baseline nasal swabs were transported to UNC at 4°C within 24 h of collection. Swabs collected between the 2nd and 14th day of the study were stored in participants’ refrigerators and picked up on day 8 and 14 of the study by a REACH community organiser. These swabs were transported to UNC at 4°C within 8 days of participant self-collection. An experiment was conducted prior to beginning this study to confirm survival of S. aureus on the swabs during an 8-day holding period (see online supplementary file). Survival of MRSA and MDRSA were not specifically investigated, although previous work has found survival of S. aureus and MRSA to be comparable.20 We presume the survival properties of MDRSA are similar to S. aureus and MRSA; however, this has not been investigated to the best of our knowledge.
Swabs were analysed for the presence of S. aureus using previously described methods21; details are available in the online supplementary file. S. aureus isolates that were positive for mecA were classified as MRSA. One isolate from each S. aureus-positive nasal swab was assessed for susceptibility to 12 classes of antibiotics. A listing of antibiotics and methods used are available in the online supplementary file. S. aureus that demonstrated complete resistance to three or more classes of antibiotics were classified as MDRSA.22 MRSA isolates meeting the definition of MDRSA were classified as multidrug-resistant MRSA.
One isolate from each S. aureus-positive nasal swab was typed based on the sequence of its staphylococcal protein A (spa) gene, a S. aureus-specific gene. spa types were then assigned to putative clonal complexes (CCs) based on the existing literature. CCs are groups of closely related strains that apparently evolved from a single founder; CC can provide insight into the epidemiology and geographic origin of a specific strain. PCR was also used to determine whether the scn gene was absent from S. aureus isolates, and whether the tet(M) gene was present among S. aureus isolates,19 as tet(M) is a proposed marker of livestock association among CC398 isolates specifically.5 Molecular analyses are described in detail in the online supplementary file.
Assessment of livestock-associated S. aureus
There are currently no established markers for livestock-associated S. aureus. Although CC398 is commonly used, this marker may not be specific or sensitive in identifying livestock-associated isolates.5 However, studies that have examined S. aureus CCs with phylogenetically distinct human and livestock clades (eg, CC398, CC5, CC97 or CC8) have detected near universal loss of scn (99–100%) among livestock-associated isolates.5 23–25 Thus, we considered absence of scn to be a proxy for livestock association among all S. aureus isolates.
Once stratified by livestock association (absence of scn), we examined the distribution of tetracycline resistance and CC among all S. aureus isolates. Tetracycline is used heavily in food animal production in the USA.26 The tet(M) gene mediating tetracycline resistance has been identified as a marker of livestock association among isolates belonging to CC398.5 Tetracycline resistance is also commonly found among S. aureus belonging to other CCs (eg, CC9) obtained from livestock.27–29
Statistical analysis
Based on nasal carriage definitions used by van Belkum et al,30 we defined persistent carriage with S. aureus, MRSA, MDRSA and scn-negative S. aureus as positivity for the outcome of interest for all, or all but one, collected nasal swab (ie, 15/15 or 14/15); intermittent carriage as positivity for 1/15 to 13/15 collected nasal swabs and non-carriage as negativity for the outcome for all collected nasal swabs. Our definition of persistent carriage allowed for one negative swab in order to minimise misclassifications resulting from laboratory or sampling error.30 Baseline swabs for three participants could not be analysed due to contamination during collection, resulting in 14 collected swabs (instead of 15). The aforementioned definitions were adjusted accordingly for these individuals.
We described the distribution of personal characteristics (age, gender, education, use of antibiotics, recent hospitalisation, participation in contact sports), household characteristics (number and age of household members, pets, location of home on a hog operation) and work exposures (time at job, average hours worked/week, number and age of hogs in contact with at work and time since last work shift) within the study population, focusing on potential risk factors related to carriage of antibiotic-resistant S. aureus. Ever-carriage of each type of S. aureus was calculated as the number of workers who carried the type at any time during the study period. We also calculated the mean daily prevalence of carriage of each S. aureus outcome by averaging the number of carriers each day. Using the aforementioned definitions, we calculated the proportions of persistent, intermittent and non-carriers of each outcome over the study period. We also examined the distribution of antibiotic resistance patterns and genetic markers of S. aureus observed among participants.
To examine associations between demographic, behavioural and work-related risk factors and carriage states (persistent, intermittent and non-carriers) we generated crude ORs using tabular methods. Crude ORs were also examined using polytomous logistic regression. We used mixed models and conditional fixed effects logistic regression models to estimate the within-person effects of time-off-work including the effects of morning versus afternoon sampling. However, the sample size was too small to produce stable results for the polytomous regression models and the mixed and fixed effects models, and therefore results are not presented.
Results
Participant characteristics
Twenty-two industrial hog operation workers participated in the study, all of whom identified as Hispanic. Most participants were between 25 and 44 years of age (73%), had completed high school (68%) and lived in households with three or more other people (86%; table 1). Twenty-three per cent (5/22) reported living on the same property as the industrial hog operation for which they worked. One worker reported using antibiotics and another reported visiting a hospital within a month of enrolment (results not shown). Most participants reported working more than 5 days a week and more than 8 h a day, resulting in a majority (59%) of participants reporting working >50 h during an average week. A majority of participants reported contact with sows, nursing or weaned pigs at work (91%), although exposure to older pigs was reported by 7/22 workers (32%).
Table 1.
Distribution of characteristics among 22 industrial hog operation workers, North Carolina
| N=22* | Per cent | |
|---|---|---|
| Personal characteristics | ||
| Age | ||
| <24 | 2 | 9.1 |
| 25–34 | 5 | 22.7 |
| 35–44 | 11 | 50.0 |
| ≥45 | 4 | 18.2 |
| Male | 12 | 54.6 |
| Education | ||
| <High school | 6 | 27.3 |
| ≥High school | 15 | 68.2 |
| Number of household members | ||
| <3 | 2 | 9.1 |
| 3–5 | 13 | 59.1 |
| ≥6 | 6 | 27.3 |
| Children <6 years old living in household | 10 | 45.5 |
| Pets inside home | 6 | 27.3 |
| Lives on same property as hog operation | 5 | 22.7 |
| Contact sports | ||
| ≥1 month ago | 10 | 45.5 |
| <1 month ago | 6 | 27.3 |
| Work characteristics† | ||
| Years employed at current hog operation | ||
| <1 | 5 | 22.7 |
| 1–5 | 7 | 31.8 |
| 6–9 | 3 | 13.6 |
| ≥10 | 4 | 18.2 |
| Average h/week | ||
| ≤40 | 3 | 13.6 |
| 41–50 | 6 | 27.3 |
| 51–60 | 11 | 50.0 |
| >60 | 2 | 9.1 |
| Life stage of hogs in contact with at work‡ | ||
| Sows/farrow piglets/wean | 20 | 90.9 |
| Feeder/finish | 7 | 31.8 |
| Average number of hogs worked with per day§ | ||
| ≤1000 | 3 | 13.6 |
| 1001–5000 | 8 | 36.4 |
| >5000 | 5 | 22.7 |
*Totals for each characteristic may not sum to the total number of participants due to missing information.
†Reported at baseline.
‡Totals do not sum to 22 because some participants had contact with pigs in multiple life stages.
§Calculated by multiplying the average number of animals per barn at operation of employment by the number of barns worked in on an average day.
Occurrence of S. aureus, MRSA and MDRSA
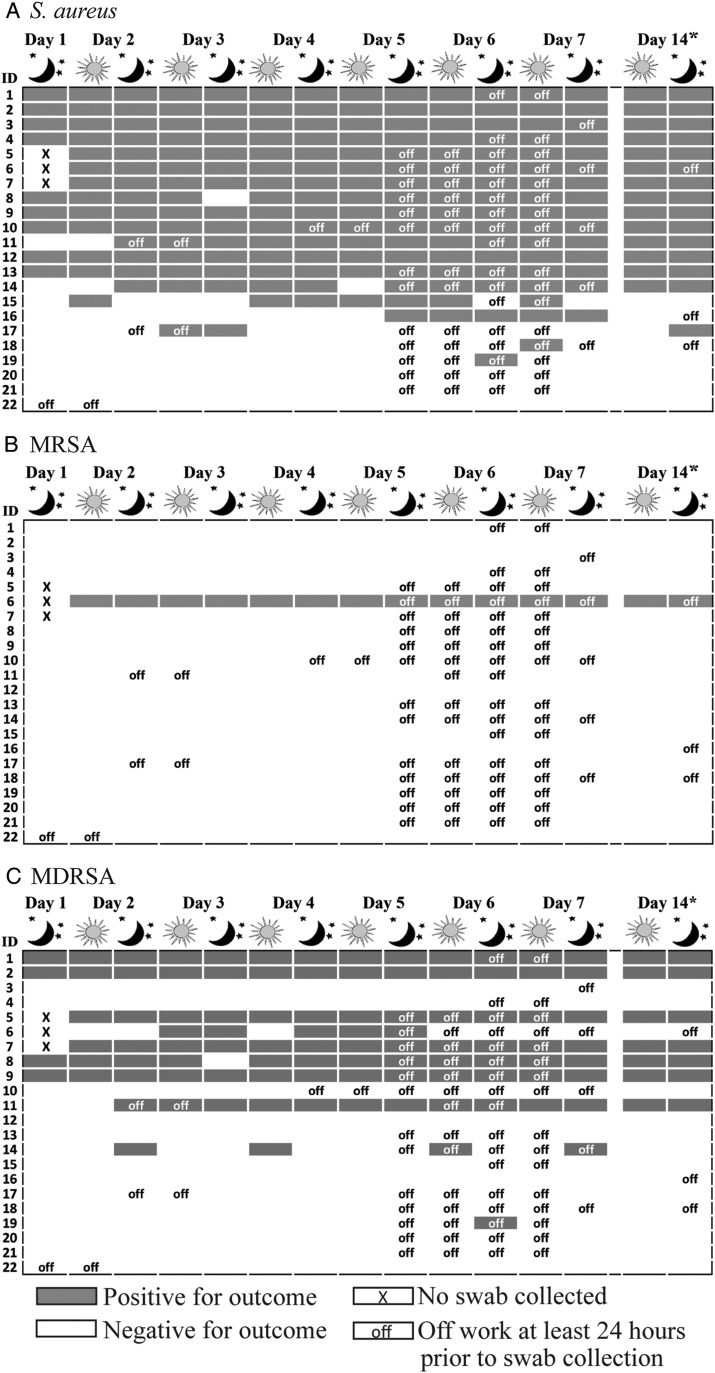
We analysed 327 nasal swabs from 22 participants; 15 nasal swabs each from 19 participants and 14 nasal swabs each from three participants. Eighty-six per cent (19/22) of workers carried S. aureus, 5% (1/22) carried MRSA and 46% (10/22) carried MDRSA during at least one sampling point of the 14-day follow-up (table 2). MRSA and MDRSA are subsets of S. aureus for this finding and all reported findings henceforth. Mean daily prevalence of S. aureus, MRSA and MDRSA was 65%, 4% and 33%, respectively. Fifty-five per cent (12/22), 5% (1/22) and 27% (6/22) of workers were persistent carriers of S. aureus, MRSA and MDRSA, respectively. The participant observed to persistently carry MRSA carried multidrug-resistant MRSA for 36% (5/14) of the sampling points. Occurrence of nasal carriage of S. aureus, MRSA and MDRSA overlaid with time away from work is presented in figure 1. Loss of carriage of S. aureus, MRSA or MDRSA did not occur in a pattern consistent with time away from work (mean 49 h; range >0–96 h).
Table 2.
Occurrence of nasal carriage, mean daily prevalence and carriage states for Staphylococcus aureus outcomes among 22 industrial hog operation workers based on 327 nasal swabs, North Carolina
| Carriage states, N (%) | |||||
|---|---|---|---|---|---|
| Outcome | Ever carriage N* (%) |
Mean daily prevalence (% (range)) | Persistent | Intermittent | Non-carrier |
| S. aureus | 19 (86.4) | 65.3 (47.4–72.7) | 12 (54.5) | 7 (31.8) | 3 (13.6) |
| scn-negative S. aureus† | 16 (72.7) | 50.6 (36.8–56.8) | 10 (45.5) | 6 (27.3) | 6 (27.3) |
| scn-positive S. aureus | 6 (27.3) | 14.7 (10.5–18.2) | 2 (9.1) | 4 (18.2) | 16 (72.7) |
| MRSA‡§ | 1 (4.5) | 4.0 (0.0–4.5) | 1 (4.5) | 0 | 21 (95.5) |
| MDRSA§ | 10 (45.5) | 32.7 (21.1–36.4) | 6 (27.3) | 4 (18.2) | 12 (54.5) |
| scn-negative MDRSA† | 9 (40.9) | 31.6 (21.1–36.4) | 6 (27.3) | 3 (13.6) | 13 (59.1) |
| scn-positive MDRSA | 1 (4.5) | 1.1 (0–2.3) | 0 | 1 (4.5) | 21 (95.5) |
*It was possible for participants to carry both scn-positive and scn-negative S. aureus and MDRSA during the study period; therefore, the sum of these subclassifications may not equal the total number of participants ever colonised by S. aureus and MDRSA.
†scn-negative isolates were considered to be livestock associated.
‡All MRSA isolates were scn-negative.
§MRSA and MDRSA are subsets of S. aureus.
MDRSA, multidrug-resistant S. aureus; MRSA, methicillin-resistant S. aureus.
Figure 1.
Occurrence of (A) Staphylococcus aureus, (B) methicillin-resistant S. aureus (MRSA) and (C) multidrug-resistant S. aureus (MDRSA) among 22 industrial hog operation workers in North Carolina over a 14-day study period. Work status in the 24 h period prior to collection of day 14 morning swabs is unknown.
Distribution of CCs and antibiotic resistance phenotypes
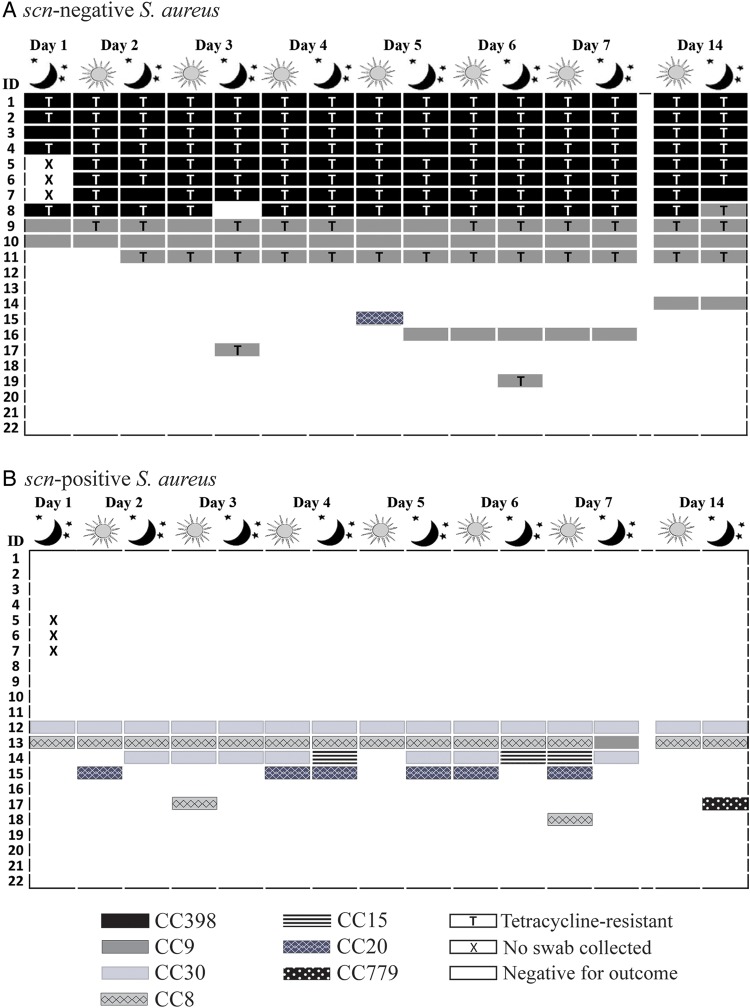
We observed a diversity of CCs among the 19 industrial hog workers who carried S. aureus at least once during the 14-day period (figure 2). CC398 was most frequently detected—carried by seven workers persistently and one intermittently, followed by CC9—carried by two workers persistently and seven intermittently. CC30, CC8, CC15, CC20 and CC779 were also observed, though less frequently. Sixty-eight per cent (15/22) of workers carried an identical CC at all S. aureus-positive sampling points. We observed a change in CC for 18% (4/22) of workers over the study period. See online supplementary file: table S3 for a listing of spa types and inferred CCs for all S. aureus isolates detected in this study.
Figure 2.
Distribution of clonal complex and tetracycline resistance among Staphylococcus aureus (S. aureus) isolated from 22 industrial hog operation workers in North Carolina over a 14-day study period, stratified by presence or absence of the scn gene (livestock-association).
We observed 12 distinct antibiotic susceptibility patterns among S. aureus-positive participants. The distribution of these patterns is depicted in online supplementary file: table S4. We observed few changes in within-participant patterns of resistance; changes in resistance patterns were typically concordant with changes in genotype.
Livestock-associated S. aureus, MRSA and MDRSA
We used absence of scn as a marker of livestock association. We detected scn-negative S. aureus at least transiently among 73% (16/22) of participants (table 2). Mean daily prevalence of scn-negative S. aureus, scn-negative MRSA and scn-negative MDRSA was 51%, 4% and 33%, respectively. During the study period, 10/22 (46%) workers were persistent and 6/22 (27%) were intermittent carriers of scn-negative S. aureus. Of the 16 workers carrying scn-negative S. aureus at least once, 6 were persistent and 3 were intermittent carriers of scn-negative MDRSA. One persistent carrier and no intermittent carriers of scn-negative MRSA were observed. Six of 22 (27%) workers were non-carriers of scn-negative S. aureus. Carriage of scn-negative S. aureus end points did not appear to change with time of sampling (morning vs evening) or time away from work (data not shown).
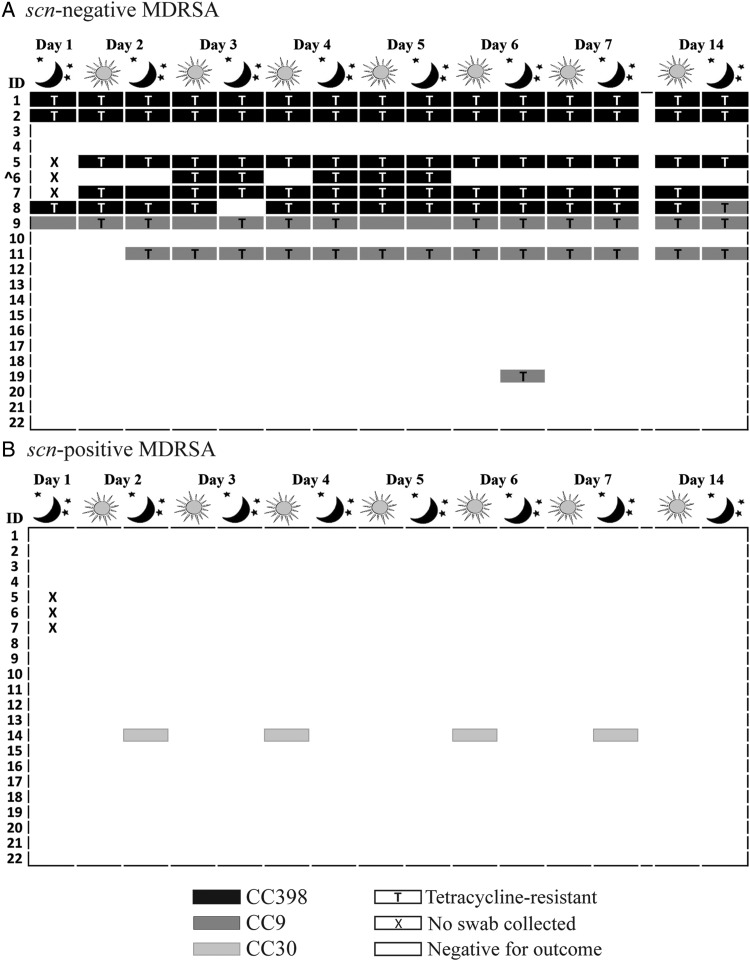
Detailed depictions of the distribution of observed CCs and tetracycline resistance by the absence of the scn gene are provided in figures 2 and 3. Only scn-negative S. aureus belonging to CC398 (n=115), CC9 (n=53) and CC20 (n=1) were observed. Among the 16 participants ever carrying scn-negative S. aureus, 15 carried either CC398 or CC9 at every S. aureus-positive sampling point. Among the six participants ever carrying scn-positive S. aureus, there was greater heterogeneity of strains, with six different CCs observed (figure 2). All MRSA (15/15) and most MDRSA isolates (106/110) were scn-negative. In addition, 82% (138/169) of scn-negative S. aureus isolates were phenotypically tetracycline-resistant, including 97% (111/115) of scn-negative CC398 isolates and 51% (27/53) of scn-negative CC9 isolates. By contrast, no scn-positive S. aureus isolates demonstrated tetracycline resistance.
Figure 3.
Distribution of clonal complex and tetracycline resistance among multidrug-resistant Staphylococcus aureus (MDRSA) isolated from 22 industrial hog operation workers in North Carolina over a 14-day study period, stratified by presence or absence of the scn gene (livestock association). ^Isolates from participant 6 were methicillin-resistant S. aureus.
Discussion
In this 14-day repeated measures study of industrial hog operation workers in North Carolina, we observed that 10/22 (45.5%) participants were persistent carriers of livestock-associated S. aureus, including six persistent carriers of livestock-associated MDRSA, and one persistent carrier of livestock-associated MRSA, which was multidrug-resistant for 5/14 of the participant's sampling points. We did not observe evidence to suggest within-person associations between time away from work and the S. aureus end points examined (including livestock-associated strains). To the best of our knowledge, this is the first study in the USA to demonstrate persistence of nasal carriage with livestock-associated S. aureus among workers employed at industrial livestock operations. The distribution of CCs and antibiotic resistance patterns observed among S. aureus, MRSA and MDRSA carried by workers may represent the population of S. aureus circulating among hogs at the industrial hog operations where participating workers are employed, as has been observed elsewhere.31 32 We did not sample animals from these operations, however, precluding confirmation of this hypothesis.
Livestock-associated MRSA was not a frequent nasal coloniser of participants in this study compared with individuals with intensive livestock exposure in other livestock-associated S. aureus persistence studies.33–35 Overall, the mean daily prevalence of S. aureus nasal carriage among participants in this study (65%) was higher than population prevalence estimates of 20–40%,16 suggesting that individuals employed at industrial hog operations are exposed to a unique reservoir of S. aureus compared with the general population, and that carriage of livestock-associated S. aureus occurs in addition to carriage of human-adapted S. aureus strains, as has been suggested elsewhere.33 Interestingly, livestock-associated S. aureus comprised the majority of S. aureus observed (mean daily prevalence: 51%). The relatively low mean daily prevalence of scn-positive S. aureus (14%) among participants in this study compared with the general population may indicate that livestock-associated S. aureus is competing with human-adapted strains to colonise the nasal passages of livestock workers in North Carolina33 or that this working population is less frequently colonised with human-adapted strains than the general population.
As this is the first study to report persistent S. aureus nasal carriage, including MRSA and MDRSA, among US livestock workers over a period of 14 days, domestic comparison is limited. One US study examined persistence of MRSA carriage among 29 veterinary students following short-term (3–4 h) exposure to pig barns; of the 22% of students who carried MRSA directly following exposure, all lost carriage within 24 h of removal from exposure.31 Similarly, recent European studies of MRSA carriage among individuals with livestock exposure found some reduction in carriage following periods of low or no exposure as short as 24 h.34 35 Graveland et al observed that 7% of 155 veal calf farmers and family members were persistent carriers and 58% intermittent carriers of MRSA over 8 weeks. The prevalence of MRSA carriage dropped by 58% during holiday periods and 24% during periods with no animals on the farm.35 A Dutch study of field workers with short-term exposure to pigs and veal calves found carriage of MRSA cleared within 24 h after contact with livestock ceased.32 These findings have led researchers to conclude that nasal carriage is an artefact of ‘contamination’ from hand-to-nose contact or bioaerosols, and that carriage is transient after short periods away from the livestock production environment.31 32 35
The results of our study do not support the ‘contamination’ hypothesis. Similar to our findings, two previous studies have observed persistence of MRSA carriage among pig farmers and veterinarians. Köck et al34 reported 59% of 35 pig farmers persistently carried MRSA spanning an average 10–12-day holiday. Verkade et al33 observed persistent MRSA CC398 carriage among 23% (32/137) and persistent MSSA carriage among 13% (18/137) of Dutch pig and veal calf veterinarians over a 2-year period. Since other studies have not reported MDRSA carriage persistence, comparisons for this outcome are not possible.
Our findings differ from previous work examining persistence of livestock-associated S. aureus among individuals exposed to industrial livestock production in multiple ways. First, we observed a relatively low prevalence of carriage of MRSA CC398 compared with previous studies of swine operation workers.31–35 Second, we observed a relatively high prevalence of CC9, a clone that has been described in livestock and livestock workers in Asia,27–29 36 but infrequently among livestock workers in the USA.21 Ninety-eight per cent of CC9 isolates were scn-negative (53/54). Our findings suggest that CC9 may circulate within a livestock reservoir in the USA; however, further research including animal sampling is needed to confirm this hypothesis. Third, while most persistent carriers were colonised with either CC398 or CC9, we observed greater heterogeneity in strain type among intermittent S. aureus carriers in this study compared with intermittent carriers observed in other studies.33–35
We used tetracycline resistance as a marker of livestock association in a previous study of carriage of S. aureus among livestock workers in North Carolina.21 We did not include tetracycline resistance as a formal marker in the present study because its capacity to distinguish human-adapted from livestock-associated S. aureus has only been described in the literature within the context of CC398.5 However, the tetracycline class of antibiotics is the most widely used in food animal production in the USA; 5.6 million kg of tetracycline were sold for domestic use in food animals in 2011.26 Consistent with knowledge about the use of tetracycline in the USA, we observed a high degree of overlap between tetracycline resistance and absence of scn (marker of livestock association used). All tetracycline-resistant isolates observed in this study (n=138) were scn-negative and most (80%) belonged to CC398, with the remaining (20%) belonging to CC9. There is emerging evidence of >95% tetracycline resistance among CC9 isolates from livestock and livestock workers in several Asian countries.27–29 36 We observed only 51% (27/53) of livestock-associated CC9 S. aureus isolates were tetracycline-resistant. Further surveillance (of feed, animals, livestock workers and humans without livestock contact) from different geographic locations may be required to determine the utility of tetracycline resistance as an indicator of livestock association beyond the scope of CC398. Continued efforts to identify and validate potential markers of livestock association will aid in improving the sensitivity and specificity of the definition of livestock association.
In the present study, as in others,33 34 we observed no change in nasal carriage status for the various S. aureus end points, including livestock-associated strains, following up to 96 h away from work. This lack of an association may indicate that time away from work is not related to loss of carriage in this population; or, is due to study limitations. Specifically, the present study was small; including 22 individuals with 14–15 sampling points each (327 observations). Small numbers of individuals experienced changes in S. aureus carriage states during the study period, resulting in a sample size insufficient for repeated measures analysis. However, even within the small sample size we were able to observe patterns of persistent, intermittent and non-carriers of S. aureus, and livestock-associated S. aureus, similar to reports from larger studies of individuals exposed to livestock,33 35 hospital inpatients and the general population.16
We also only assessed one S. aureus isolate per nasal swab for genotype, antibiotic resistance phenotype and absence of scn. Assessment of only one isolate does not reflect the contemporaneous diversity of S. aureus that may occur within participants’ noses37 and may be responsible for some of our anomalous S. aureus outcomes (eg, only one observation each of scn-negative CC20 and scn-positive CC9). In addition, the design of the study resulted in an up to 8-day holding time between self-swabbing and laboratory analysis of nasal swabs. A S. aureus survival study revealed that this holding time may have resulted in false-negative outcomes if swabs were inoculated with 102 colony forming units or fewer (see online supplementary file). We allowed for one ‘false’ negative in our definition of persistent carriers to reduce potential misclassification of carriage states based on holding time. Allowing for one false negative also accounted for potential error in self collection of nasal swabs, a method that has been previously used and validated.38
Participants independently completed journal entries reporting daily work activities which were used as proxy measures of exposure to S. aureus in the industrial hog production environment. Ideally, we would have complemented this exposure information with samples from pigs and the barn environment in order to increase the certainty that S. aureus detected among participants was present in the livestock production environment. As the presence of S. aureus, and MRSA specifically, has been known to vary between herds and operations,39 it is possible that our assumption that these proxy measures were representative of exposure to S. aureus at work was incorrect. Overall, misclassification of outcomes or exposures could have introduced bias to our results. However, no evidence exists to indicate that misclassification was differential with respect to outcome or exposure.
Differences between our findings and other studies could be related to several aspects of industrial swine production in North Carolina. Prior studies of persistence of livestock-associated S. aureus carriage included individuals with short-term exposure31 32 or extended time away from livestock or work.34 35 More than 2 days away from work was uncommon in our study population and when it occurred, it was unplanned. Workers reported that having 24 h away from work more than 1–2 times/month was rare, precluding evaluation of the effects of longer times away from work in this population. Most pigs in North Carolina are owned by vertically-integrated companies that determine the animals’ breed, feed and antibiotic administration, and they are often transported between confinements dedicated to life stages of animal growth (eg, farrowing, wean-to-feeder, feeder-to-finish), all factors that could affect animal carriage of S. aureus. Previous studies at industrial hog operations in North Carolina have been conducted at specific operations with permission from corporate producers. Our study was not conducted with corporate producers and therefore the generalisability of our findings is not restricted to the types of operations that agree to participate in research.
Further work is needed to improve the state of knowledge about the temporal dynamics of nasal carriage of livestock-associated S. aureus, including MRSA and MDRSA, among livestock workers in the USA. Our findings suggest that a study with a follow-up period longer than 2 weeks among a population of workers with greater variability in time away from work may be necessary to observe changes in nasal carriage of these end points. Examining the temporal dynamics of nasal carriage among a larger cohort is also necessary to evaluate measures of association between carriage states and personal and work-related characteristics or activities. In addition, to properly evaluate the magnitude and severity of the public health risk posed by livestock-associated S. aureus, livestock-associated MRSA and livestock-associated MDRSA, research is needed to determine whether there is an association between persistence of nasal carriage of these bacteria and the occurrence of infections among industrial hog operation workers and their contacts.
Although further work is required, our findings lay an important foundation for future efforts to examine the public health implications of livestock-associated S. aureus nasal carriage. Our findings indicate that nasal carriage of livestock-associated S. aureus, including MRSA and MDRSA, can persist among industrial hog operation workers in the USA even during periods away from industrial hog operation work. These findings support the need for future surveillance studies of carriage persistence and infection dynamics among livestock workers in the USA.
Supplementary Material
Acknowledgments
This study would not have been possible without a strong partnership between researchers and community-based organisations that have the trust of members of communities in areas where the density of industrial hog production is high. The authors would like to thank the workers who participated in this study. The authors would also like to acknowledge Norma Mejia, Paul Baker and Sherri Basnight with the Rural Empowerment Association for Community Help for assistance with data collection; Elena Popowitch from the UNC Hospital's Molecular Microbiology Laboratory and Dr Karen Carroll, Nicole Kwiatkowsi, Tsigereda Tekle and Tracy Howard from the Johns Hopkins Hospital Medical Microbiology Laboratory for assistance with microbiology procedures and sample analysis; and David Richardson with the Department of Epidemiology, University of North Carolina at Chapel Hill for guidance on data analysis and presentation of findings.
Footnotes
Contributors: All authors contributed to writing the manuscript, have read the manuscript, agree that the work is ready for submission to Occupational and Environmental Medicine and accept responsibility for the manuscript's contents.
Funding: Funding for this study was provided by the North Carolina Occupational Safety and Health and Education and Research Center, National Institute for Occupational Health and Safety (NIOSH) grant 1K01OH010193-01A1, a directed research award from the Johns Hopkins Center for a Livable Future, and NSF grant 1316318 as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases programme. MN was supported by a Royster Society fellowship and an EPA Science to Achieve Results fellowship. JLR and LH-M were supported by the National Institute of Environmental Health Sciences (NIEHS) award no. T32ES007018. NP was supported by NIEHS award no. 5T32ES007141-30. KEN and DCL were supported by a gift from the GRACE Communications Foundation. CDH was supported by NIOSH grant 1K01OH010193-01A1.
Competing interests None.
Patient consent: Obtained.
Ethics approval: UNC Public Health Nursing Institutional Review Board approved this study (IRB: 12-0712).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.MacDonald J, McBride W. The transformation of U.S. Livestock Agriculture: Scale, efficiency, and risks. In: United States Department of Agriculture, ed 2009. http://www.ers.usda.gov/publications/eib-economic-information-bulletin/eib43.aspx#.UqY1JvTudBl [Google Scholar]
- 2.Reimer JJ. Vertical integration in the pork industry. Am J Agric Econ 2006;88:234–48. [Google Scholar]
- 3.Feedstuffs. Hog Density by County. 24 May 2010. http://fdsmagissues.feedstuffs.com/fds/PastIssues/FDS8221/fds14_8221.pdf [Google Scholar]
- 4.Census of Agriculture. 2007 Census of Agriculture. Washington DC: United States Department of Agriculture (USDA), 2007. http://www.agcensus.usda.gov/ [Google Scholar]
- 5.Price LB, Stegger M, Hasman H, et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 2012;3:pii: e00305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollis A, Ahmed Z. Preserving antibiotics, rationally. N Engl J Med 2013;369:2474–6. [DOI] [PubMed] [Google Scholar]
- 7.Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 2011;24:718–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TC, Pearson N. The emergence of Staphylococcus aureus ST398. Vector Borne Zoonotic Dis 2011;11:327–39. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs SG, Green CF, Tarwater PM, et al. Isolation of antibiotic-resistant bacteria from the air plume downwind of a swine confined or concentrated animal feeding operation. Environ Health Perspect 2006;114:1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham JP, Price LB, Evans SL, et al. Antibiotic resistant enterococci and staphylococci isolated from flies collected near confined poultry feeding operations. Sci Total Environ 2009;407:2701–10. [DOI] [PubMed] [Google Scholar]
- 11.Rule AM, Evans SL, Silbergeld EK. Food animal transport: a potential source of community exposures to health hazards from industrial farming (CAFOs). J Infect Public Health 2008;1:33–9. [DOI] [PubMed] [Google Scholar]
- 12.Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998;339: 520–32. [DOI] [PubMed] [Google Scholar]
- 13.Harrison EM, Paterson GK, Holden MTG, et al. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol Med 2013;5:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rijen MM, Bosch T, Verkade EJ, et al. Livestock-associated MRSA carriage in patients without direct contact with livestock. PLoS ONE 2014;9:e100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Meurs ML, Schellekens JJ, de Neeling AJ, et al. Real-time PCR to distinguish livestock-associated (ST398) from non-livestock-associated (methicillin-resistant) Staphylococcus aureus. Infection 2013;41:339–46. [DOI] [PubMed] [Google Scholar]
- 16.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005;5:751–62. [DOI] [PubMed] [Google Scholar]
- 17.Sung JM, Lloyd DH, Lindsay JA. Staphylococcus aureus host specificity: comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology. 2008;154(Pt 7):1949–59. [DOI] [PubMed] [Google Scholar]
- 18.Verkaik NJ, Benard M, Boelens HA, et al. Immune evasion cluster-positive bacteriophages are highly prevalent among human Staphylococcus aureus strains, but they are not essential in the first stages of nasal colonization. Clin Microbiol Infect 2011;17:343–8. [DOI] [PubMed] [Google Scholar]
- 19.Stegger M, Liu CM, Larsen J, et al. Rapid differentiation between livestock-associated and livestock-independent Staphylococcus aureus CC398 clades. PLoS ONE 2013;8:e79645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis 2008;8: 101–13. [DOI] [PubMed] [Google Scholar]
- 21.Rinsky JL, Nadimpalli M, Wing S, et al. Livestock-associated methicillin and multidrug resistant Staphylococcus aureus is present among industrial, not antibiotic-free livestock operation workers in North Carolina. PLoS ONE 2013;8:e67641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- 23.Resch G, François P, Morisset D, et al. Human-to-bovine jump of Staphylococcus aureus CC8 is associated with the loss of a β-hemolysin converting prophage and the acquisition of a new staphylococcal cassette chromosome. PLoS ONE 2013;8:e58187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowder BV, Guinane CM, Ben Zakour NL, et al. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc Natl Acad Sci USA 2009;106:19545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spoor LE, McAdam PR, Weinert LA, et al. Livestock origin for a human pandemic clone of community-associated methicillin-resistant Staphylococcus aureus. mBio 2013;4:pii:e00356–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food and Drug Administration. Summary report on antimicrobials sold or distributed for use in food-producing animals. Washington DC: Department of Health and Human Services, 2011. http://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM338170.pdf [Google Scholar]
- 27.Patchanee P, Tadee P, Arjkumpa O, et al. Occurence and characterization of livestock associated-methicillin resistant Staphylococcus aureus in pig industries of northern Thailand. J Vet Sci Published Online First: 20 Jun 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen J, Imanishi M, Hinjoy S, et al. Methicillin-resistant Staphylococcus aureus ST9 in pigs in Thailand. PLoS ONE 2012;7:e31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neela V, Mohd Zafrul A, Mariana NS, et al. Prevalence of ST9 methicillin-resistant Staphylococcus aureus among pigs and pig handlers in Malaysia. J Clin Microbiol 2009;47:4138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Belkum A, Verkaik NJ, de Vogel CP, et al. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 2009;199:1820–6. [DOI] [PubMed] [Google Scholar]
- 31.Frana TS, Beahm AR, Hanson BM, et al. Isolation and characterization of methicillin-resistant Staphylococcus aureus from pork farms and visiting veterinary students. PLoS ONE 2013;8:e53738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Cleef BAGL, Graveland H, Haenen APJ, et al. Persistence of livestock-associated methicillin-resistant staphylococcus aureus in field workers after short-term occupational exposure to pigs and veal calves. J Clin Microbiol 2011;49:1030–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verkade E, van Benthem B, den Bergh MK, et al. Dynamics and determinants of Staphylococcus aureus carriage in livestock veterinarians: a prospective cohort study. Clin Infect Dis 2013;57:e11–17. [DOI] [PubMed] [Google Scholar]
- 34.Köck R, Loth B, Köksal M, et al. Persistence of nasal colonization with livestock-associated methicillin-resistant Staphylococcus aureus in pig farmers after holidays from pig exposure. Appl Environ Microbiol 2012;78:4046–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graveland H, Wagenaar JA, Bergs K, et al. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS ONE 2011;6:e16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui S, Li J, Hu C, et al. Isolation and characterization of methicillin-resistant Staphylococcus aureus from swine and workers in China. J Antimicrob Chemother 2009;64:680–3. [DOI] [PubMed] [Google Scholar]
- 37.Cespedes C, Said-Salim B, Miller M, et al. The clonality of Staphylococcus aureus nasal carriage. J Infect Dis 2005;191:444–52. [DOI] [PubMed] [Google Scholar]
- 38.Gamblin J, Jefferies JM, Harris S, et al. Nasal self-swabbing for estimating the prevalence of Staphylococcus aureus in the community. J Med Microbiol 2013; 62(Pt 3):437–40. [DOI] [PubMed] [Google Scholar]
- 39.Smith TC, Male MJ, Harper AL, et al. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in Midwestern U.S. swine and swine workers. PLoS ONE 2009;4:e4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.