Background: The PDZ scaffolding protein EBP50 promotes Akt-dependent cell proliferation through the S-phase kinase associated protein-2 Skp2.
Results: Akt phosphorylates threonine 156 of EBP50, allosterically promoting the binding of Skp2 to the first PDZ domain of EBP50.
Conclusion: The interaction with EBP50 regulates the localization and stability of Skp2 and promotes cell proliferation.
Significance: These studies define a regulatory mechanism contributing to Akt-dependent cell proliferation.
Keywords: Akt PKB, Cell Proliferation, PDZ Domain, Phosphorylation, Vascular Smooth Muscle Cells, EBP50, Skp2, nherf, nherf1
Abstract
The regulation of the cell cycle by the ubiquitin-proteasome system is dependent on the activity of E3 ligases. Skp2 (S-phase kinase associated protein-2) is the substrate recognition subunit of the E3 ligase that ubiquitylates the cell cycle inhibitors p21cip1 and p27kip1 thus promoting cell cycle progression. Increased expression of Skp2 is frequently observed in diseases characterized by excessive cell proliferation, such as cancer and neointima hyperplasia. The stability and cellular localization of Skp2 are regulated by Akt, but the molecular mechanisms underlying these effects remain only partly understood. The scaffolding protein Ezrin-Binding Phosphoprotein of 50 kDa (EBP50) contains two PDZ domains and plays a critical role in the development of neointimal hyperplasia. Here we report that EBP50 directly binds Skp2 via its first PDZ domain. Moreover, EBP50 is phosphorylated by Akt on Thr-156 within the second PDZ domain, an event that allosterically promotes binding to Skp2. The interaction with EBP50 causes cytoplasmic localization of Skp2, increases Skp2 stability and promotes proliferation of primary vascular smooth muscle cells. Collectively, these studies define a novel regulatory mechanism contributing to aberrant cell growth and highlight the importance of scaffolding function of EBP50 in Akt-dependent cell proliferation.
Introduction
The ubiquitin-proteasome system is a major regulator of the cell cycle (1, 2). One of the critical E3 ligases involved in cell cycle progression is the Skp1/Cul-1/Rbx-1/Skp2 complex. Within this complex, S-phase kinase-associated protein 2 (Skp2)2 is the substrate recognition subunit that binds the cell cycle inhibitors p21cip1 and p27kip1 and promotes their ubiquitylation and proteasomal degradation (3–6). Skp2 overexpression is observed frequently in disease states associated with aberrant cell proliferation. Among these, the pro-oncogenic action of Skp2 is the best characterized. Increased Skp2 expression is detected in various types of cancer (including prostate, breast, ovarian, and lung cancers, and lymphomas (7–11)) and correlates with poor prognosis (11, 12).
Similarly, Skp2 has profound effects during vascular remodeling. After the surgical resolution of an atherosclerotic plaque by angioplasty, local increases in growth factors and cytokines initiate cell cycle entry, often leading to restenosis. Skp2 activity is essential for the development of intimal hyperplasia (13, 14). Skp2 expression is low in healthy uninjured arteries but increases rapidly upon vascular injury and promotes vascular smooth muscle cell (VSMC) growth (14, 15). Conversely, Skp2 inhibition leads to significant reduction of neointimal lesions (16). These findings underscore the central role for Skp2 in vascular pathologies (17) but little is known on the mechanisms regulating Skp2 expression during vascular remodeling (13).
EBP50 is a PDZ-domain scaffolding protein that regulates mineral ions homeostasis and microvilli formation (18–22). We reported that EBP50 expression increases in restenotic vessels upon endoluminal injury (23, 24) and participates in intimal hyperplasia. In EBP50−/− mice we observed an 80% reduction in neointima formation compared with WT mice following wire injury (23). Unexpectedly, we found that EBP50 promotes vascular smooth muscle cells (VSMC) proliferation by regulating the stability of Skp2. Thus, Skp2 levels are reduced in EBP50−/− VSMC, resulting in increased expression of the cell cycle inhibitor p21cip1, both in primary cells and in vivo (23). These observations suggest that EBP50 is a critical regulator of Skp2 and consequently cell proliferation. Yet, the mechanisms by which this regulation occurs are not known. Here we describe a novel phosphorylation-dependent interaction between Skp2 and EBP50 that controls Skp2 localization, stability, and function.
EXPERIMENTAL PROCEDURES
Plasmids and Mutagenesis
The plasmid encoding N-terminal Flag-human EBP50 was described previously (25). The mutants S1, S2, ΔERM, S1/S2, and T156A EBP50 constructs were made from Flag-EBP50 by using the QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, CA). [L424A]Skp2 mutant construct was also made by mutagenesis from Flag-tagged Skp2 (a gift from Dr. Michele Pagano, New York University School of Medicine). All plasmid DNA sequences were confirmed by sequence analysis (GeneWiz).
Cell Culture and Transfection
Primary VSMC were isolated from murine thoracic aortic explants and cultured in Dulbecco's modified eagle media (DMEM) containing 10% fetal bovine serum (FBS) in 5% CO2 at 37 °C. All experiments were performed with cells between passages 3 and 15. Cells were transfected with siRNA for Skp2 (0.1 μm) using DharmaFECT Duo transfection reagent (Dharmacon, Thermo Scientific) and used for experiments 72 h after transfection. YFP-tagged and Flag-tagged EBP50 constructs, Flag-tagged Skp2, and constitutively active Myr-Akt (a gift from Dr. Daniel Altschuler, University of Pittsburgh School of Medicine) were introduced in primary VSMC (1 × 106) by electroporation using an AMAXA electroporator and the Basic Nucleofect kit for primary smooth muscle cells (Lonza). CHO cells were cultured in Ham's F-12 medium supplemented with 10% FBS. EBP50 constructs and Myc-Akt were transfected in CHO cells using Fugene6 (Promega).
Immunofluorescence
Cells on glass coverslips were fixed with 4% paraformaldehyde and incubated with blocking buffer containing 5% goat serum and 0.2% Nonidet P-40 (Nonidet P-40) in PBS. Primary rabbit anti-Skp2 (Santa Cruz Biotechnology, 1:1000) or anti-Flag (Santa Cruz Biotechnology, 1:500) were applied in the same buffer overnight at 4 °C. Coverslips were washed with PBS, incubated with Alexa546-conjugated anti-rabbit secondary antibody (Molecular Probes, 1:1000) and 4′,6-diamidino-2-phenylindole (DAPI, 0.1 μg/ml; Sigma) for 2 h and washed again. Coverslips were mounted for immunofluorescence microscopy and analyzed with an Olympus Fluoview confocal laser-scanning microscope with an 63× oil immersion objective. Image analysis was performed with ImageJ software (National Institutes of Health).
Western Blot Analysis
Cells were lysed in urea lysis buffer (4 m urea, 62.5 mm Tris, 2% SDS, 1 mm EDTA) containing a protease inhibitor mixture. The cell lysates were resolved by SDS-PAGE. Proteins were transferred onto nitrocellulose membranes, which were then subjected to two sequential incubations with appropriate primary antibodies (1:500 dilution for EBP50, p21cip1, and 1:1000 dilution for Skp2 (all from Santa Cruz Biotechnology); 1:1000 dilution for p27kip1, pAkt, Akt antibodies (Cell Signaling); 1:5000 dilution for βactin (Sigma)) and horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibody (1:2000, Cell Signaling). Immunoreactivity was detected by incubation with Immune-Star ECL (Bio-Rad). Quantitation of band intensity was performed with the Image J software (National Institutes of Health). For co-immunoprecipitation assay, cells were lysed in RIPA buffer (Santa Cruz Biotechnology) containing protease inhibitor mixture. Lysates were incubated with the indicated antibodies overnight and with protein A/G beads for 2 h. Immuno-bead complexes were washed twice with Nonidet P-40 buffer (1 m Tris-base, 150 mm NaCl, 5 mm EDTA, and 0.5% Nonidet P-40). Bound proteins were then released with 2× Laemmli sample buffer with 5% β-mercaptoethanol.
In-gel Overlay Assay
Cell lysates were immunoprecipitated with anti-Skp2 antibody or anti-HA (negative control). Immunoprecipitated protein were separated by SDS-PAGE and transferred to nylon membranes. Membranes were overlaid with His-tagged EBP50 protein (1 μg/ml) and incubated with anti-His-HRP-conjugated antibody (Amersham Biosciences, 1:4000). Interactions of EBP50 with Skp2 were visualized by chemiluminescence.
Peptide Synthesis
The synthesis of the 22-amino acid carboxyl-terminal peptide of Skp2a was carried out by solid phase methodology using standard Fmoc (N-(9 fluorenyl)methoxycarbonyl) chemistry (0.1 mmol scale) on an Applied Biosystems AB433 peptide synthesizer. After synthesis, the peptidyl resin was treated overnight with 4 Eq of carboxytetramethylrhodamine in the presence of HBTU/HOBt/DIEA. Following standard trifluoroacetic acid cleavage, the product was purified by HPLC on a Vydac C-18 reverse phase column and lyophilized. The final product was characterized by electron spray mass spectrometry.
Fluorescence Polarization Assay
Purified recombinant EBP50 or the isolated PDZ domains (PDZ1 and PDZ2) in PBS were added to 96-well polystyrene plates. Rhodamine-labeled Skp2 peptide was added to each well at a final concentration of 2 μm. Fluorescence polarization was measured at an excitation of 544 nm and emission at 595 nm. Recordings were performed at room temperature under equilibrium conditions.
Nuclear Magnetic Resonance
All spectra were acquired on a Bruker Avance III 700 MHz equipped with a TCI cryoprobe. Samples for the EBP50 PDZ1/Skp2 peptide interaction study contained 300 μm Skp2 carboxyl-terminal 22 amino acids peptide (NKKNQEIWGIKCRLTLQKPSCL) and 50 μm EBP50 PDZ1 (amino acids 1–140), in 25 mm sodium phosphate, 50 mm NaCl, 0.1 mm TCEP, 0.02% NaN3, 5% v/v D2O, at pH 6.8. 1H,15N HSQC experiments were used to map peptide binding through chemical shift perturbations. Thirty-two scans were collected with 1024 points in the 1H-dimension and 128 in the 15N-dimension. The comparison of 1H-15N-HSQC spectra utilized Sparky 3.115 (T. D. Goddard and D. G. Kneller, SPARKY 3, University of California, San Francisco).
Molecular Dynamics Simulations
The starting structure for molecular dynamics simulations was generated using Chimera (26). The structures of EBP50 PDZ1 (PDB ID: 1G9O) and the PDZ of PSD95 bound to the peptide KKETPV (1TP3) were overlaid. The 5 C-terminal residues of Skp2 were then superimposed to the KKETPV peptide. Structures were energy minimized for 50,000 steps (0.1 ns), followed by 5,000,000 steps (10 ns) of molecular dynamics (2 fs step size) at 310 K and 1.013 bar. Analysis of molecular dynamics data were carried out in VMD 1.9.1 and Chimera 1.6.1.
Fractionation of Nuclear or Cytoplasm Proteins
Cells were harvested by trypsinization and washed twice with ice-cold PBS followed by resuspension of the cell pellet in hypotonic buffer A (10 mm HEPES-K+ pH 7.5, 10 mm KCl, 1.5 mm MgCl2, 0.5 mm DTT) in the presence of protease inhibitor mixture. Cells were pelleted by spinning at 1,000 rpm for 5 min. The cells were lysed in ice-cold 0.5% Nonidet P-40 containing buffer A on ice for 10 min. The nuclei were isolated by centrifugation at 3,000 rpm for 2 min. The supernatant (containing the cytoplasmic protein) was collected and frozen at −80 °C. The nuclear pellets were washed with buffer A (without Nonidet P-40), followed by resuspension in buffer C (20 mm HEPES-K+ pH 7.9, 420 mm NaCl, 0.2 mm EDTA, 1.5 mm MgCl2, 0.5 mm DTT, 25% glycerol). Nuclei were incubated on ice for 30 min, and vortexed periodically. Supernatants containing nuclear protein were collected by spinning at 13,000 rpm for 10 min at 4 °C and then snap frozen for further use. Antibodies against histone H3 and tubulin were used as nuclear and cytoplasmic markers, respectively.
Phosphorylation Assays
In vitro. Recombinant active Akt was incubated with 10 μCi [32P]ATP and 1 μg recombinant EBP50. Reactions were incubated at 30 °C and phosphorylation was visualized by autoradiography. For fluorescence polarization assays, recombinant Akt and EBP50 were incubated in the absence or presence of ATP. In cells. CHO cells transfected with Flag-tagged WT or [T156A]EBP50 were stimulated with 5% FBS, lysed, and immunoprecipitated with anti-Flag antibodies. Phosphorylation was visualized by anti-AKT substrate antibody (RXRXXpS/pT).
Proliferation Assays
Cells on 24-well plates were transfected as indicated and cultured until 70–80% confluent. Cells were incubated with 1 μCi/ml [3H]thymidine in the culture media for 18 h at 37 °C, rinsed with PBS and exposed to 10% trichloroacetic acid (TCA) for 10 min. TCA was removed and the cell monolayers dissolved in 1 N NaOH for the determination of radioactivity. For BrdU incorporation experiments, cells were grown on coverslips and incubated in the presence of 100 μm BrdU for 16 h. After fixation with 4% paraformaldehyde, cells were permeabilized in 0.5% Triton X-100/PBS for 30 min and incubated with anti-BrdU antibody (1:100, Biodesign International) in blocking solution (10% promega RQ1 DNaseI, 1% BSA, 1% FBS in PBS) at 37 °C for 1 h. Flag-EBP50 was visualized with anti-Flag antibody (Sigma, 1:1000) in the same buffer. Cells were rinsed in PBS, incubated with secondary antibody anti-sheep Alexa 594 (1:1000, Molecular Probes) for BrDU or anti-rabbit Alexa 488 (1:1000, Molecular Probes) for EBP50 in PBS containing 1% BSA, 1% FBS.
RESULTS
Skp2 Binds the PDZ1 Domain of EBP50
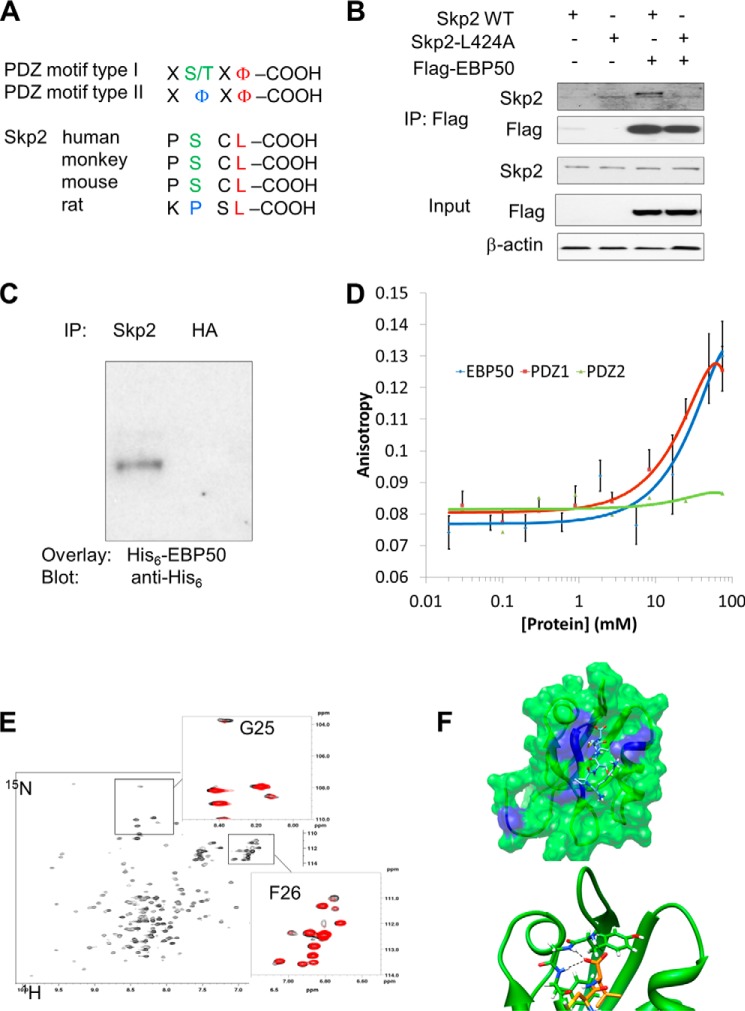
EBP50 contains two tandem PDZ domains: PDZ1 (residues 11–97) and PDZ2 (residues 149–236). PDZ domains typically recognize signature sequences at the extreme C terminus of target proteins. Class I PDZ domains interact with the sequence [S/T]-X-Φ, where X is any amino acid and Φ is a hydrophobic residue. Class II PDZ domains preferentially interact with the sequence X- Φ-X- Φ. Examination of the Skp2 sequence revealed a C-terminal class I (PSCL; in human, primates and mouse) or class II (KPSL; in rat) PDZ-binding motif (Fig. 1A). Consistent with our previous report (23), we found that Skp2 interacts with EBP50 in co-immunoprecipitation experiments (Fig. 1B). This interaction is dependent on the PDZ ligand of Skp2 because it was abrogated by mutation of the C-terminal leucine (Leu-424) to alanine (Fig. 1B). We then performed a series of additional experiments to further characterize the interaction between Skp2 and EBP50. In-gel overlay of purified His6-tagged EBP50 to immunoprecipitated Skp2 established that the interaction is direct (Fig. 1C). Fluorescence polarization experiments using a Rhodamine-labeled peptide comprising the last 22 amino acids of Skp2 confirmed the direct interaction with EBP50 (Fig. 1D). Identical binding was observed for the isolated PDZ1 domain, whereas we detected no interaction with the PDZ2 domain (Fig. 1D). We then characterized the details of the interaction between Skp2 and the first PDZ domain of EBP50 by NMR. 1H,15N correlation spectra (HSQC) of PDZ1 both free (Fig. 1E, in black) and bound to the C-terminal 22 amino acids of Skp2 (Fig. 1E, in red) were acquired. Clear perturbations of the resonances of G25 and F26 of PDZ1 (residues comprising the “core” GYGF binding motif) were observed upon binding of the C-terminal Skp2 peptide. The molecular model of the Skp2 peptide bound to PDZ1 confirms that the experimental observations are consistent with a canonical binding mode of the C-terminal region of Skp2 to first PDZ domain of EBP50 (Fig. 1F). Collectively, these experiments show that the C-terminal region of Skp2 interacts directly with the first PDZ domain of EBP50.
FIGURE 1.
Interaction between Skp2 and EBP50. A, sequence analysis of the C-terminal region of Skp2 from several species, containing common PDZ binding motif type I and II. B, interaction between Skp2 and EBP50 is dependent on the PDZ binding motif of Skp2. CHO cells were transiently transfected with Flag-EBP50, and either Skp2 or [L424A]Skp2, as indicated. Cells were lysed, and immunoprecipitation experiments were performed with anti-Flag antibody followed by SDS-PAGE and immunoblotting with anti-Flag or anti-Skp2 antibodies. C, overlay assay. Lysates of primary VSMC were immunoprecipitated with anti-Skp2 antibody or control antibody (anti-HA). The membrane was incubated with purified His-tagged EBP50 (1 mg/ml) followed by anti-His antibody. D, fluorescence polarization experiments were conducted with purified EBP50, PDZ1 or PDZ2 domains. E, 1H,15N-HSQC spectrum of EBP50 PDZ1. The expanded regions show the overlay of the spectra in the absence (black) and presence (red) of the Skp2 peptide. Residues affected by the peptide binding are labeled. F, chemical shift perturbation observed upon Skp2 peptide binding is mapped on the molecular model of the EBP50 PDZ1/Skp2 peptide complex (top panel, affected residues are in blue). The bottom panel shows the details of the interaction of the C-terminal residues of the Skp2 peptide with the GYGF binding loop of EBP50 PDZ1.
Akt Phosphorylates EBP50 and Increases the Interaction with Skp2
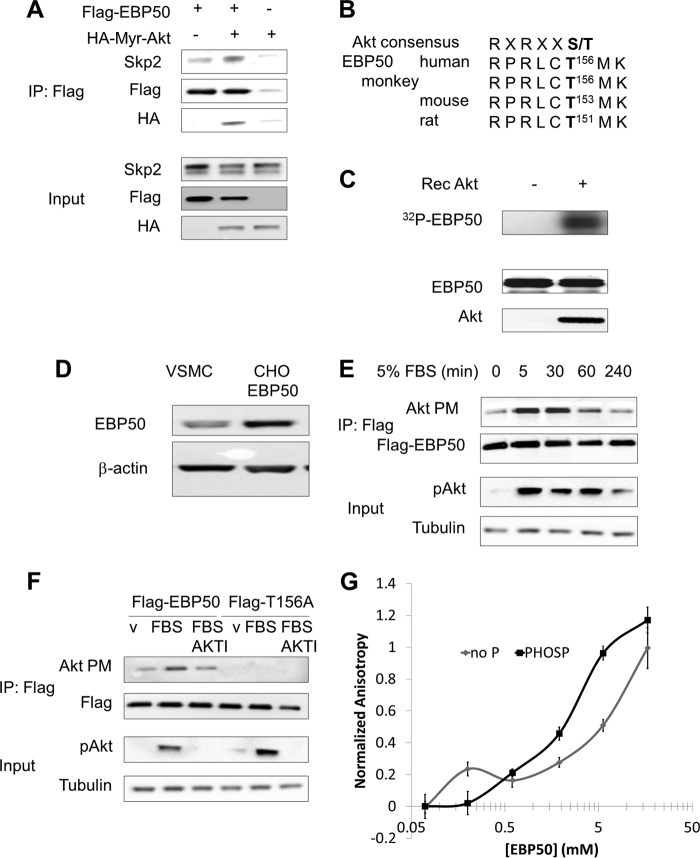
The proliferative effect of EBP50 in VSMC is Akt-dependent (23). We therefore hypothesized that Akt activity affects the interaction between EBP50 and Skp2. Co-immunoprecipitation assays showed that expression of a constitutively active myristolated form of Akt (HA-Myr-Akt) increased the interaction between EBP50 and Skp2 (Fig. 2A). Interestingly, we detected HA-Myr-Akt in the same EBP50 immunoprecipitates, suggesting that phosphorylation of EBP50 by Akt may favor the formation of the complex with Skp2. This possibility was further supported by the presence of a conserved Akt consensus motif (RXRXXT/S, where X is any amino acid) comprising threonine 156 (Thr-156) as a putative phosphorylation site (Fig. 2B). In vitro phosphorylation assays showed that recombinant Akt phosphorylates purified EBP50 (Fig. 2C). To demonstrate that Akt phosphorylates EBP50 in vivo, we used CHO cells expressing Flag-EBP50. The levels of EBP50 expressed under these conditions were approximately three times higher than primary VSMC (Fig. 2D), and were similar to those observed under inflammatory conditions (27) and in vessels (23, 24). Cells were incubated with FBS after overnight serum starvation to induce Akt activation (Fig. 2E, third panel). Following immunoprecipitation, EBP50 phosphorylation was detected with an antibody specific for phosphorylated Akt consensus motif (Akt PM) (Fig. 2E, upper panels). Furthermore, the Akt inhibitor AKTI prevented FBS-induced phosphorylation of EBP50 and mutation of Thr-156 to alanine abolished phosphorylation (Fig. 2F). Next, the effect of EBP50 phosphorylation by Akt on the interaction with Skp2 was determined by fluorescence polarization. As shown in Fig. 2G, the affinity of Skp2 for EBP50 increased 10-fold upon phosphorylation. Thus, Akt phosphorylates EBP50 on Thr-156 and enhances the interaction with Skp2.
FIGURE 2.
EBP50 is directly phosphorylated by Akt. A, interaction between Skp2 and EBP50 is increased by Akt activation. CHO cells were transfected with constitutively active HA-tagged Myr-Akt and Flag-EBP50, as indicated. Cells were lysed, and immunoprecipitation experiments were performed with anti-Flag antibody followed by SDS-PAGE and immunoblotting with anti-Flag, anti-HA, and anti-Skp2 antibodies. B, sequence analysis of the Akt consensus sequence in the second PDZ domain of EBP50. C, in vitro kinase assay for the phosphorylation of EBP50 by recombinant active Akt. Phosphorylated EBP50 was visualized by autoradiography. D, EBP50 expression in primary VSMC and in CHO cells transfected with Flag-EBP50. E, phosphorylation of EBP50 in cells. CHO cells were transfected with Flag-EBP50 and incubated with 5% FBS for the indicated time after overnight serum starvation. After immuno-precipitation with anti-Flag antibody and SDS-PAGE, membranes were blotted with an antibody for the phosphorylated Akt consensus motif (Akt PM). F, Akt phosphorylates Thr156 of EBP50. CHO cells were transfected with Flag-tagged WT or [T156A]EBP50. Cells were serum starved overnight, pretreated with 5 μm Akt inhibitor (AKTI) for 1 h (as indicated), and stimulated with FBS for 5 min. Phosphorylation of EBP50 was observed by anti-AKT substrate antibody (Akt PM). G, purified EBP50 was phosphorylated in vitro by recombinant active Akt and fluorescence polarization experiments were conducted with the Rhodamine-tagged C-terminal Skp2 peptide.
EBP50 Regulates the Cellular Localization of Skp2
The C-terminal domain of EBP50 (called EBD) interacts strongly with cytoskeletal components such as ezrin, radixin and moesin. In both primary VSMC and CHO cells EBP50 localization is primarily determined by the cytoskeleton and we found no evidence of EBP50 in the nucleus (Fig. 3, panels C and D). We therefore speculated that the interaction with EBP50 may cause a re-localization of Skp2 from the nucleus to the cytoplasm. To test this hypothesis we performed cell fractionation studies in CHO cells (that do not express EBP50 endogenously) and in primary VSMC from WT and EBP50−/− mice. In both cell types EBP50 significantly increased the fraction of Skp2 in the cytoplasm relative to that in the nucleus (Fig. 3, A and B). Complementary studies using confocal microscopy in primary EBP50−/− VSMC electroporated with mCherry-EBP50 or control mRFP and immunostained for endogenous Skp2 confirmed these observations (Fig. 3C). To determine if phosphorylation of T156 in EBP50 by Akt affects Skp2 localization we used primary VSMC from EBP50−/− mice expressing either WT or [T156A]EBP50. As shown in Fig. 3D, the fraction of cells with nuclear Skp2 was greater when [T156A]EBP50 was expressed than in EBP50-expressing cells. We then used a similar approach to determine the specific domains in EBP50 that mediate Skp2 localization. Expression of either WT or an EBP50 mutant with inactivating mutations in the core binding motif (GYGF to GAGA) of PDZ2 (S2) reduced the fraction of cells with nuclear Skp2 (Fig. 3E). In contrast, expression of a truncated form of EBP50 lacking the C-terminal EBD (ΔEBD) or of EBP50 constructs were either PDZ1 (S1) or both PDZ1 and 2 (S1S2) are mutated had no effect on Skp2 localization (Fig. 3E). Collectively, these experiments show that the interaction between EBP50 and Skp2 causes a redistribution of Skp2 from the nucleus to the cytoplasm. This effect requires both the first PDZ domain and the EBD of EBP50 and is dependent on the Akt-mediated phosphorylation of Thr-156.
FIGURE 3.
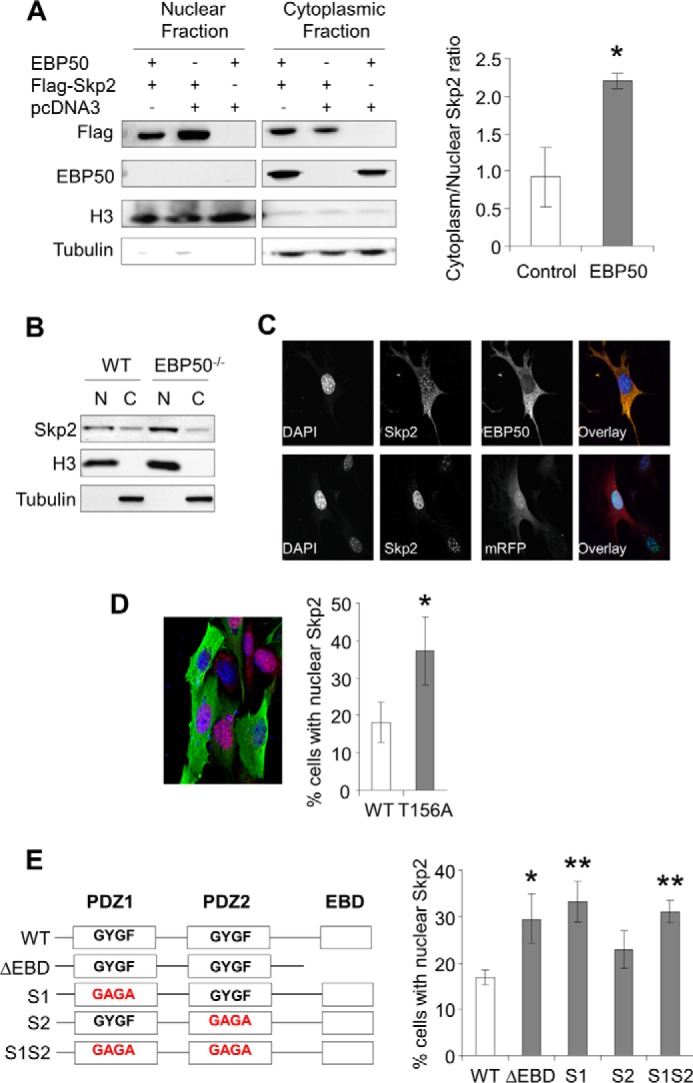
EBP50 regulates the subcellular localization of Skp2. A, nuclear fractionation. CHO cells were transfected with EBP50, and either Flag-Skp2 or vector (pcDNA3) as indicated. Nuclear and cytoplasmic fractions analyzed for Flag, EBP50, H3 (nuclear marker) and Tubulin (cytoplasmic marker) by Western blot. Graph shows the average ratio (cytoplasm/nuclear) of Skp2 in cells with pcDNA3 or EBP50 overexpression from 4–5 different experiments. *, p < 0.05. B, Skp2 levels in nuclear (N) and cytoplasmic (C) fractions of primary VSMC from WT and EBP50−/− mouse. C, primary VSMC from EBP50−/− mice were electroporated with mCherry-EBP50 or control mRFP and immunostained for endogenous Skp2 (in green) and EBP50 (in red). DAPI was used for nuclear staining (in blue). D, primary VSMC from EBP50−/− mice were electroporated with Flag-tagged WT or [T156A]EBP50 and immunostained for endogenous Skp2 (in red) and Flag (in green). Nuclei are visualized with DAPI (in blue). Graph shows the percentage (± S.E.) of transfected cells with nuclear Skp2. *, p < 0.0003, n = 3. E, left, schematic representation of EBP50 and mutant forms lacking the ezrin-binding domain (ΔEBD) and harboring inactivating mutations (GYGF to GAGA) in PDZ1 (S1), PDZ2 (S2), or both PDZ domains (S1S2). Right, primary VSMC from EBP50−/− mice were electroporated with the indicated Flag-tagged EBP50 mutants and immunostained as in D. The graph shows the percentage (± S.E.) of transfected cells with nuclear Skp2. *, p < 0.05; **, p < 0.01 n = 6.
The Interaction with EBP50 Increases Skp2 Stability and Function
Cellular Skp2 levels are controlled by the ubiquitin-proteasome system. Skp2 is actively degraded by the Cdh1/APC complex, which is principally located in the nucleus (28, 29). Our previous experiments indicate that, upon phosphorylation by Akt, EBP50 induces relocalization of Skp2 from the nucleus to the cytosol (Fig. 3). We therefore tested the hypothesis that Akt-dependent phosphorylation of EBP50 increases Skp2 stability and function. The stability of Skp2 was determined in primary EBP50−/− VSMC expressing control empty vector (pcDNA3), WT EBP50, or the phosphorylation-resistant [T156A]EBP50. We found that Skp2 was significantly more stable in cells expressing EBP50 than either empty vector or [T156A]EBP50 (Fig. 4A). The degradation of Skp2 in these cells was inhibited by MG132 (Fig. 4B), supporting that proteasomal degradation mediates the effect of EBP50 on cellular Skp2 levels. Consistent to our previous report (23), expression of EBP50 in VSMC increased proliferation (Fig. 4C). In contrast, no effect was observed for [T156A]EBP50 (Fig. 4C). Of note, the mitogenic effect of EBP50 was entirely dependent on Skp2, because it was abrogated in cells in which Skp2 expression was reduced by siRNA (Fig. 4C). These experiments indicate that Akt-phosphorylated EBP50 increases Skp2 stability and mitogenic action. To determine if the interaction with EBP50 directly affects the function of Skp2 we overexpressed WT Skp2 and the mutant [L424A]Skp2 that does not bind EBP50 (Fig. 1B). As shown in Fig. 4D, both Skp2 constructs were equally effective in inducing degradation of p21cip1 and p27kip1, two well-characterized targets of Skp2. Also, overexpression of both WT and [L424A]Skp2 increased VSMC proliferation (Fig. 4E). Thus, EBP50 is not directly required for substrate recognition or function of Skp2-containing SCF E3 ligases.
FIGURE 4.
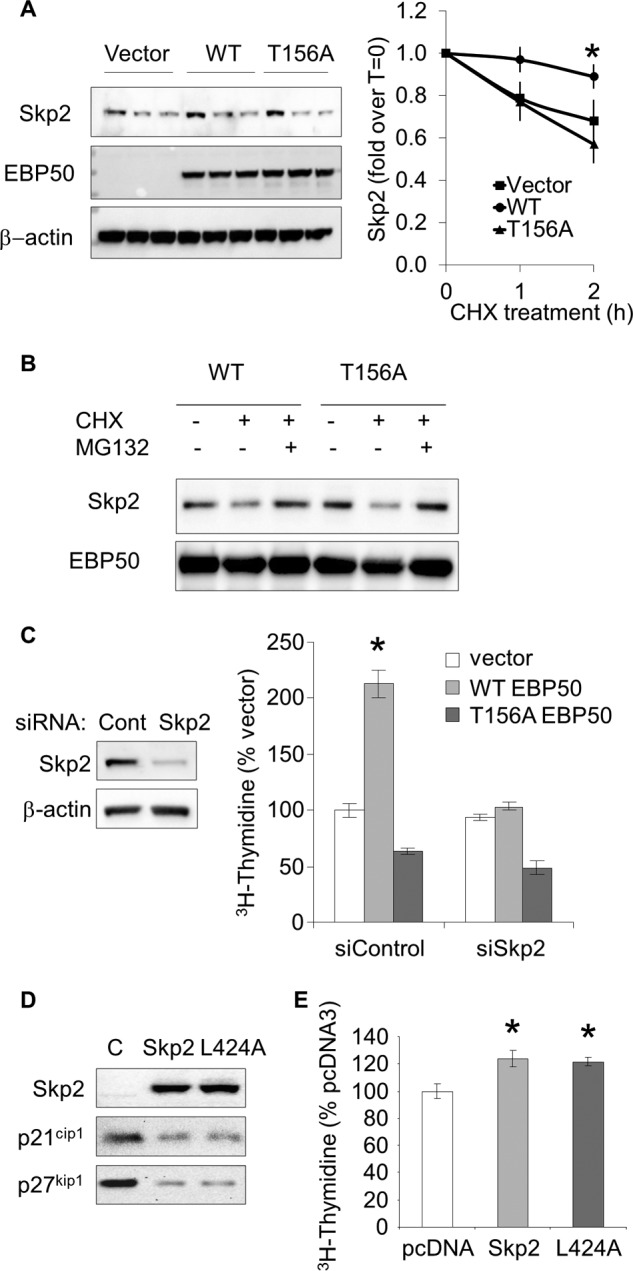
EBP50 regulates Skp2 stability and VSMC proliferation in an Akt-dependent manner. A, primary VSMC from EBP50−/− mice, were electroporated with control empty vector (pcDNA3), WT, or [T156A]EBP50 and treated with cycloheximide (10 μg/ml) for the indicated times. Equal amounts of proteins were analyzed by Western blot for Skp2 expression. Data show the mean (± S.E.) of Skp2 intensity (normalized by β-actin) relative to time 0. *, p < 0.05, n = 3. B, VSMC transfected as in A, were treated with cycloheximide (10 μg/ml) in the absence or presence of the proteasome inhibitor MG132 (2 μm) for 2 h, followed by immunoblotting for Skp2 and Flag expression. C, VSMC transfected with WT and [T156A]EBP50 were treated with control siRNA or siSkp2. Proliferation was determined by [3H]thymidine incorporation. *, p < 0.05 versus vector, n = 3. D, Skp2 overexpression decreases p21cip1 and p27kip1 expression. Primary VSMC were electroporated with WT or [L424A]Skp2 and analyzed for the expression of p21cip1 and p27kip1 by Western blot. E, proliferation of VSMC overexpressing WT or [L424A]Skp2. *, p < 0.05 versus vector, n = 3.
DISCUSSION
The mechanisms underlying Akt-dependent Skp2 stabilization are incompletely understood. Other mechanisms have been proposed to explain the Akt-dependent re-localization of Skp2 in cancer cells. Both direct phosphorylation by Akt (30, 31) and p300-mediated acetylation (32) of specific residues within the nuclear localization signal of Skp2 have been identified and proposed to regulate the nuclear-cytoplasmic trafficking as well as the ubiquitylation activity of Skp2. Yet, these mechanisms remain controversial (33–35) and do not explain the effect of EBP50 on Skp2 (23). The experiments reported here support a novel mechanism for the Akt-dependent regulation of Skp2. A schematic of the proposed model is shown in Fig. 5. Akt phosphorylates EBP50 on Thr-156, which is located in PDZ2 only a few residues removed from the core binding groove (GYGF). However, the C terminus of Skp2 interacts with PDZ1 of EBP50, and inactivating mutations in PDZ1, but not in PDZ2, prevent EBP50-dependent re-localization of Skp2. These data suggest that phosphorylation of Thr-156 exerts an allosteric effect on PDZ1, leading to increased affinity for Skp2. Consistent with this model is the observation that a human mutation in the Akt consensus motif of EBP50 (R153Q) significantly decreases the interaction with the sodium/phosphate transporter Npt2a which, like Skp2, binds exclusively to PDZ1 (25).
FIGURE 5.
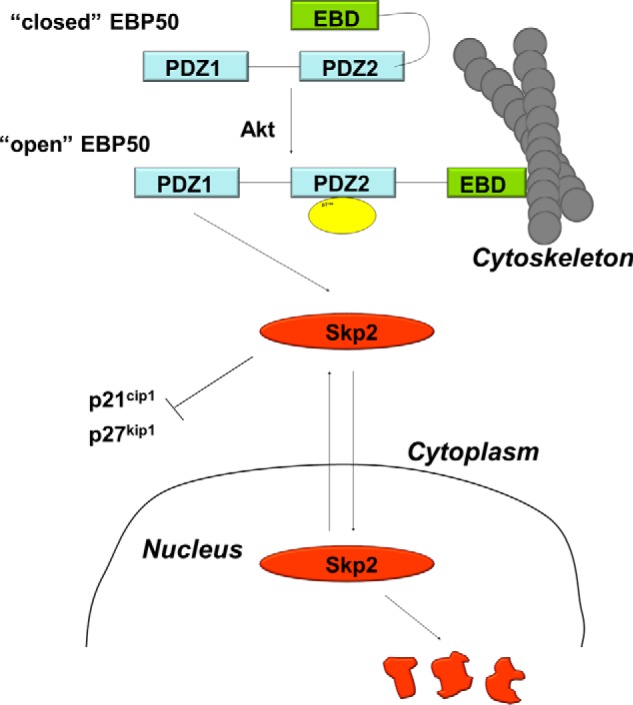
Schematic representation of the regulation of Skp2 by EBP50. Phosphorylation of Thr-156 in the PDZ2 domain of EBP50 by Akt allosterically promotes the interaction of Skp2 with PDZ1. Because EBP50 strongly interacts with cytoskeletal proteins this interaction induces cytoplasmic localization of Skp2 and increases Skp2 stability, ultimately leading to cell cycle progression.
Degradation of Skp2 by the Cdh1/APC complex occurs in the nucleus (29). We show here that the interaction with EBP50 causes cytoplasmic accumulation of Skp2 leading to its stabilization. A key element of this mechanism is the ability of EBP50 to interact with cytoskeletal components (such as ezrin, radixin, and moesin) via its C-terminal EBD. Thus, a form of EBP50 lacking EBD was unable to induce cytoplasmic localization of Skp2. Notably, in the cell systems used in this work (VSMC and CHO cells) EBP50 is localized predominantly along the cytoskeleton, and we did not observe EBP50 in the nucleus.
The increased stability of Skp2 in EBP50 expressing cells leads to increased degradation of p21cip1 and cell cycle entry (23). However, our data indicate that the Akt-dependent interaction with EBP50 has no effect on the ability of Skp2 to function within the E3 ligase complex. This is not surprising because in vitro reconstitution of the ubiquitylation activity of Skp2-containing SFC ligases requires neither Akt nor EBP50 (3). However, because EBP50 regulates the cellular localization of Skp2 it is possible that it may affect the selectivity of the substrates that undergo degradation. It has been shown that cytoplasmic Skp2 induces degradation of membrane-bound E-cadherin (32) and phosphorylated (i.e. cytoplasmic) FOXO1 (36) thus regulating cell motility, proliferation and survival. Further studies are required to determine the effect of EBP50 on substrate selection by Skp2-containing E3 ligases.
The regulation of Skp2 by EBP50 has important consequences in the responses of the vasculature to surgical interventions. The expression of Skp2 increases rapidly in balloon-injured rat carotids where it regulates VSMC proliferation (16). Interestingly, a similar increase in EBP50 expression occurs during neointima formation in both rats and mice (23, 24), suggesting that the concomitant expression of Skp2 and EBP50 contributes to the aberrant mitogenic response. Our data indicate that EBP50 acts upstream of Skp2 in regulating the mitogenic response of VSMC to injury. Interestingly, it has been recently shown that in breast cancer cells Akt undergoes Skp2-dependent K-63-linked ubiquitylation (37). This modification is non-proteolitic and leads to Akt activation and cell proliferation. It is possible that this mechanism may also be involved in the mitogenic actions of EBP50 in the vasculature, since we have found decreased Akt activation and proliferation in EBP50−/− VSMC and because VSMC proliferation is dependent on Akt (23).
In addition to the effect on cell proliferation, EBP50 promotes VSMC motility (38) and the production of pro-inflammatory mediators in macrophages and of adhesion molecules in VSMC and endothelial cells (27). Collectively, these studies support the notion that EBP50 is a critical regulator of vascular remodeling. The definition of the molecular events and structural determinants by which EBP50 regulates Skp2 provides both the rationale and specific targets for the development of EBP50 inhibitors for the treatment of inflammatory and proliferative diseases.
This work was supported in part by National Institutes of Health Grants S10 RR-025085 (to D. F. M.) and R01-DK069998 (to P. A. F.), and by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education [2013R1A1A2061607]. This work was also supported by a Pilot Project Program in Hemostasis and Vascular Biology (to A. B.) supported by grants from the Institute for Transfusion Medicine and the Hemophilia Society of Western Pennsylvania and the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005.
- Skp2
- S-phase kinase-associated protein 2
- VSMC
- vascular smooth muscle cells
- EBD
- ezrin-binding domain
- EBP50
- ezrin-binding phosphoprotein 50
- NMR
- nuclear magnetic resonance
- PDZ
- PSD-95, Discs-large, and ZO1.
REFERENCES
- 1. Hershko A. (2005) The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture). Angewandte Chemie 44, 5932–5943 [DOI] [PubMed] [Google Scholar]
- 2. Frescas D., Pagano M. (2008) Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nature Reviews. Cancer 8, 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrano A. C., Eytan E., Hershko A., Pagano M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biology 1, 193–199 [DOI] [PubMed] [Google Scholar]
- 4. Sutterlüty H., Chatelain E., Marti A., Wirbelauer C., Senften M., Müller U., Krek W. (1999) p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nature Cell Biology 1, 207–214 [DOI] [PubMed] [Google Scholar]
- 5. Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278, 25752–25757 [DOI] [PubMed] [Google Scholar]
- 6. Yu Z. K., Gervais J. L., Zhang H. (1998) Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc. Natl. Acad. Sci. U. S. A. 95, 11324–11329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Signoretti S., Di Marcotullio L., Richardson A., Ramaswamy S., Isaac B., Rue M., Monti F., Loda M., Pagano M. (2002) Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Investig. 110, 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Gstaiger M., Jordan R., Lim M., Catzavelos C., Mestan J., Slingerland J., Krek W. (2001) Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl. Acad. Sci. U. S. A. 98, 5043–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latres E., Chiarle R., Schulman B. A., Pavletich N. P., Pellicer A., Inghirami G., Pagano M. (2001) Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl. Acad. Sci. U. S. A. 98, 2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shigemasa K., Gu L., O'Brien T. J., Ohama K. (2003) Skp2 overexpression is a prognostic factor in patients with ovarian adenocarcinoma. Clinical Cancer Res. 9, 1756–1763 [PubMed] [Google Scholar]
- 11. Zhu C. Q., Blackhall F. H., Pintilie M., Iyengar P., Liu N., Ho J., Chomiak T., Lau D., Winton T., Shepherd F. A., Tsao M. S. (2004) Skp2 gene copy number aberrations are common in non-small cell lung carcinoma, and its overexpression in tumors with ras mutation is a poor prognostic marker. Clinical Cancer Res. 10, 1984–1991 [DOI] [PubMed] [Google Scholar]
- 12. Li J. Q., Wu F., Masaki T., Kubo A., Fujita J., Dixon D. A., Beauchamp R. D., Ishida T., Kuriyama S., Imaida K. (2004) Correlation of Skp2 with carcinogenesis, invasion, metastasis, and prognosis in colorectal tumors. Int. J. Oncol. 25, 87–95 [PubMed] [Google Scholar]
- 13. Bond M., Sala-Newby G. B., Wu Y. J., Newby A. C. (2006) Biphasic effect of p21Cip1 on smooth muscle cell proliferation: role of PI 3-kinase and Skp2-mediated degradation. Cardiovascular Res. 69, 198–206 [DOI] [PubMed] [Google Scholar]
- 14. Bond M., Sala-Newby G. B., Newby A. C. (2004) Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. A novel mechanism regulating smooth muscle cell proliferation. J. Biol. Chem. 279, 37304–37310 [DOI] [PubMed] [Google Scholar]
- 15. Wu Y. J., Bond M., Sala-Newby G. B., Newby A. C. (2006) Altered S-phase kinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circulation Res. 98, 1141–1150 [DOI] [PubMed] [Google Scholar]
- 16. Wu Y. J., Sala-Newby G. B., Shu K. T., Yeh H. I., Nakayama K. I., Nakayama K., Newby A. C., Bond M. (2009) S-phase kinase-associated protein-2 (Skp2) promotes vascular smooth muscle cell proliferation and neointima formation in vivo. J. Vasc. Surg. 50, 1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bond M., Wu Y. J. (2011) Proliferation unleashed: the role of Skp2 in vascular smooth muscle cell proliferation. Front. Biosci. 16, 1517–1535 [DOI] [PubMed] [Google Scholar]
- 18. Seidler U., Singh A., Chen M., Cinar A., Bachmann O., Zheng W., Wang J., Yeruva S., Riederer B. (2009) Knockout mouse models for intestinal electrolyte transporters and regulatory PDZ adaptors: new insights into cystic fibrosis, secretory diarrhoea and fructose-induced hypertension. Exp. Physiol. 94, 175–179 [DOI] [PubMed] [Google Scholar]
- 19. Weinman E. J., Cunningham R., Wade J. B., Shenolikar S. (2005) The role of NHERF-1 in the regulation of renal proximal tubule sodium-hydrogen exchanger 3 and sodium-dependent phosphate cotransporter 2a. J. Physiol. 567, 27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ardura J. A., Friedman P. A. (2011) Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol. Rev. 63, 882–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reczek D., Berryman M., Bretscher A. (1997) Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 139, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinman E. J., Hall R. A., Friedman P. A., Liu-Chen L. Y., Shenolikar S. (2006) The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu. Rev. Physiol. 68, 491–505 [DOI] [PubMed] [Google Scholar]
- 23. Song G. J., Barrick S., Leslie K. L., Bauer P. M., Alonso V., Friedman P. A., Fiaschi-Taesch N. M., Bisello A. (2012) The scaffolding protein EBP50 promotes vascular smooth muscle cell proliferation and neointima formation by regulating Skp2 and p21(cip1). Arteriosclerosis, Thromb., Vasc. Biol. 32, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song G. J., Barrick S., Leslie K. L., Sicari B., Fiaschi-Taesch N. M., Bisello A. (2010) EBP50 inhibits the anti-mitogenic action of the parathyroid hormone type 1 receptor in vascular smooth muscle cells. J. Mol. Cell. Cardiol. 49, 1012–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang B., Means C. K., Yang Y., Mamonova T., Bisello A., Altschuler D. L., Scott J. D., Friedman P. A. (2012) Ezrin-anchored protein kinase A coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport. J. Biol. Chem. 287, 24148–24163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J. Computat. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 27. Leslie K. L., Song G. J., Barrick S., Wehbi V. L., Vilardaga J. P., Bauer P. M., Bisello A. (2013) Ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) and nuclear factor-κB (NF-κB): a feed-forward loop for systemic and vascular inflammation. J. Biol. Chem. 288, 36426–36436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei W., Ayad N. G., Wan Y., Zhang G. J., Kirschner M. W., Kaelin W. G., Jr. (2004) Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428, 194–198 [DOI] [PubMed] [Google Scholar]
- 29. Zhou Y., Ching Y. P., Chun A. C., Jin D. Y. (2003) Nuclear localization of the cell cycle regulator CDH1 and its regulation by phosphorylation. J. Biol. Chem. 278, 12530–12536 [DOI] [PubMed] [Google Scholar]
- 30. Lin H. K., Wang G., Chen Z., Teruya-Feldstein J., Liu Y., Chan C. H., Yang W. L., Erdjument-Bromage H., Nakayama K. I., Nimer S., Tempst P., Pandolfi P. P. (2009) Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nature Cell Biology 11, 420–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao D., Inuzuka H., Tseng A., Chin R. Y., Toker A., Wei W. (2009) Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nature Cell Biol. 11, 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inuzuka H., Gao D., Finley L. W., Yang W., Wan L., Fukushima H., Chin Y. R., Zhai B., Shaik S., Lau A. W., Wang Z., Gygi S. P., Nakayama K., Teruya-Feldstein J., Toker A., Haigis M. C., Pandolfi P. P., Wei W. (2012) Acetylation-dependent regulation of Skp2 function. Cell 150, 179–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bashir T., Pagan J. K., Busino L., Pagano M. (2010) Phosphorylation of Ser72 is dispensable for Skp2 assembly into an active SCF ubiquitin ligase and its subcellular localization. Cell Cycle 9, 971–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boutonnet C., Tanguay P. L., Julien C., Rodier G., Coulombe P., Meloche S. (2010) Phosphorylation of Ser72 does not regulate the ubiquitin ligase activity and subcellular localization of Skp2. Cell Cycle 9, 975–979 [DOI] [PubMed] [Google Scholar]
- 35. Wang H., Cui J., Bauzon F., Zhu L. (2010) A comparison between Skp2 and FOXO1 for their cytoplasmic localization by Akt1. Cell Cycle 9, 1021–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang H., Regan K. M., Wang F., Wang D., Smith D. I., van Deursen J. M., Tindall D. J. (2005) Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl. Acad. Sci. U.S.A. 102, 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan C. H., Li C. F., Yang W. L., Gao Y., Lee S. W., Feng Z., Huang H. Y., Tsai K. K., Flores L. G., Shao Y., Hazle J. D., Yu D., Wei W., Sarbassov D., Hung M. C., Nakayama K. I., Lin H. K. (2012) The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell 149, 1098–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song G. J., Leslie K. L., Barrick S., Bougoin S., Taboas J. M., Bisello A. (2012) EBP50 promotes focal adhesion turnover and vascular smooth muscle cells migration. J. Mol. Cell. Cardiol. 53, 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]