Background: MicroRNA (miR)-146a and miR-146b (miR-146a/b) expression is induced upon human monocyte differentiation into dendritic cells (DCs).
Results: miR-146a/b negatively regulates DC apoptosis and cytokine production.
Conclusion: The miR-146a/b-TRAF6/IRAK1-NF-κB axis is responsible for DC apoptosis.
Significance: Our data reveal a novel negative feedback regulation in DCs and may have implications in the pathogenesis and treatment of autoimmune diseases.
Keywords: Apoptosis, Cytokine, Dendritic Cell, Differentiation, MicroRNA (miRNA), NF-kappa B (NF-κB), TNF Receptor-associated Factor (TRAF), IL-1 Receptor-associated Kinases (IRAK), miR-146
Abstract
We have previously reported 27 differentially expressed microRNAs (miRNAs) during human monocyte differentiation into immature dendritic cells (imDCs) and mature DCs (mDCs). However, their roles in DC differentiation and function remain largely elusive. Here, we report that microRNA (miR)-146a and miR-146b modulate DC apoptosis and cytokine production. Expression of miR-146a and miR-146b was significantly increased upon monocyte differentiation into imDCs and mDCs. Silencing of miR-146a and/or miR-146b in imDCs and mDCs significantly prevented DC apoptosis, whereas overexpressing miR-146a and/or miR-146b increased DC apoptosis. miR-146a and miR-146b expression in imDCs and mDCs was inversely correlated with TRAF6 and IRAK1 expression. Furthermore, siRNA silencing of TRAF6 and/or IRAK1 in imDCs and mDCs enhanced DC apoptosis. By contrast, lentivirus overexpression of TRAF6 and/or IRAK1 promoted DC survival. Moreover, silencing of miR-146a and miR-146b expression had little effect on DC maturation but enhanced IL-12p70, IL-6, and TNF-α production as well as IFN-γ production by IL-12p70-mediated activation of natural killer cells, whereas miR-146a and miR-146b overexpression in mDCs reduced cytokine production. Silencing of miR-146a and miR-146b in DCs also down-regulated NF-κB inhibitor IκBα and increased Bcl-2 expression. Our results identify a new negative feedback mechanism involving the miR-146a/b-TRAF6/IRAK1-NF-κB axis in promoting DC apoptosis.
Introduction
Dendritic cells (DCs)2 are specialized antigen-presenting cells derived from hematopoietic progenitor cells, influencing both innate and adaptive immune responses as well as maintaining self-tolerance (1–3). Hematopoietic progenitor cell-derived monocytes have a unique genetic signature and can develop into immature (imDCs) and mature (mDCs) DCs (4, 5). mDCs have a high surface expression of co-stimulatory molecules and produce proinflammatory cytokines. mDCs also have the unique ability to present antigens to naïve T cells (1–3). Due to their unique role in linking the innate and adaptive immunity, numerous studies on DC development, phenotypes, and functional manipulation have been performed (2). However, the underlying molecular mechanisms involved in the regulation of DC development and function are not fully elucidated to date.
MicroRNAs (miRNAs) are a family of 19–24-nucleotide noncoding RNAs that post-transcriptionally modulate gene expression and have important biologic functions (6). They regulate gene expression through targeting the 3′-UTR of mRNA, resulting in either translational repression or mRNA degradation, or both (7). At present, >100 miRNAs that are selectively expressed in cells of the innate and adaptive immune systems have been identified (8), and the miRNA expression profile of bone marrow- or monocyte-derived DCs has been further examined at different stages of DC differentiation (9–11).
miR-146 was identified as one of the miRNAs induced in response to cytokines and/or pathogen products such as LPS in macrophages and DCs (12–14), and it is transcriptionally induced by NF-κB in response to activation of innate immune signaling in monocytes (12). NF-κB has been reported to play a critical role in the regulation of DC maturation and immune functions (15, 16). NF-κB also mediates protection of DCs from apoptosis (17). Interestingly, miR-146a is known to silence TRAF6 (TNF receptor-associated factor 6) and IRAK1/2 (IL-1 receptor-associated kinase 1/2) (12, 13, 18, 19), key adaptor proteins of the NF-κB signaling cascade, resulting in inhibition of NF-κB activation (18, 20).
miR-146 has been proposed to be an anti-inflammatory miRNA in DCs (12, 13, 21). However, further study is needed to dissect other mechanisms of miR-146 in DCs. Using miRNA microarrays, we previously demonstrated that expression of miR-146a and miR-146b is up-regulated upon human monocyte differentiation into imDCs and mDCs (10). Therefore, we aimed to identify additional functional roles of miR-146a and miR-146b during human monocyte differentiation into imDCs and mDCs. Here, we provide evidence demonstrating that miR-146a and miR-146b promote DC apoptosis and inhibit IL-12p70, IL-6, and TNF-α production by targeting TRAF6 and IRAK1, with subsequent inhibition of the NF-κB signaling cascade.
EXPERIMENTAL PROCEDURES
DC Differentiation and Maturation
Human leukocytes were purchased from the New York Blood Center. Isolation of monocytes and in vitro human monocyte differentiation into DCs were performed as described previously (10, 22). Purified monocytes (>95% purity) using anti-CD14 MicroBeads (Miltenyi Biotec) were cultured at 37 °C in 6-well plates (1 × 106 cells/well) in 3 ml of serum-free AIM V medium (Invitrogen) containing human GM-CSF (100 ng/ml; Bayer HealthCare Pharmaceuticals) and human IL-4 (20 ng/ml; PeproTech). A total of 1 ml of fresh medium with GM-CSF and IL-4 was added to the cell cultures at day 3. Old medium was replaced with 3 ml of fresh medium with GM-CSF and IL-4 at day 5. DCs were matured with IL-1β (10 ng/ml; PeproTech), IL-6 (10 ng/ml; PeproTech), TNF-α (10 ng/ml; PeproTech), and prostaglandin E2 (PGE2; 1 μg/ml; Sigma-Aldrich) on day 6, and DCs were harvested on day 8 or at the indicated time.
Real-time PCR Analysis
Cells were washed twice with ice-cold phosphate-buffered saline and lysed with QIAzol reagent to isolate total RNA, from which miRNA was isolated using an miRNeasy kit (Qiagen). Single-stranded cDNA was synthesized using an NCode miRNA first-strand cDNA synthesis kit (Invitrogen). miRNA-specific primers were purchased from Qiagen, and primer sequences for 5 S RNA and comparative real-time PCR analysis have been described previously (10). Comparative real-time PCR using SYBR Green SuperMix (Invitrogen) was performed in a 96-well plate and run in a CFX96 real-time PCR system (Bio-Rad) at 50 °C for 2 min and at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 57 °C for 1 min. Each sample was analyzed in duplicate or triplicate. The level of miRNA expression was measured using the threshold cycle (CT) according to the ΔΔCT method (23). To normalize the relative abundance of miRNAs, 5 S RNA was used as an endogenous control. Standard curves for mature miR-146a and miR-146b were prepared by quantitative RT-PCR analysis using qSTAR miR-146a and miR-146b template standard kits (OriGene Technologies, Rockville, MD). Ten ng of RNA was subjected to quantitative RT-PCR, and absolute miRNA copy numbers were calculated using the miRNA standard curve (24).
Flow Cytometry
Cells were harvested at the indicated time points, washed, and labeled with allophycocyanin-annexin V or FITC-annexin V (eBioscience) and propidium iodide (Invitrogen) or 7-aminoactinomycin D (eBioscience). Flow cytometric analysis was carried out on a MACSQuant analyzer (Miltenyi Biotec) and analyzed using FlowJo software (Tree Star).
Caspase-3/7 Assay
Cells were transfected with the indicated miRNA mimics or inhibitors in a 96-well format for 48 h and then processed using a caspase-3/7 kit (Promega) according to the manufacturer's protocol.
Transfection of miRNA Mimics or Inhibitors and siRNA
miRNA mimics and inhibitors for hsa-miR-146a and hsa-miR-146b and negative controls of miRNA mimics (negative control 1) and inhibitors were purchased from Ambion. TRAF6, IRAK1, and scrambled control siRNAs were purchased from Santa Cruz Biotechnology. miRNA mimics/inhibitors and respective controls, as well as TRAF6, IRAK1, or scrambled control siRNA, were transfected into the cells at the indicated concentrations for the indicated times using Lipofectamine RNAiMAX (Invitrogen).
Western Blotting
Western blotting has been described previously (10). Anti-TRAF6, anti-IRAK1, anti-GAPDH, and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology. Anti-IκB and anti-Bcl-2 antibodies were purchased from Cell Signaling. Blots were quantified by densitometry using NIH ImageJ software (rsb.info.nih.gov/ij/) and normalized to GAPDH.
Lentiviral Vector and DC Transduction
Following New York Medical College Institutional Biosafety Committee approval, full-length human cDNA clones of TRAF6 (accession no. NM_004620) and IRAK1 (accession no. BC054000) were purchased from Open Biosystems and OriGene Technologies, respectively. The ORFs of TRAF6 and IRAK1 were cloned into an HIV-1-based bidirectional vector (pEhfflucmCNsin) to replace humanized firefly luciferase for regulation of TRAF6 or IRAK1 and truncated nerve growth factor receptor (ΔNGFR) expression (pETRAF6mCNsin and pEIRAK1mCNsin), respectively (see Fig. 4E) (25). The lentiviral constructs were verified by sequencing analysis, and expression of TRAF6 and IRAK1 was confirmed in 293T cells (American Type Culture Collection) by Western blotting. Lentiviral production in 293T cells was performed as described previously (26). Viral supernatants of 293T cells were concentrated by ultracentrifugation and the viral titer was determined, which ranged from 2.1 to 9.6 × 107 transducing units/ml. imDCs at days 4 and 6 were transduced by spinoculation for 1 h with lentiviruses for TRAF6/NGFR, IRAK1/NGFR, TRAF6/NGFR + IRAK1/NGFR, and firefly luciferase/NGFR (as a control) at multiplicities of infection of 20–30 in 24-well plates coated with RetroNectin. The transduced day 4 and 6 imDCs were supplemented with GM-CSF and IL-4 with or without the maturation mixture and incubated for an additional 48 h at 37 °C and 5% CO2. The transduction efficiency was determined to be >80% NGFR+ by flow cytometry.
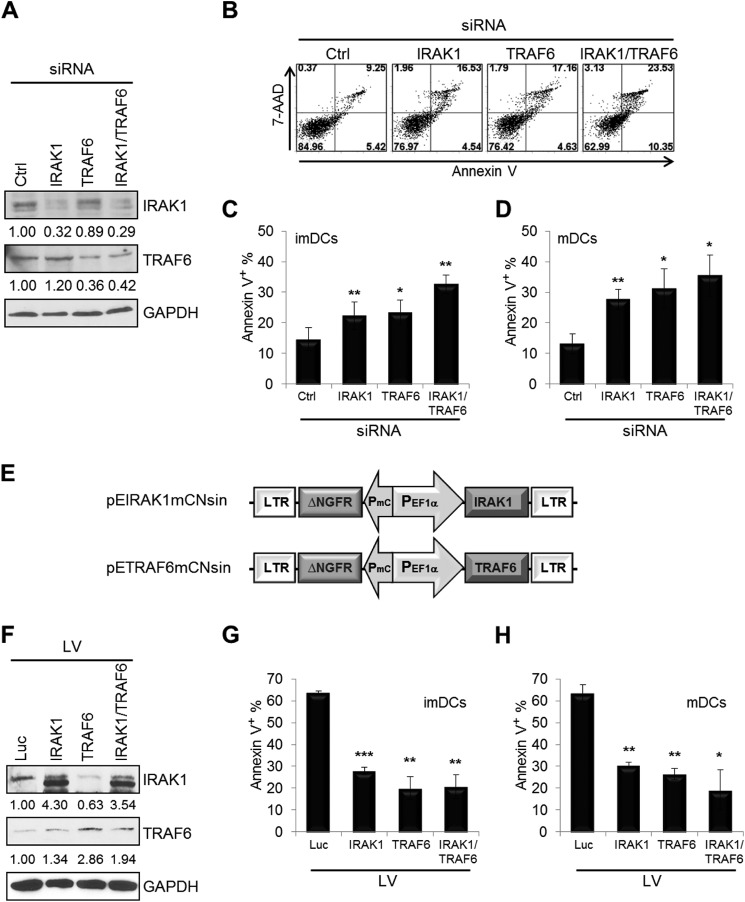
FIGURE 4.
TRAF6 and IRAK1 modulate DC apoptosis. imDCs at day 4 were transfected with TRAF6 and/or IRAK1 siRNA and scrambled control (Ctrl) siRNA at a final concentration of 30 nm for 40 h. A, reduced expression of TRAF6 and/or IRAK1 was analyzed by Western blotting. B, all cells were analyzed for apoptosis by staining with FITC-annexin V and 7-aminoactinomycin D (7-AAD). C, the percentages of annexin V+ cells (including annexin V+/propidium iodide− and annexin V+/propidium iodide+) were determined as in B. D, imDCs at day 6 were transfected with TRAF6 and/or IRAK1 siRNA and scrambled control siRNA, followed by the maturation mixture for 40 h, and cells were then analyzed for apoptosis as described above. E, schematic representation of the pEhfflucmCNsin lentiviral vector expressing human IRAK1 and TRAF6. LTR, long terminal repeat; mC, minimal core promoter element; EF1α, elongation factor 1α. imDCs at days 4 (G) and 6 (F and H) were transduced with the control lentiviral vector (Luc) and with the lentiviral vector (LV) expressing TRAF6 and/or IRAK1, and cells were then incubated for 48 h with (F and H) or without (G) the maturation mixture. The transduction efficiency was determined to be >80% NGFR+ by flow cytometry. Cells were analyzed for Western blotting (F) or apoptosis (G and H) by staining with FITC-annexin V and allophycocyanin-NGFR. NGFR+ cells were used only to determine the percentages of annexin V+ cells. High baseline apoptosis in the control lentiviral vector-transduced imDCs and mDCs was due to use of Polybrene for lentiviral transduction and serum-free AIM V medium for DC culture. Data shown are mean percentages ± S.E. and are representative of four (A–D) or two (F–H) independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005 (by paired t test).
ELISA Assay
Cells were transfected with the indicated miRNA mimics or inhibitors and, 6 h later, treated with either LPS (0.1 μg/ml; Sigma-Aldrich) and imidazoquinoline compound R848 (2.5 μg/ml; InvivoGen) or poly(I:C) (20 μg/ml; InvivoGen) and the maturation mixture in AIM V medium supplemented with human GM-CSF and IL-4 for an additional 16 h. Cell culture supernatants were recovered and evaluated in duplicate for IL-12p70 levels using ELISA kits (BD Biosciences) and for IL-6 and TNF-α levels using chemiluminescent kits (Quansys Biosciences).
DC/Natural Killer (NK) Co-culture Assay
imDCs in 24-well plates (2.5 × 105 cells/well) were transfected with the indicated miRNA inhibitors and a negative control. Six h later, DCs were stimulated with the maturation mixture for 12 h. The old medium was then removed, and transfected DCs were co-cultured with NK cells (5 × 105), which were isolated from human peripheral blood mononuclear cells using an NK cell negative isolation kit (Miltenyi Biotec). Cells were co-cultured for 24 h, and supernatants were harvested for quantification of selected cytokines by ELISA.
Statistical Analysis
All results were calculated as the means ± S.E. Data were analyzed using a two-sample t test, and differences with a p value of <0.05 were regarded as significant.
RESULTS
miR-146a and miR-146b Expression Is Up-regulated upon Monocyte Differentiation
To investigate miR-146a and miR-146b expression during human monocyte differentiation into imDCs and mDCs, monocytes from four healthy donors were differentiated into imDCs with GM-CSF and IL-4 for 6 days and matured with IL-1β, IL-6, TNF-α, and PGE2 for 2 days (10). Monocytes, differentiating DCs (at days 2, 4, and 6), and mature DCs (at day 8) were analyzed for expression of miR-146a and miR-146b by real-time PCR (Fig. 1A). Expression of both miR-146a and miR-146b was significantly increased upon monocyte differentiation into imDCs (at day 6: miR-146a, 10-fold; miR-146b, 37-fold; n = 4, p < 0.05) and mDCs (miR-146a, 51-fold; miR-146b, 79-fold; n = 4, p < 0.005). Consistent with these results, the copy numbers of both miR-146a and miR-146b were significantly increased upon monocyte differentiation into imDCs and mDCs (Fig. 1B).
FIGURE 1.
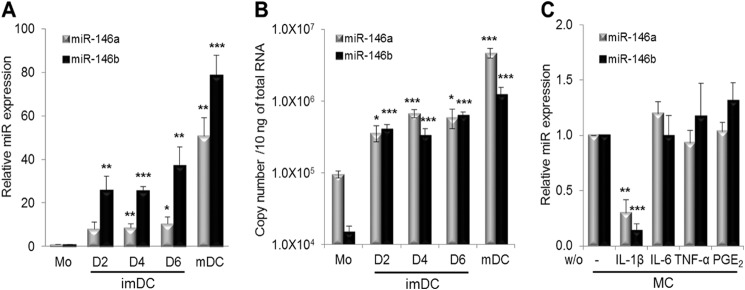
miR-146 expression during monocyte differentiation into imDCs and mDCs. A, miR-146a and miR-146b expression in human monocytes (Mo) at day (D) 0; imDCs at days 2, 4, and 6; and mDCs at day 8 was analyzed by real-time PCR analysis. B, copy numbers of miR-146a and miR-146b. C, effect of the individual component in the maturation mixture on miR-146a and miR-146b expression. imDCs at day 6 were treated for 2 days with maturation mixture (MC) consisting of 10 ng/ml IL-1β, IL-6, and TNF-α and 1 μg/ml PGE2, from which the individual cytokine was excluded. The relative expression of miR-146a and miR-146b was quantified by real-time PCR and normalized to mDCs that had been treated with the full maturation mixture. Data shown are mean percentages ± S.E. of four (A), two (B), or three (C) independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005. w/o, without.
Because both miR-146a and miR-146b expression is greatly increased in mDCs, we investigated which components of the maturation mixture regulate the expression of miR-146a and miR-146b. After incubation for 2 days in the maturation mixture from which individual cytokines were excluded, expression of miR-146a and miR-146b was analyzed by real-time PCR (Fig. 1C). Expression of both miR-146a and miR-146b was significantly decreased using the maturation mixture without IL-1β, whereas the maturation mixture without IL-6, TNF-α, or PGE2 had no or little effect on miR-146a and miR-146b expression in mDCs (n = 3, p < 0.01). These results indicate that expression of miR-146a and miR-146b in mDCs is predominantly mediated by IL-1β but not by IL-6, TNF-α, or PGE2.
miR-146a/b Functions as a Pro-apoptotic Factor during Human Monocyte Differentiation into imDCs and mDCs
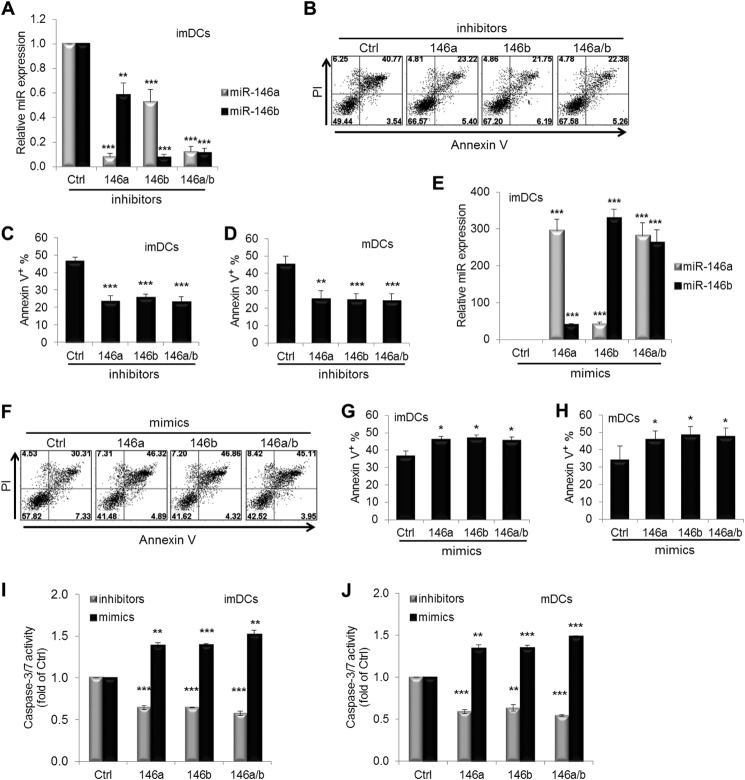
DC apoptosis is important for self-tolerance and immunity (27). Recent studies have demonstrated that miR-146a provides negative feedback inhibition of both innate and adaptive immune responses (28, 29). Therefore, we evaluated the effect of altering miR-146a and/or miR-146b expression levels on DC apoptosis by annexin V/propidium iodide staining. imDCs at day 4 were transfected with miR-146a, miR-146b, or both or with scrambled inhibitors for 40 h. A reduction in miR-146a and miR-146b expression was confirmed by real-time PCR analysis. miR-146a or miR-146b expression levels were significantly decreased to ∼90% of control levels in imDCs transfected with miR-146a or miR-146b inhibitors (Fig. 2A). Transfection of miR-146a or miR-146b inhibitors also reduced the expression levels of miR-146b and miR-146a by 40–50% compared with control groups. Transfection of both miR-146a and miR-146b inhibitors suppressed both miR-146a and miR-146b levels by 90%. Compared with scrambled control inhibitor-transfected cells, the proportion of apoptotic cells after transfection with miR-146a, miR-146b, or both miR-146a and miR-146b inhibitors was significantly decreased, although we could not find a synergic effect by transfection of both miR-146a and miR-146b inhibitors (24 ± 3.1%, 26 ± 1.7%, and 23 ± 2.8% versus 46 ± 2.3% (control) of annexin V+ populations; n = 8, p < 0.005) (Fig. 2, B and C). Silencing of miR-146a, miR-146b, or both in mDCs also led to a significant reduction in DC apoptosis compared with the scrambled control (25 ± 4.7%, 25 ± 3.5%, and 24 ± 4.1% versus 45 ± 5.0%; n = 8, p < 0.05) (Fig. 2D).
FIGURE 2.
miR-146 modulates DC apoptosis. imDCs at day 4 were transfected with miR-146a and/or miR-146b and scrambled control (Ctrl) inhibitors (A–C) or mimics (E–G) at a final concentration of 30 nm for 40 h. A Cy3-labeled negative control was used to monitor the transfection efficiency (close to 80%) of these miRNA inhibitors or mimics. A and E, the relative expression of miR-146a and miR-146b was quantified by real-time PCR. C and G, the percentages of annexin V+ cells (including annexin V+/propidium iodide− (PI) and annexin V+/propidium iodide+) were determined as in B or F. imDCs at day 6 were transfected with miR-146a and/or miR-146b and scrambled control inhibitors (D) or mimics (H), followed by the maturation mixture for 40 h, and cells were then analyzed for apoptosis as described above. Caspase-3/7 activity was measured with the Caspase-Glo 3/7 assay after transfection of miR-146a and/or miR-146b and scrambled control inhibitors or mimics for 40 h in imDCs (I) and mDCs (J). Data shown are mean percentages ± S.E. of eight (C), four (A and E), six (D, G, and H), or two (I and J) independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005 (by paired t test).
To further demonstrate the effect of miR-146a and miR-146b on DC apoptosis, imDCs were transfected with miR-146a and/or miR-146b mimics or the scrambled control for 40 h, and ∼300-fold increased expression of miR-146a and miR-146b compared with the control was confirmed by real-time PCR analysis (Fig. 2E). As anticipated, overexpression of miR-146a, miR-146b, or both in imDCs significantly increased the proportion of apoptotic cells (46 ± 1.9%, 47 ± 2.1%, and 45 ± 2.3% versus 36 ± 3.3% (control); n = 6, p < 0.05) (Fig. 2, F and G). Similarly, overexpression of miR-146a, miR-146b, or both in mDCs also significantly increased DC apoptosis compared with scrambled control mimic-transfected cells (46 ± 5.0%, 48 ± 5.1%, and 47 ± 5.1% versus 34 ± 8.3%; n = 6, p < 0.01) (Fig. 2H). Furthermore, the effect of miR-146a and miR-146b on DC apoptosis was confirmed by activation of caspase-3/7 (Fig. 2, I and J). Taken together, these results indicate that miR-146a and miR-146b may function as pro-apoptotic regulators during human monocyte differentiation into imDCs and mDCs.
Transient Silencing or Overexpression of miR-146a and miR-146b Has Little Effect on Expression of HLA and Co-stimulatory Molecules in DCs
Because both miR-146a and miR-146b were up-regulated in mDCs (Fig. 1, A and B), we hypothesized that these miRNAs may influence DC maturation. Flow cytometric analysis of DC surface molecules, including CD40, CD80, CD83, CD86, and HLA class II (HLA-DR) on imDCs matured for 2 days with miR-146a, miR-146b, or both silenced, showed no significant differences in expression levels compared with the scrambled control except for increased CD40 expression (n = 6) (data not shown). The only consistent difference in expression of DC surface molecules on imDCs overexpressing miR-146a, miR-146b, or both was a slight down-regulation of CD 40 (n = 3) (data not shown). Because we observed only a small effect of miR-146a/b on DC maturation for 2 days, we tested the effect of miR-146a/b on DC maturation for 4 days. Again, silencing both miR-146a and miR-146b in imDCs showed no difference in expression of DC surface molecules except for CD40 expression (n = 2) (data not shown) even after 4 days of maturation. These results were further confirmed using LPS plus R848 and poly(I:C) plus the maturation mixture (n = 2) (data not shown). Importantly, even with 4 days of maturation, we demonstrated significantly decreased expression of both miR-146a and miR-146b by real-time PCR (n = 2) (data not shown). Therefore, these results indicate that expression of miR-146a and miR-146b has little effect on DC maturation.
TRAF6 and IRAK1 Expression Inversely Correlates with miR-146a and miR-146b Expression
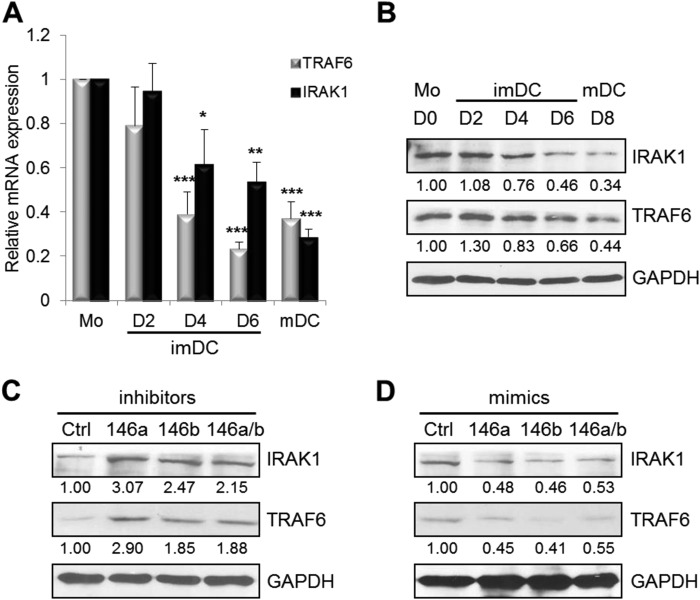
It is known that the NF-κB pathway regulates DC development, function, and survival and that TRAF6 and IRAK1 are major signal transducers in the NF-κB pathway (17, 30). In addition, both TRAF6 and IRAK1 are known target genes of miR-146a (12, 18). Therefore, we investigated whether there is an inverse correlation between TRAF6 and IRAK1 and miR-146a and miR-146b during human monocyte differentiation into imDCs and mDCs by real-time PCR and Western blotting. Expression of both TRAF6 and IRAK1 at the mRNA level was significantly decreased upon monocyte differentiation into imDCs and mDCs (n = 4) (Fig. 3A). Consistent with decreased mRNA levels, expression of TRAF6 and IRAK1 at the protein level was also significantly down-regulated upon DC maturation (Fig. 3B). In contrast to TRAF6 and IRAK1 expression, miR-146a and miR-146b expression increased during monocyte differentiation into imDCs and mDCs (Fig. 1, A and B).
FIGURE 3.
Ectopic expression of miR-146 affects TRAF6 and IRAK1 expression. TRAF6 and IRAK1 expression in human monocytes (Mo) at day (D) 0; imDCs at days 2, 4, and 6; and mDCs at day 8 was analyzed by real-time PCR analysis (A) and Western blotting (B). There was a significant difference in TRAF6 protein (but not mRNA) expression between days 6 and 8 (p < 0.05). There was a significant difference in both IRAK1 mRNA and protein expression between days 6 and 8 (p < 0.05). imDCs at day 4 were transfected with miR-146a and/or miR-146b and scrambled control (Ctrl) inhibitors (C) or mimics (D) at a final concentration of 30 nm. Forty h later, cells were analyzed for TRAF6 and IRAK1 expression by Western blotting. Data shown are mean percentages ± S.E. and are representative of four (A and B) or three (C and D) independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
To determine whether miR-146a and miR-146b regulate TRAF6 and IRAK1 in DCs, we evaluated the expression of TRAF6 and IRAK1 after silencing or overexpression of miR-146a and/or miR-146b in imDCs and mDCs by real-time PCR (data not shown) and Western blotting (Fig. 3, C and D). Compared with scrambled inhibitor-transfected cells, TRAF6 and IRAK1 expression at the mRNA and protein levels was slightly increased after transfection with miR-146a, miR-146b, or both miR-146a and miR-146b inhibitors in imDCs (Fig. 3C) (data not shown). Silencing of miR-146a, miR-146b, or both in mDCs also led to a significant increase in expression of both TRAF6 and IRAK1 compared with the scrambled control (data not shown). Consistently, overexpression of miR-146a, miR-146b, or both in imDCs caused a significant reduction in expression of TRAF6 and IRAK1 at the mRNA and protein levels (Fig. 3D) (data not shown). Overexpression of miR-146a, miR-146b, or both in mDCs also significantly decreased expression of both TRAF6 and IRAK1 compared with scrambled miRNA control-transfected cells (data not shown). Taken together, these data indicate that miR-146a and miR-146b expression is inversely correlated with TRAF6 and IRAK1 expression, suggesting that TRAF6 and IRAK1 could be the potential target molecules of miR-146a and miR-146b in DC apoptosis.
Silencing of TRAF6 and IRAK1 Expression Induces DC Apoptosis
To determine whether TRAF6 and IRAK1 are directly involved in regulating miR-146a/b-mediated DC apoptosis, we silenced TRAF6 and/or IRAK1 expression by siRNA in imDCs and mDCs to evaluate the effect on DC apoptosis. As shown in Fig. 4A, TRAF6 and/or IRAK1 expression was significantly reduced after siRNA silencing in imDCs. Compared with control siRNA-transfected cells, the proportion of apoptotic cells after transfection with TRAF6, IRAK1, or both TRAF6 and IRAK1 siRNAs in imDCs was significantly increased (23 ± 4.1%, 22 ± 4.4%, and 33 ± 3.0% versus 15 ± 3.7% (control) of annexin V+ populations; n = 4; p < 0.05) (Fig. 4, B and C), which was in agreement with the data obtained with miR-146-overexpressing cells (Fig. 2). Silencing of TRAF6, IRAK1, or both in mDCs also led to induction of apoptosis compared with the scrambled control (31 ± 6.4%, 28 ± 3.3%, and 35 ± 6.7% versus 13 ± 3.3%; n = 4, p < 0.05) (Fig. 4D). In addition, overexpression of TRAF6, IRAK1, or both in imDCs and mDCs by lentiviral transduction led to increased DC survival compared with control lentivirus-transduced cells (Fig. 4, E–H). These observations thus strongly suggest that both miR-146a and miR-146b induce apoptosis during human DC development by targeting TRAF6 and IRAK1.
To confirm that miR-146a/b-induced human DC apoptosis is involved in suppression of the NF-κB pathway, at least in part through down-regulation of the NF-κB signaling transducers TRAF6 and IRAK1, we examined the protein level of IκB as a negative regulator of NF-κB. After transfection of miR-146a and/or miR-146b inhibitors, IκB expression was significantly decreased by Western blotting (Fig. 5A), whereas IκB was accumulated upon transfection of miR-146a and/or miR-146b mimics (Fig. 5B) or TRAF6 and/or IRAK1 siRNA in imDCs (Fig. 5C). Overexpression of TRAF6, IRAK1, or both in mDCs by lentiviral transduction also led to decreased IκB expression (Fig. 5D). These data suggest that miR-146a and miR-146b inhibit the NF-κB pathway. In addition, we also investigated the expression level of Bcl-2 as a known downstream anti-apoptotic molecule of the NF-κB pathway. Transfection with the miR-146a and/or miR-146b inhibitors induced an increase in Bcl-2 expression (Fig. 5A), whereas transfection of miR-146a and/or miR-146b mimics (Fig. 5B) or TRAF6 and/or IRAK1 siRNA (Fig. 5C) resulted in a decrease in Bcl-2 protein levels in imDCs. Increased expression of Bcl-2 was confirmed by overexpression of TRAF6, IRAK1, or both in mDCs by lentiviral transduction (Fig. 5D). Taken together, these results indicate that miR-146a and miR-146b modulate DC apoptosis through inhibition of NF-κB activation via targeting TRAF6 and IRAK1.
FIGURE 5.
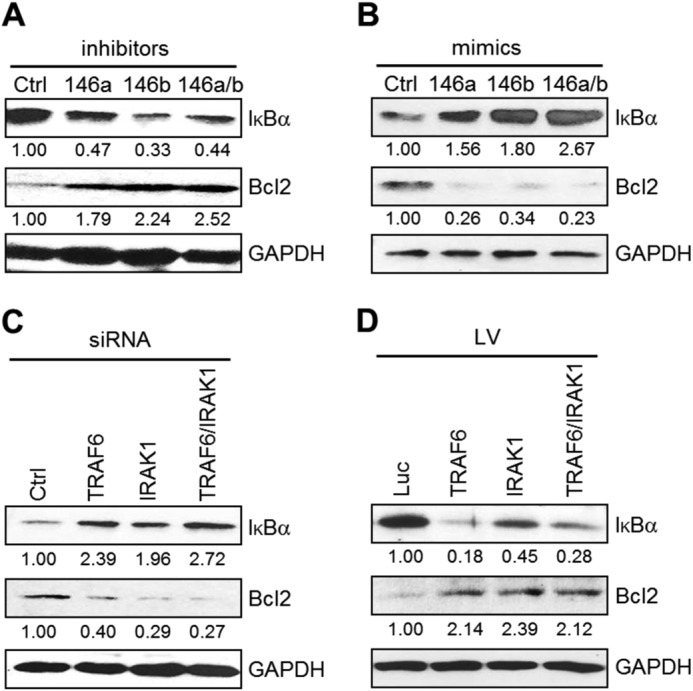
miR-146 induces apoptosis via the NF-κB pathway in human DCs. imDCs at day 4 were transfected with miR-146a and/or miR-146b and scrambled control (Ctrl) inhibitors (A) or mimics (B) or with TRAF6 and/or IRAK1 siRNA and scrambled control siRNA (C) at a final concentration of 30 nm. Forty h later, cells were analyzed for IκB and Bcl-2 expression by Western blotting. D, imDCs at day 6 were transduced with the control lentiviral vector (Luc) and with the lentiviral vector (LV) expressing TRAF6 and/or IRAK1, and cells were then incubated for 48 h with the maturation mixture. Blots were quantified by densitometry and normalized to GAPDH. Data shown are representative of four (A–C) or two (D) independent experiments.
miR-146a and miR-146b Modulate Proinflammatory Cytokine Production in mDCs
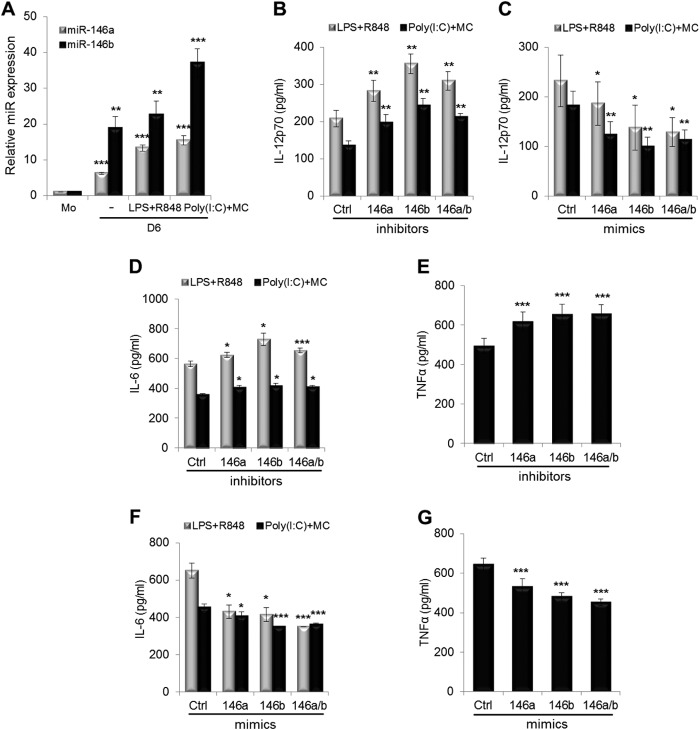
We next investigated whether miR-146a and miR-146b regulate proinflammatory cytokine production in DCs. IL-12, a proinflammatory cytokine, induces the production of IFN-γ primarily from NK and T cells and favors the promotion of Th1 cell immunity (31). We found that IL-12p70 production was slightly enhanced after miR-146a and/or miR-146b silencing, whereas IL-12p70 production was slightly reduced after miR-146a and/or miR-146b overexpression during DC maturation (data not shown). To confirm these data, we used potent IL-12 inducers, including LPS plus R848 or poly(I:C) plus the maturation mixture (32, 33), because the maturation mixture alone induces a very low level of IL-12 production in human DCs. Expression of miR-146a and miR-146b was increased by these stimulations (Fig. 6A). We found that IL-12p70 production was significantly enhanced after miR-146a and/or miR-146b silencing during DC maturation (Fig. 6B). By contrast, IL-12p70 production was greatly reduced after miR-146a and/or miR-146b overexpression (Fig. 6C).
FIGURE 6.
miR-146 modulates cytokine production in mDCs. A, imDCs at day 5 were treated with the LPS + R848 or with poly(I:C) + maturation mixture (MC) in AIM V medium supplemented with human GM-CSF and IL-4 for 16 h. miR-146a and miR-146b expression in human monocytes (Mo) at day 0, imDCs at day 6, and mDCs by LPS + R848 or poly(I:C) + maturation mixture at day 6 was analyzed by real-time PCR analysis. imDCs at day 5 were transfected with miR-146a and/or miR-146b and scrambled control (Ctrl) inhibitors (B, D, and E) or mimics (C, F, and G). Six h later, cells were treated with LPS + R848 (B–D and F) or with poly(I:C) + maturation mixture (B–G) in AIM V medium supplemented with human GM-CSF and IL-4 for 16 h. Supernatants were harvested, and IL-12p70, IL-6, and TNF-α were measured by ELISA. Data shown are mean percentages ± S.E. of three (A), four (B and C), or two (D–G) independent experiments. There is a large variation between individual donors in C. *, p < 0.05; **, p < 0.01; ***, p < 0.005 (by paired t test).
We also measured the effect of alteration of miR-146a and miR-146b expression on IL-6 and TNF-α production during DC maturation. Both IL-6 and TNF-α levels were significantly increased after miR-146a and/or miR-146b silencing (Fig. 6, D and E), whereas IL-6 and TNF-α levels were significantly decreased after miR-146a and/or miR-146b overexpression during DC maturation (Fig. 6, F and G).
To investigate whether silencing of miR-146a and miR-146b could enhance the ability of mDCs to activate NK cells, mDCs treated to silence miR-146a, miR-146b, or both were co-cultured with CD3−CD56+ NK cells. NK cells co-cultured with mDCs that were transfected with inhibitors of miR-146a, miR-146b, or both had significantly increased IL-12p70 production (Fig. 7, A and B) and secreted significantly higher levels of IFN-γ compared with control cultures (p < 0.005). These results suggest that miR-146a and miR-146b silencing can enhance the ability of mDCs to activate NK cell production of IFN-γ.
FIGURE 7.
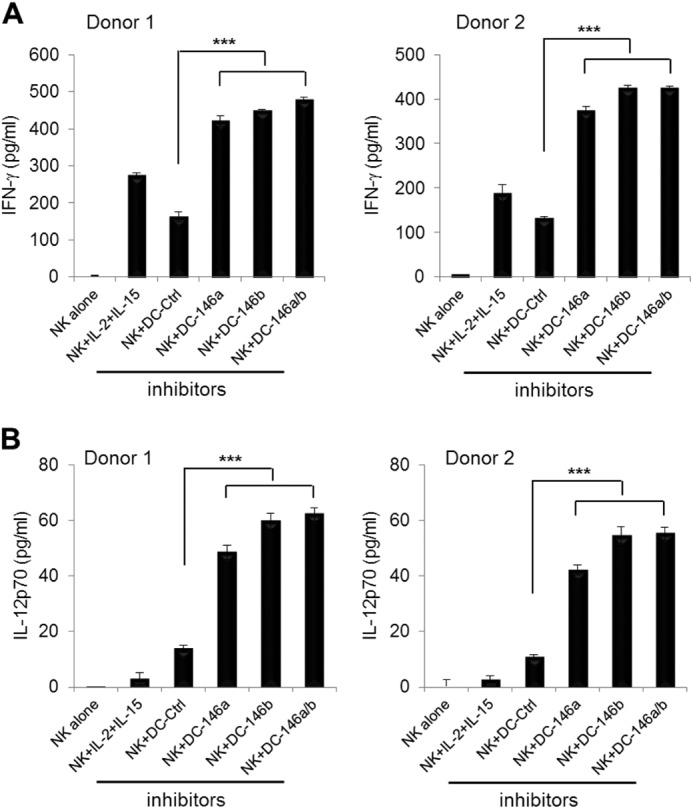
miR-146 modulates IFN-γ production by NK cells through increased IL-12p70 production by DCs. imDCs at day 6 were transfected with miR-146a and/or miR-146b and scrambled control (Ctrl) inhibitors. Six h later, cells were treated with the maturation mixture for another 12 h. After washing, DCs were co-cultured with NK cells at a 1:2 ratio for 24 h. Supernatants were harvested, and IFN-γ (A) and IL-12p70 (B) were measured by ELISA. Data shown are mean percentages ± S.E. of two independent experiments with two different donors. ***, p < 0.005.
DISCUSSION
In this study, we have demonstrated three important findings concerning the role of miR-146a and miR-146b in human DC differentiation and function. First, expression of both miR-146a and miR-146b is up-regulated during human monocyte differentiation into imDCs and mDCs, although an earlier report suggested that only miR-146a is induced upon activation of human monocytes (12). We also demonstrated that increased expression of both miR-146a and miR-146b during human DC differentiation is significantly inversely correlated with TRAF6 and IRAK1 expression. Second, miR-146a and miR-146b may be critical regulators of DC apoptosis and cytokine production. miR-146a and/or miR-146b significantly promotes DC apoptosis and inhibits production of IL-12p70, IL-6, and TNF-α. Silencing of miR-146a and/or miR-146b in mDCs leads to higher levels of IL-12p70 secretion by DCs and increased IFN-γ production by NK cells. Third, mechanistically, miR-146a and miR-146b target TRAF6 and IRAK1, leading to inhibition of NF-κB and reduced expression of Bcl-2. We have thus demonstrated for the first time that miR-146a and miR-146b regulate human DC apoptosis and cytokine production, uncovering a new negative feedback mechanism for miR-146 in controlling overstimulation of the immune responses (Fig. 8).
FIGURE 8.
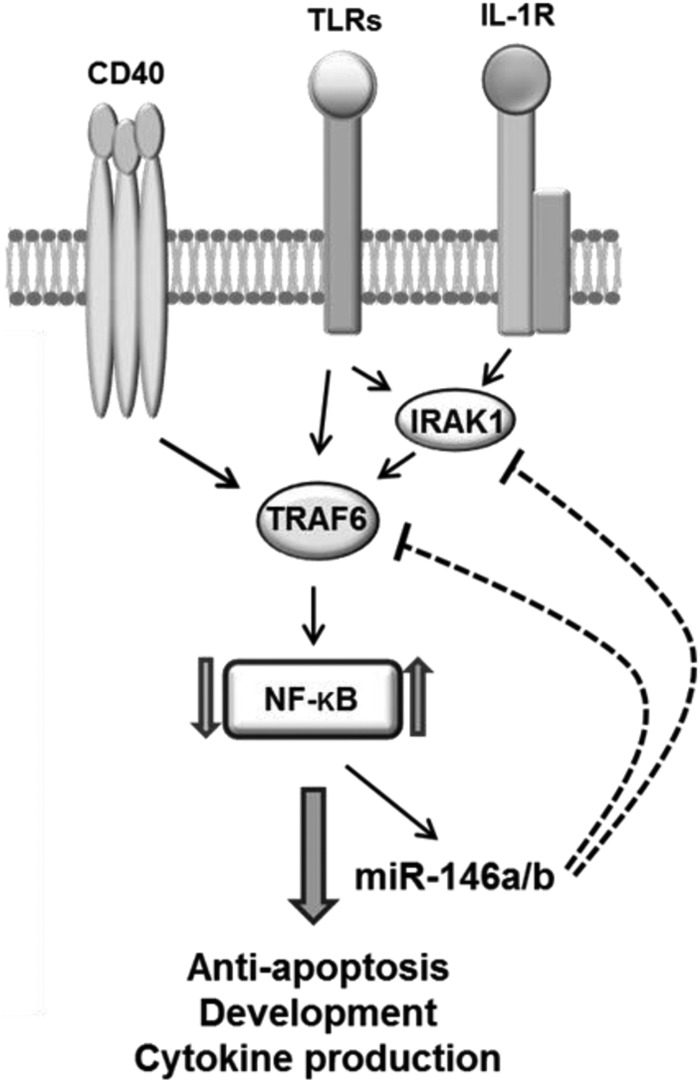
Working model for miR-146a/b-mediated regulation of DC functions. In human monocytes, miR-146a and miR-146b are expressed at low levels. During differentiation of monocytes into imDCs and mDCs, stimulation of TRAF6 and IRAK1 via various signals such as the TLR/IL-1 receptor and CD40 leads to subsequent activation of NF-κB, which up-regulates miR-146a and miR-146b expression. The increased miR-146a and miR-146b levels in turn suppress NF-κB activity through inhibition of TRAF6 and IRAK1 expression. This negative feedback loop regulates DC survival, maturation, and cytokine production.
miR-146a has emerged as a negative master regulator of Toll-like receptor (TLR) activation (12). The miR-146 family consists of two evolutionarily conserved miRNA genes, miR-146a and miR-146b on chromosomes 5 and 10, respectively, and their mature products differ only by 2 nucleotides in the 3′-end (34). Baltimore and colleagues (12) have shown that the expression of both miR-146a and miR-146b was rapidly induced in human monocytic THP-1 cells in response to TLR stimuli (e.g. LPS) and proinflammatory cytokines (e.g. TNF-α and IL-1β). Induction of miR-146a was shown to be NF-κB-dependent. They also identified that TRAF6 and IRAK1, which are two key adaptor molecules in the TLR or cytokine receptor/NF-κB signaling pathway, are direct targets of miR-146a and miR-146b through a negative feedback regulation loop (12). However, only miR-146a in the precursor and mature forms, but not miR-146b, was detected to be up-regulated after 8 h of LPS treatment in two human myeloid cell lines (THP-1 and HL-60). Failure to detect miR-146b could be due to a short period of LPS treatment and/or use of cell lines, but not primary cells. A recent kinetic study showed that induction of miR-146a in human primary monocytes by LPS stimulation was faster compared with miR-146b and that miR-146b induction could be detected at 12 h post-stimulation (35). By contrast, in our study, both miR-146a and miR-146b were up-regulated upon monocyte differentiation into imDCs and mDCs, and both were involved in terminating DC function through apoptosis and dampening of cytokine production.
Our study also identified the mechanisms underlying miR-146a and miR-146b regulation of DC apoptosis through the miR-146a/b-TRAF6/IRAK1-NF-κB axis. We identified that miR-146a/b up-regulation upon DC maturation was inversely correlated with TRAF6 and IRAK1 expression. Overexpression of miR-146a and/or miR-146b or silencing of TRAF6 and/or IRAK1 led to a significant increase in the expression of IκB in DCs. Interestingly, the phosphorylation of IκB on Ser-32 is essential for its degradation, and this phosphorylation is reduced by overexpression of miR-146a or miR-146b in MDA-MB-231 breast cancer cells (18). Up-regulation of IκB by overexpression of miR-146a has also been shown in NK/T cell lymphoma cells (36). Furthermore, TRAF6 functions as an E3 ubiquitin ligase that activates IκB kinase (37). These findings support the notion that miR-146a and miR-146b serve as NF-κB negative regulators through down-regulation of TRAF6 and IRAK1 functions.
Our study supports published data claiming that miR-146 regulates cell survival. It has been reported that miR-146a regulates survival and maturation of human plasmacytoid DCs (pDCs) and can be induced upon TLR7/9 ligation in this cell type (38). Furthermore, ectopic miR-146a expression impairs TLR-mediated signaling by diminished production of proinflammatory cytokines (IL-6 and IFN-β), increased apoptosis, and reduced expression of co-stimulatory molecules and HLA class II due to reduced activation of NF-κB. However, the majority of these results were obtained with a pDC cell line, and it was unclear if miR-146b was also involved in pDC survival. Our results demonstrate that both miR-146a and miR-146b are critical regulators in the survival of conventional monocyte-derived DCs.
Our results may provide new insights into how miR-146a/b regulates DC function. DCs activate lymphocytes to fight infection and subsequently progress to cell death to maintain self-tolerance and prevent autoimmunity. It has been shown that a defect in DC apoptosis in animals and patients with mutations in the gene for caspase-10 can lead to DC accumulation, chronic lymphocyte activation, and systemic autoimmune manifestations (39). Our results appear to make physiological sense because induction of miR-146a and miR-146b upon maturation of DCs could lead to their apoptosis and reduced cytokine production, suggesting that both miR-146a and miR-146b could act as a regulatory mechanism to prevent overstimulation of the proinflammatory response in human DCs.
In line with our findings, miR-146a-deficient mice show an increase in the number of regulatory T cells that exhibit impaired capacity to suppress the Th1 response (40). Also, these mice display spontaneous autoimmune disorders with loss of T cell tolerance, massive myeloproliferation, and cancer (21). Furthermore, miR-146a deficiency in T cells causes hyper-responsiveness of both acute antigenic responses and chronic inflammatory autoimmune responses (41). These effects of miR-146a deficiency are at least in part related to the absence of negative feedback regulation through TRAF6 and IRAK1 (20, 21, 41). In fact, this hypothesis is further supported by several recent findings. miR-146a-deficient mice produce excessive amounts of proinflammatory cytokines such as TNF-α and IL-6 in response to LPS (21). DCs from TRAF6-deficient mice show significantly impaired production of IL-6 and IL-12 by stimulation of TLR ligands or CD40L (42). Finally, as discussed above, overexpression of miR-146a in the pDC CAL-1 cell line increases cell apoptosis and decreases expression of IFN-β and IL-6 mRNAs (38).
Although our results demonstrated that miR-146a and miR-146b negatively regulate DC apoptosis by targeting TRAF6 and IRAK1, we cannot exclude the possibility that other molecules targeted by miR-146a/b may contribute to DC apoptosis and cytokine production. We performed a computational search using miRanda (43) and TargetScan (44), and we found that CD40LG, TLR4, FADD, FAS, and SMAD4 are predicted targets for miR-146a and miR-146b. It has been reported that CD40L increases DC survival, up-regulates MHC expression, and induces the expression of a variety of cytokines such as IL-12 in DCs (45). Our data demonstrated that production of IL-12 by DCs and of IL-12-mediated IFN-γ by NK cells was significantly enhanced via silencing of miR-146a and miR-146b. Therefore, CD40L could be a target of miR-146a and miR-146b for DC apoptosis and cytokine production. Further work is needed to determine whether CD40L or other target molecules play a role in miR-146a/b-mediated DC apoptosis and cytokine production.
We have previously demonstrated that miR-155 is induced in human mDCs, leading to increased DC apoptosis and IL-12p70 production (10). In this study, we demonstrated that both miR-146a and miR-146b play a key role in the regulation of DC survival and inflammatory activation. It remains to be determined whether miR-146a and miR-146b, as well as miR-155, play a redundant or distinct role in DC apoptosis. These miRNAs may be required to achieve an additive effect on DC apoptosis, as each individual miRNA serves as a fine-tuning regulator. Future research is also needed to understand the precise in vivo role of miR-146a/b-mediated DC apoptosis in self-tolerance and autoimmunity. Our findings may have therapeutic implications for autoimmune diseases and/or cancer via manipulating the miR-146a/b-TRAF6/IRAK1-NF-κB pathway in DCs.
Acknowledgments
We thank Drs. Carl Hamby and Mary Petzke for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant AI092220 (to X. Z.). This work was also supported by the Pediatric Cancer Research Foundation (to M. S. C.).
- DC
- dendritic cell
- imDC
- immature DC
- mDC
- mature DC
- miRNA/miR
- microRNA
- PGE2
- prostaglandin E2
- NGFR
- NGF receptor
- NK
- natural killer
- TLR
- Toll-like receptor
- pDC
- plasmacytoid DC.
REFERENCES
- 1. Lanzavecchia A., Sallusto F. (2004) Lead and follow: the dance of the dendritic cell and T cell. Nat. Immunol. 5, 1201–1202 [DOI] [PubMed] [Google Scholar]
- 2. Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. (2000) Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 [DOI] [PubMed] [Google Scholar]
- 3. Steinman R. M., Hawiger D., Nussenzweig M. C. (2003) Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 [DOI] [PubMed] [Google Scholar]
- 4. Jiang H., Van De Ven C., Satwani P., Baxi L. V., Cairo M. S. (2004) Differential gene expression patterns by oligonucleotide microarray of basal versus lipopolysaccharide-activated monocytes from cord blood versus adult peripheral blood. J. Immunol. 172, 5870–5879 [DOI] [PubMed] [Google Scholar]
- 5. Jiang H., van de Ven C., Baxi L., Satwani P., Cairo M. S. (2009) Differential gene expression signatures of adult peripheral blood vs cord blood monocyte-derived immature and mature dendritic cells. Exp. Hematol. 37, 1201–1215 [DOI] [PubMed] [Google Scholar]
- 6. Bartel D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 7. Pasquinelli A. E. (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 13, 271–282 [DOI] [PubMed] [Google Scholar]
- 8. O'Connell R. M., Rao D. S., Chaudhuri A. A., Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 [DOI] [PubMed] [Google Scholar]
- 9. Hashimi S. T., Fulcher J. A., Chang M. H., Gov L., Wang S., Lee B. (2009) MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood 114, 404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu C., Huang X., Zhang X., Roensch K., Cao Q., Nakayama K. I., Blazar B. R., Zeng Y., Zhou X. (2011) miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood 117, 4293–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun Y., Varambally S., Maher C. A., Cao Q., Chockley P., Toubai T., Malter C., Nieves E., Tawara I., Wang Y., Ward P. A., Chinnaiyan A., Reddy P. (2011) Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood 117, 6172–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. (2006) NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 12481–12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou J., Wang P., Lin L., Liu X., Ma F., An H., Wang Z., Cao X. (2009) MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 183, 2150–2158 [DOI] [PubMed] [Google Scholar]
- 14. Jurkin J., Schichl Y. M., Koeffel R., Bauer T., Richter S., Konradi S., Gesslbauer B., Strobl H. (2010) miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. J. Immunol. 184, 4955–4965 [DOI] [PubMed] [Google Scholar]
- 15. Kawai T., Akira S. (2006) TLR signaling. Cell Death Differ. 13, 816–825 [DOI] [PubMed] [Google Scholar]
- 16. Rescigno M., Martino M., Sutherland C. L., Gold M. R., Ricciardi-Castagnoli P. (1998) Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 188, 2175–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van de Laar L., van den Bosch A., van der Kooij S. W., Janssen H. L., Coffer P. J., van Kooten C., Woltman A. M. (2010) A nonredundant role for canonical NF-κB in human myeloid dendritic cell development and function. J. Immunol. 185, 7252–7261 [DOI] [PubMed] [Google Scholar]
- 18. Bhaumik D., Scott G. K., Schokrpur S., Patil C. K., Campisi J., Benz C. C. (2008) Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene 27, 5643–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nahid M. A., Pauley K. M., Satoh M., Chan E. K. (2009) miR-146a is critical for endotoxin-induced tolerance. Implication in innate immunity. J. Biol. Chem. 284, 34590–34599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao J. L., Rao D. S., Boldin M. P., Taganov K. D., O'Connell R. M., Baltimore D. (2011) NF-κB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc. Natl. Acad. Sci. U.S.A. 108, 9184–9189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boldin M. P., Taganov K. D., Rao D. S., Yang L., Zhao J. L., Kalwani M., Garcia-Flores Y., Luong M., Devrekanli A., Xu J., Sun G., Tay J., Linsley P. S., Baltimore D. (2011) miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 208, 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thurner B., Röder C., Dieckmann D., Heuer M., Kruse M., Glaser A., Keikavoussi P., Kämpgen E., Bender A., Schuler G. (1999) Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 223, 1–15 [DOI] [PubMed] [Google Scholar]
- 23. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 24. Baseler W. A., Thapa D., Jagannathan R., Dabkowski E. R., Croston T. L., Hollander J. M. (2012) miR-141 as a regulator of the mitochondrial phosphate carrier (Slc25a3) in the type 1 diabetic heart. Am. J. Physiol. Cell Physiol. 303, C1244–C1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang X., Guo H., Kang J., Choi S., Zhou T. C., Tammana S., Lees C. J., Li Z. Z., Milone M., Levine B. L., Tolar J., June C. H., Scott McIvor R., Wagner J. E., Blazar B. R., Zhou X. (2008) Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol. Ther. 16, 580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou X., Cui Y., Huang X., Yu Z., Thomas A. M., Ye Z., Pardoll D. M., Jaffee E. M., Cheng L. (2003) Lentivirus-mediated gene transfer and expression in established human tumor antigen-specific cytotoxic T cells and primary unstimulated T cells. Hum. Gene Ther. 14, 1089–1105 [DOI] [PubMed] [Google Scholar]
- 27. Chen M., Wang J. (2010) Programmed cell death of dendritic cells in immune regulation. Immunol. Rev. 236, 11–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams A. E., Perry M. M., Moschos S. A., Larner-Svensson H. M., Lindsay M. A. (2008) Role of miRNA-146a in the regulation of the innate immune response and cancer. Biochem. Soc. Trans. 36, 1211–1215 [DOI] [PubMed] [Google Scholar]
- 29. Curtale G., Citarella F., Carissimi C., Goldoni M., Carucci N., Fulci V., Franceschini D., Meloni F., Barnaba V., Macino G. (2010) An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood 115, 265–273 [DOI] [PubMed] [Google Scholar]
- 30. Xiong H., Zhu C., Li F., Hegazi R., He K., Babyatsky M., Bauer A. J., Plevy S. E. (2004) Inhibition of interleukin-12 p40 transcription and NF-κB activation by nitric oxide in murine macrophages and dendritic cells. J. Biol. Chem. 279, 10776–10783 [DOI] [PubMed] [Google Scholar]
- 31. Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 [DOI] [PubMed] [Google Scholar]
- 32. Kim H. J., Kim H. O., Lee K., Baek E. J., Kim H. S. (2010) Two-step maturation of immature DCs with proinflammatory cytokine cocktail and poly(I:C) enhances migratory and T cell stimulatory capacity. Vaccine 28, 2877–2886 [DOI] [PubMed] [Google Scholar]
- 33. Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. (2002) Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 [DOI] [PubMed] [Google Scholar]
- 34. Garzon R., Calin G. A., Croce C. M. (2009) MicroRNAs in Cancer. Annu. Rev. Med. 60, 167–179 [DOI] [PubMed] [Google Scholar]
- 35. Curtale G., Mirolo M., Renzi T. A., Rossato M., Bazzoni F., Locati M. (2013) Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc. Natl. Acad. Sci. U.S.A. 110, 11499–11504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paik J. H., Jang J. Y., Jeon Y. K., Kim W. Y., Kim T. M., Heo D. S., Kim C. W. (2011) MicroRNA-146a downregulates NFκB activity via targeting TRAF6 and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin. Cancer Res. 17, 4761–4771 [DOI] [PubMed] [Google Scholar]
- 37. Yang W. L., Wang J., Chan C. H., Lee S. W., Campos A. D., Lamothe B., Hur L., Grabiner B. C., Lin X., Darnay B. G., Lin H. K. (2009) The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 325, 1134–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karrich J. J., Jachimowski L. C., Libouban M., Iyer A., Brandwijk K., Taanman-Kueter E. W., Nagasawa M., de Jong E. C., Uittenbogaart C. H., Blom B. (2013) MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood 122, 3001–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang J., Zheng L., Lobito A., Chan F. K., Dale J., Sneller M., Yao X., Puck J. M., Straus S. E., Lenardo M. J. (1999) Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell 98, 47–58 [DOI] [PubMed] [Google Scholar]
- 40. Lu L. F., Boldin M. P., Chaudhry A., Lin L. L., Taganov K. D., Hanada T., Yoshimura A., Baltimore D., Rudensky A. Y. (2010) Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142, 914–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang L., Boldin M. P., Yu Y., Liu C. S., Ea C. K., Ramakrishnan P., Taganov K. D., Zhao J. L., Baltimore D. (2012) miR-146a controls the resolution of T cell responses in mice. J. Exp. Med. 209, 1655–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kobayashi T., Walsh P. T., Walsh M. C., Speirs K. M., Chiffoleau E., King C. G., Hancock W. W., Caamano J. H., Hunter C. A., Scott P., Turka L. A., Choi Y. (2003) TRAF6 is a critical factor for dendritic cell maturation and development. Immunity 19, 353–363 [DOI] [PubMed] [Google Scholar]
- 43. John B., Enright A. J., Aravin A., Tuschl T., Sander C., Marks D. S. (2004) Human microRNA targets. PLoS Biol. 2, e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lewis B. P., Burge C. B., Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 45. van Kooten C., Banchereau J. (1997) Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 9, 330–337 [DOI] [PubMed] [Google Scholar]