Background: TRPM8 channels are highly expressed in prostate tissues, where the role of this cold receptor is not well understood.
Results: Testosterone directly interacts with the TRPM8 protein.
Conclusion: TRPM8 is a testosterone receptor.
Significance: TRPM8 channels may be implicated in various physiological processes regulated by androgens.
Keywords: Androgen, Calcium Channel, Ion Channel, Testosterone, Transient Receptor Potential Channels (TRP Channels), Cold and Menthol Receptor TRPM8, Transient Receptor Potential Melastatin 8 Channel
Abstract
The transient receptor potential ion channel of the melastatin subfamily, TRPM8, is a major cold receptor in the peripheral nervous system. Along with the sensory neurons, the TRPM8 protein is highly expressed in the prostate epithelial cells, and this expression is regulated by androgens. Here we investigated the expression and intracellular localization of the TRPM8 channel in relationship to androgens. We performed experiments using human prostate tissues obtained from healthy individuals and patients with prostate cancer at various stages of the disease as well as in cultured cells. Using an immunohistochemistry approach, we detected an intensive colocalization pattern of the TRPM8 protein with endogenous androgens in all tissues tested, suggesting possible interactions. Co-immunoprecipitation experiments performed using cultured prostate epithelial cells, prostate cancer cells, and HEK-293 cells stably expressing TRPM8 further confirmed direct binding of the steroid hormone, testosterone, to the TRPM8 protein. Applications of picomolar concentrations of testosterone to the primary human prostate cells, endogenously expressing TRPM8, elicited Ca2+ responses and channel currents, and those were inhibited in the presence of TRPM8 antagonist, N-(2-aminoethyl)-N-(4-(benzyloxy)-3-methoxybenzyl)thiophene-2-carboxamide hydrochloride. These results indicate that the TRPM8 channel is physically associated with testosterone and suggest that, in addition to a genomic role, testosterone plays a role in direct regulation of the TRPM8 channel function.
Introduction
TRPM8 (transient receptor potential melastatin family member 8) is the major receptor for a wide range of cold temperatures in the peripheral nervous system (1–3). TRPM8 is also responsible for sensing chemical compounds such as menthol, icilin, eucalyptol, geraniol, and linalool (4–6).
The role and function of TRPM8 are well established in the somatosensory nervous system. The TRPM8 protein expression, however, is not limited to the sensory neurons alone and has been also observed in organ-specific epithelial tissues. Particularly, TRPM8 is highly expressed in the prostate gland and in the prostate cancer tissues (7). However, the role of this cold and menthol receptor in prostate remains elusive.
In our study, we aimed to address the role of TRPM8 in the prostate epithelial cells. It has been previously recognized that TRPM8 expression is androgen-dependent, where the androgen receptor (AR)2 protein is an important transcription factor for TRPM8 expression in prostate cells (8). In general, androgens are important regulators of reproductive organs and are also involved in the pathogenesis of prostate cancer, including progression and metastasis (9). The classic genomic actions of these steroid hormones are mediated through the AR protein, a member of the nuclear receptor family of transcription factors (10, 11). The steroid-AR complex is translocated to the nucleus, where it binds to promoters and stimulates gene expression (12).
In attempts to identify androgen regulation of TRPM8 expression, we first performed immunohistochemistry experiments to visualize both the protein and androgen distribution in human prostate tissues. Surprisingly, we detected high levels of colocalization between TRPM8 and endogenous androgens on the human prostate samples. Furthermore, co-immunoprecipitation and enzyme-linked immunosorbent assay (ELISA) experiments showed that testosterone directly interacts with the channel in the prostate epithelial cells, prostate cancer cells, and HEK-293 cells stably expressing the TRPM8 protein. These findings suggest that TRPM8 might play the role of a membrane testosterone receptor.
EXPERIMENTAL PROCEDURES
Cell Culture
HEK-293 cells were maintained in minimal essential medium solution (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin. The cells were transfected with the rat TRPM8 cDNA using the Effectene reagent (Qiagen, Chatsworth, CA). The TRPM8 stable cell line was developed with TRPM8 tagged with Myc on the N terminus as described previously (13).
The primary human prostate epithelial cells were purchased from Lonza and cultured in the prostate epithelial basal medium with supplements and growth factors, as directed (Lonza Inc., Allendale, NJ).
The prostate cancer cell lines LNCaP, PC-3, and RWPE2 were obtained from the American Type Culture Collection (Manassas, VA) and cultured as directed. LNCaP cells were cultured in RPMI medium supplemented with 2 mm l-glutamine, 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, 10 mm HEPES, and 1.0 mm sodium pyruvate (Invitrogen). PC3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM)/F12K (1:1). Both media contained 10% fetal bovine serum (Invitrogen) and 5% penicillin/streptomycin. RWPE-2 cells were cultured in keratinocyte serum-free medium supplemented with 0.05 mg/ml bovine pituitary extract and 5 ng/ml epidermal growth factor. All of the cells were maintained in a 37 °C incubator in a 5% CO2 humidified atmosphere.
Preparation of the TRPM8 Protein from HEK-293 Cells
HEK-293 cells stably expressing Myc-tagged TRPM8 were grown to 70–80% confluence, washed, and collected with cold PBS. Cells were harvested and resuspended in NCB buffer, containing 500 mm NaCl, 50 mm NaH2PO4, 20 mm Hepes, 10% glycerol, pH 7.5, with the addition of 1 mm protease inhibitor PMSF, 5 mm β-mercaptoethanol. Then the cells were lysed by the freeze-thawing method and centrifuged at low speed to remove cell debris and DNA. The supernatant was further centrifuged at 40,000 × g for 2.5 h, and the pellet was resuspended in NCB buffer with the addition of a protease inhibitor mixture (Roche Applied Science), 0.1% Nonidet P-40 (Roche Applied Science), and 0.5% dodecylmaltoside (Calbiochem). The suspension was incubated overnight at 4 °C on a shaker with gentle agitation and then centrifuged for 1 h at 40,000 × g. Further, the TRPM8 protein was purified by immunoprecipitation with anti-Myc IgG conjugated to protein A/G magnetic beads (Pierce, Thermo Scientific), following the procedure provided by the manufacturer. All steps of purification were performed at 4 °C. Protein was eluted with Myc-peptide (50 μg/ml), in NCB buffer containing 0.1% Nonidet P-40 and 0.03% lauryl maltose neopentyl glycol (LMNG) (Anatrace, Maumee, OH).
TRPV1 was obtained from HEK-293 cells stably expressing the Myc-tagged TRPV1 protein, using the same purification procedure used for TRPM8 and as described previously (14).
Immunoblotting and Immunoprecipitation Assay
Western blot analysis was done using equal amounts of protein separated by SDS-PAGE, transferred onto nitrocellulose or PVDF membranes (Bio-Rad), incubated with a 1:1000 dilution of primary antibodies, and subsequently incubated with a 1:1000 dilution of species-specific, HRP-conjugated secondary antibody according to the standard protocol (15). Immunoprecipitation assays were carried out by incubating about 500 μg of membrane extracts with the required specific primary antibody (1 μg) overnight at 4 °C on a rotating shaker. Around 50 μl of protein A/G-agarose beads (Miltenyi Biotech, Auburn, CA) were added to the above complex and incubated on ice for 30 min. These beads were passed through MACS 20-μm columns (Miltenyi Biotech), and the bound immunoprecipitates were eluted according to the manufacturer's instructions. The immunoprecipitates were then immunoblotted using specific primary antibodies. IP was performed using sheep anti-testosterone/DHT (Fisher) antibody, and the eluate was immunoprobed using rabbit anti-TRPM8 antibody (Phoenix Pharmaceuticals, Burlingame, CA) to show the association of testosterone/DHT with TRPM8. The immunoblots for the AR protein were developed using antibodies specific for AR. Flotillin was used as a loading control.
Immunohistochemistry
A prostate cancer tissue microarray (US Biomax, Rockville, MD) containing 60 cases of prostate adenocarcinoma (grades 1–4) and nine cases of normal prostate tissues were processed for immunohistochemical analysis according to the standard protocol (16). The pathology description on Gleason score and grade is provided by US Biomax. The tissue sections were deparaffinized in xylene and rehydrated in graded ethanol solutions. Antigen retrieval was carried out with 10 mm citrate buffer (pH 6) at boiling temperature for 60 min and permeabilization in 0.1% Triton X-100. The sections were blocked using 10% BSA in 1× PBS and were incubated with primary antibodies overnight at 4 °C, washed with PBS, and incubated with fluorescence-labeled, species-specific secondary antibodies (Alexa Fluor) at 1:500 dilution for 1 h at room temperature. Before mounting, the slides were washed with PBS and incubated for 5 min with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining and analyzed using confocal microscopy (Zeiss LSM 510 upright confocal microscope, Toronto, Canada) at ×40 magnification.
Immunocytochemistry
HEK-293 cells transiently expressing Myc-TRPM8 were grown on 25-mm round glass coverslips and fixed with 2 ml of 4% paraformaldehyde in PBS at room temperature for 30 min. The cells were washed three times with PBS (2 ml) and treated with 2 ml of 100 mm glycine in PBS for 30 min. After washing with PBS twice, the cells were permeabilized with 300 μl of cold methanol at −20 °C for 5 min and then washed twice with 2 ml of PBS. The cells were then blocked with 1% gelatin in PBS for 1 h at room temperature, followed by incubation with anti-Myc antibody (Sigma-Aldrich) (1:2500) in 1 ml of the 1% gelatin-PBS for overnight at room temperature. The next day, the cells were washed with PBS three times and then treated with the secondary antibodies (1:3000) (Alexa Fluor, Invitrogen); with testosterone/BSA/FITC (Sigma-Aldrich) in the ratio testosterone (10 nm), BSA/FITC (1 nm); or with control BSA/FITC (Sigma-Aldrich) (1 nm) for 1 h at room temperature. The cells were washed three times with 2 ml of PBS, washed once with distilled water, and mounted on a glass slide with Immu-Mount (Pierce, Thermo Scientific) mounting medium. The images were obtained with an Olympus BX61 confocal microscope (Minneapolis, MN) (×60 objective).
Indirect ELISA Using Purified TRPM8
Microtiter polystyrene plates (96-well; Evergreen Scientific, Los Angeles, CA) were directly coated with the purified TRPM8 or TRPV1 proteins (78, 156, or 625 ng in 100-μl well) in bicarbonate/carbonate (100 mm) coating buffer at 37 °C for 2 h and overnight at 4 °C. Wells were then blocked by incubation with 1% (w/v) gelatin in PBS. After further washing, agonist/antagonists (200 nm testosterone, 200 nm DHT, 1 μm M8-B, 50 μm menthol, 10 μm icilin) at various dilutions in PBS were added to the plates for 15 min at 37 °C. Next, the wells only containing M8-B/menthol/icilin were replaced with testosterone/DHT without washing, and the plate was incubated for 1 h at 37 °C. Binding of testosterone/DHT was then detected using a sheep polyclonal anti- testosterone/DHT antibody (Pierce, Thermo Scientific) diluted 1:500 in 1% gelatin in PBS, followed by a peroxidase-conjugated rabbit anti-sheep IgG (diluted 1:2000 in 1% gelatin in PBS). Incubations were performed at 37 °C for 1 h. After each step, the wells were washed extensively with PBS containing 0.05% (v/v) Tween 20 (PBS-T). Wells with the purified protein plus anti-sheep IgG and protein plus substrate only served as negative controls. Bound peroxidase was detected with 3,3′,5,5′-tetramethylbenzidine chromogenic substrate (Sigma-Aldrich), the blue color developed was stopped using 2 m H2SO4 stop solution, and the absorbance was read at 450 nm.
Whole-cell Patch Clamp Recordings
The whole-cell patch clamp experiments were performed as described previously (13, 17). The standard extracellular solution used in experiments contained 137 mm NaCl, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, and 10 mm glucose, pH 7.4 (adjusted with NaOH). The pipette (intracellular) solution contained 140 mm potassium gluconate, 5 mm EGTA, 1 mm MgCl2, 2 mm Na-ATP, and 10 mm HEPES, pH 7.3 (adjusted with KOH). Patch pipettes were pulled from borosilicate glass capillaries (1.5-mm outer diameter; WPI) on a PIP5 pipette puller (HEKA), and pipette resistance was 3–5 megaohms when filled with pipette solution. After formation of gigaohm resistance seals, the whole-cell configuration was established, and currents were measured at a holding potential of −60 mV using an Axopatch 200B amplifier (Axon Instruments), and 800-ms duration voltage ramps from −100 to +100 mV were delivered at desired time points. Currents were low pass-filtered at 2 kHz and digitized using a Digidata 1322A unit (Axon Instruments). No series resistance compensation was performed. All recordings were performed at room temperature (24–25 °C). Data were collected and analyzed with pCLAMP and further analyzed and plotted with Origin version 9.0 (Microcal Software Inc., Northampton, MA).
Intracellular Ca2+ Measurements
The extracellular solution used in ratiometric [Ca2+]i measurements contained 137 mm NaCl, 5 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm Hepes, pH 7.4. Cells were incubated with 2 μm Fura-2 acetoxymethyl ester (Tef Labs, Austin, TX) for 30 min at room temperature. The fluorescence signals of the cells grown on the coverslips were measured using alternating excitation at 340 and 380 nm, and emission was detected at 510 nm. The ratio of fluorescence (340 nm/380 nm) was plotted against time. The obtained values of ratios from each coverslips were first analyzed, and then the mean values of stimuli-induced signals were combined, and statistically averaged values with mean errors were plotted in the summary graphs. The total numbers of measurements/coverslips (n) are indicated in the figure legends. The measurements were performed using a Photon Technology International (Birmingham, NJ) imaging system mounted on a Zeiss-AXIO Observed D1 microscope, equipped with a DeltaRAM excitation light source or with a Ratiomaster 5 imaging system (Photon Technology International) equipped with a Cool-snap HQ2 (Roper) camera.
Statistical Analysis
Statistical analysis was performed using Origin version 9.0 software (Microcal Software Inc.). Statistical significance was calculated using one-way analysis of variance followed by Fisher's least significant differences test, and data were expressed as mean ± S.E.
RESULTS
Biochemical Evidence for the Binding of Testosterone to TRPM8
TRPM8 was initially found in prostate cells (7). However, no specific function was recognized for this channel in cells other than excitatory neurons. We sought to determine the biological role of TRPM8 in the prostate and utilized immunodetection probes to visualize the channel and its interaction network.
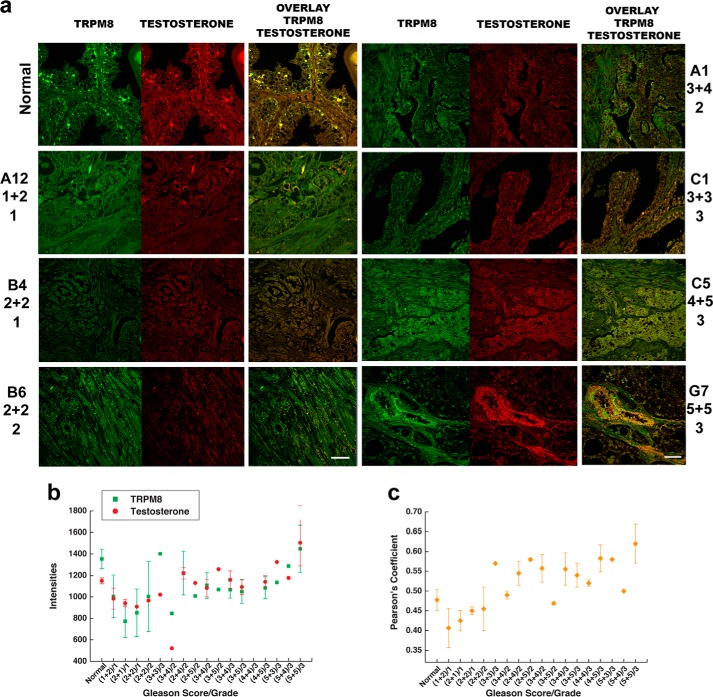
Immunohistochemistry experiments revealed high levels of TRPM8 in human prostate tissues from both healthy individuals and patients with prostate cancer (stages 1–4) (Fig. 1, TRPM8 column), which is consistent with previous reports (8, 18–20). However, the concurrent labeling of TRPM8 and androgens resulted in surprising observations. In all of the tested prostate tissue samples, the TRPM8 protein and endogenous testosterone were highly colocalized, indicating plausible interactions (Fig. 1, a–c; additional and enlarged images are shown in Fig. 2, a–f).
FIGURE 1.
Testosterone colocalization with the TRPM8 protein in human prostate tissues. Shown is immunohistochemistry analysis of a prostate cancer tissue microarray containing 60 cases (180 cores) of prostate adenocarcinoma (grades 1–4) and nine cases (27 cores) of normal prostate tissues using Alexa Fluor anti-TRPM8 (green) and anti-testosterone/DHT (red) secondary antibodies, as described under “Experimental Procedures.” Microscopic examination was performed (Zeiss LSM 510 upright confocal microscope) at ×40 magnification. a, immunohistochemical detection of the TRPM8 protein and testosterone obtained from the tissue array. b, relative intensities obtained for the TRPM8 protein (green) and testosterone/DHT (red). The values were averaged from the samples obtained from the same patients and from several regions within one sample because prostate cancer tissues are highly heterologous. The Pearson coefficients are plotted against the Gleason score/grade in c. The Pearson coefficient was calculated in ImageJ using the colocalization finder plugin to evaluate the relative colocalization pattern between TRPM8 and testosterone. Scale bar, 20 μm. Error bars, S.E.
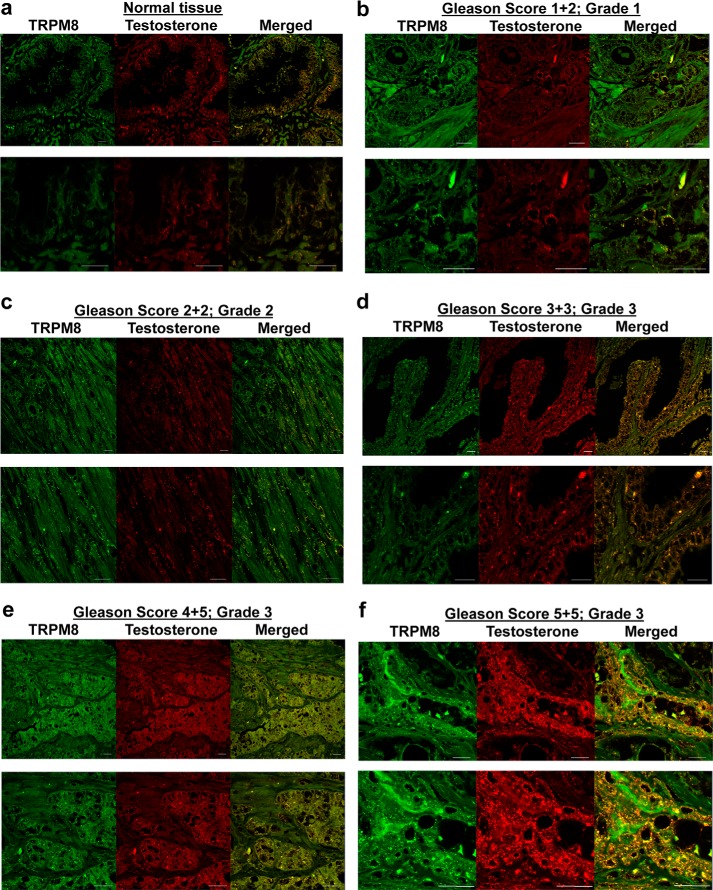
FIGURE 2.
Testosterone colocalization with TRPM8 in human prostate tissues. Images additional to and enlarged from those presented in Fig. 1 show immunohistochemistry analysis of the prostate cancer tissue microarray (US Biomax) containing 60 cases (180 cores) of prostate adenocarcinoma (grades 1–4) and nine cases (27 cores) of normal prostate tissues using Alexa Fluor anti-TRPM8 (green) and anti-testosterone/DHT (red) secondary antibodies as described under “Experimental Procedures.” Microscopic examination was done (Zeiss LSM 510 upright confocal microscope) at ×40 magnification. a, representative images obtained from normal prostate; b, prostate cancer tissues (Gleason score 1 + 2, grade 1); c, Gleason score 2 + 2, grade 2; d, Gleason score 3 + 3, grade 3; e, Gleason score 4 + 5, grade 3; f, Gleason score 5 + 5, grade 3. The bottom panels are enlarged images of the top panels. Scale bars, 20 μm.
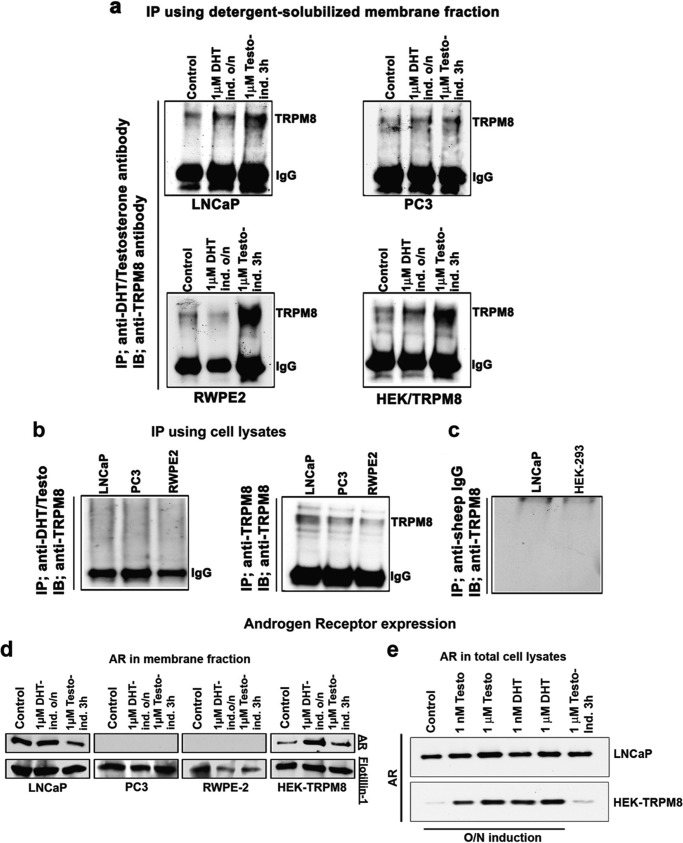
To determine the specificity of the TRPM8-testosterone correlation observed in the prostate tissue samples, we next performed IP experiments. Numerous sets of IP probes were applied to various cell lines, including the prostate cancer cells LNCaP and PC3, the prostate epithelial cell line RWPE-2, and, as a control, HEK-293 cells stably expressing TRPM8. IP with anti-DHT/testosterone IgG resulted in high levels of TRPM8 precipitation from all of the tested cell lines (Fig. 3a). Incubation of the cells with testosterone (1 μm) for 3 h resulted in higher levels of the precipitated TRPM8 protein in RWPE-2 and LNCaP cells, whereas the effect of DHT (1 μm) was more evident after overnight incubation (Fig. 3a), which may also account for the genomic induction of TRPM8 expression. Importantly, TRPM8 precipitation was achieved only from the membrane fractions solubilized in detergent and not the total cell lysates (Fig. 3, a and b). The membranes were solubilized overnight in the presence of Nonidet P-40 (0.1%) and dodecylmaltoside (0.5%), under similar conditions used for the TRPM8 protein purification (21, 22). This observation suggests that accessibility of the TRPM8-testosterone complex to the antibody can be achieved with the protein solubilized in detergent.
FIGURE 3.
Testosterone directly binds to the TRPM8 protein. Shown are IP and immunoblot (IB) analysis using the membrane extracts of LNCaP, PC3, RWPE-2, and HEK-TRPM8 control and treated (DHT and testosterone) cells. a, IP was performed using the detergent-solubilized fractions, containing 0.1% Nonidet P-40 and 0.5% dodecylmaltoside. The membrane fractions were obtained from the epithelial prostate cells RWPE-2, prostate cancer cells PC3 and LNCaP, and HEK-293 stably expressing TRPM8. To extract protein into detergent micelles, the membrane fractions were incubated overnight at 4 °C on a shaker. IP was performed using a sheep anti-testosterone/DHT antibody (Fisher), and the eluate was immunoprobed using a rabbit anti-TRPM8 antibody to show the association of testosterone/DHT with TRPM8 as described under “Experimental Procedures.” The protein band that corresponds to TRPM8 monomer was detected at ∼130 kDa. IgG denotes the anti-IgG heavy chain antibody control in the IP assays. b, IP performed on the total cell lysates did not result in detectable levels of TRPM8 with testosterone. c, IP was performed using negative control anti-sheep IgG, and the immunoprecipitated proteins were immunoblotted against anti-TRPM8 IgG with the secondary anti-rabbit antibody. d, the immunoblots from the membrane fractions were developed using antibodies specific for the AR; flotillin was used as a loading control for detergent-solubilized membrane fractions. e, AR in total cell lysates of LNCaP and HEK-293 cells after androgen treatment: Western blot analysis for the AR protein using 40 μg of total cell lysates from control-, testosterone-, and DHT-treated LNCaP and HEK-293 cells stably expressing TRPM8. The blots are representatives from three independent experiments. O/N, overnight.
In this assay, the cell membrane lysates were also analyzed for the presence of the classic AR protein (Fig. 3d). In LNCaP cells, the AR levels were unchanged after overnight incubation with DHT but were lower after the testosterone treatment, possibly due to a competitive binding of testosterone to TRPM8 in the membrane fraction. The PC3 and RWPE-2 cell lines both had no androgen receptor, which is in agreement with the literature (8). Surprisingly, HEK-293 cells showed positive staining for AR, which has not been shown previously (Fig. 3d). The total cell lysates of LNCaP cells and HEK-293 cells stably expressing TRPM8 demonstrated dose-dependent increase in the AR levels upon overnight induction with both the steroids (Fig. 3e).
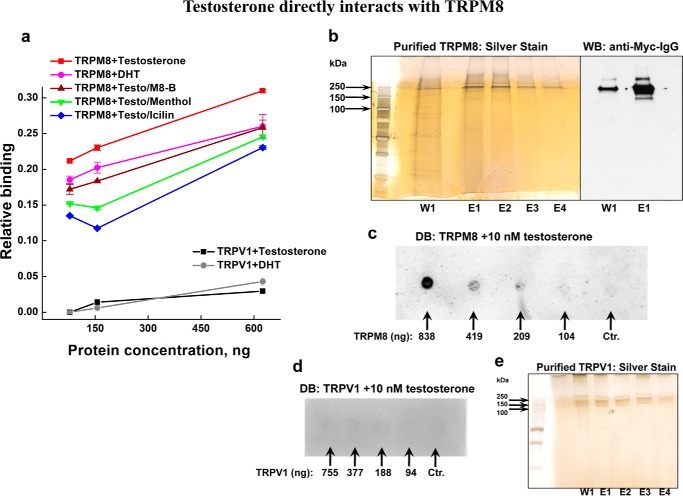
Next, to confirm that testosterone directly binds TRPM8, we performed ELISA experiments using the purified TRPM8 protein. Various binding assays for testosterone-TRPM8 were assembled, where TRPM8 was incubated with testosterone alone or in the presence of the agonist menthol or icilin or TRPM8 inhibitor M8-B (Fig. 4a). In this experiment, we detected direct interaction of TRPM8 with testosterone, which was lower with DHT and also was reduced when the channel was preincubated with either its agonists or antagonist, indicating either competition for the same binding site or some degree of allostery (Fig. 4a). The silver staining and Western blot of the purified TRPM8 used for ELISA are shown in Fig. 4b.
FIGURE 4.
Direct interaction between the purified TRPM8 protein and testosterone in the presence or absence of the TRPM8-specific inhibitor M8-B and the TRPM8 agonists menthol and icilin. a, ELISA plates coated with TRPM8 or TRPV1 (78, 156, or 625 ng/well) were incubated with various TRPM8 agonist/antagonists (200 nm testosterone, 200 nm DHT, 1 μm M8-B, 50 μm menthol, or 10 μm icilin) at various dilutions in PBS as mentioned under “Experimental Procedures.” After washing, binding of testosterone or DHT to immobilized TRPM8 or TRPV1 was detected with a sheep polyclonal anti-testosterone/DHT antibody. The binding of testosterone/DHT to TRPM8 and TRPV1 is represented graphically. The negative control anti-IgG did not show any binding to immobilized TRPM8 and TRPV1. The OD values were deducted from the test values. b, silver staining and Western blot (WB) of the purified TRPM8 samples. TRPM8 protein was eluted with NCB buffer containing Myc peptide (50 μg/ml), 0.1% Nonidet P-40, and 0.03% LMNG. TRPM8 shows high stability in the presence of LMNG, and even after incubating with the SDS-loading buffer for 10 min at 95 °C, it runs on the SDS-gel preferably in the form of dimer. The silver-stained gel demonstrates wash 1 (W1), done with NCB/Nonidet P-40/LMNG buffer; eluates 1–4 (E1–E4) were obtained with the NCB/Nonidet P-40/LMNG elution buffer containing Myc peptide. c, dot blot (DB) was done on the purified TRPM8 protein treated with 10 nm testosterone. 2 μl of TRPM8 (in various concentrations, indicated on the blot) diluted with the NCB elution buffer containing the same detergent concentration were applied onto the nitrocellulose membrane, dried for 30 min at 37 °C, and then incubated with 10 nm testosterone in 3% BSA-PBS buffer for 1 h at 37 °C. The blot was then developed with anti-DHT/testosterone IgG. The control sample contained NCB elution buffer, with all of the additives and detergents described in b. d, dot blot performed on the purified TRPV1 protein treated with 10 nm testosterone. The conditions are the same as described in c. 2 μl of TRPV1 were used in various dilutions done with the NCB elution buffer. The protein concentrations are indicated on the blot. The control sample represents NCB elution buffer with the same detergent composition. e, silver staining of TRPV1, obtained under the same purification conditions used for TRPM8, those described in b. Error bars, S.E.
To visualize direct binding of testosterone to TRPM8, we also performed dot blot on the purified TRPM8 protein treated with 10 nm testosterone and immunoblotted with anti-DHT/testosterone antibodies (Fig. 4c). The decrease of the testosterone signal with the decreased amount of TRPM8 further supports the specific interaction between TRPM8 and testosterone. As a control, we performed similar binding experiments on the vanilloid TRP channel representative, the heat and capsaicin receptor TRPV1. The TRPV1 protein was purified under the identical conditions and with the same detergent composition as used for TRPM8 (Fig. 4e). We found no appreciable binding of testosterone or DHT to TRPV1 channels as seen from the ELISA or dot blot probes (Fig. 4, a and d).
Alternatively, we performed immunocytochemistry experiments to visualize testosterone interaction with TRPM8. HEK-293 cells transiently expressing Myc-tagged TRPM8 were immunodetected with anti-Myc-antibody along with treatment of the cells with testosterone/BSA/FITC and control label BSA/FITC. Fig. 5 shows testosterone/BSA/FITC or BSA/FITC and TRPM8 labeling and their merged images. The signals from FITC were observed only in the presence of testosterone, and those were highly colocalized with TRPM8 (Fig. 5). Collectively, these results indicate a direct molecular interaction between testosterone and the TRPM8 protein.
FIGURE 5.
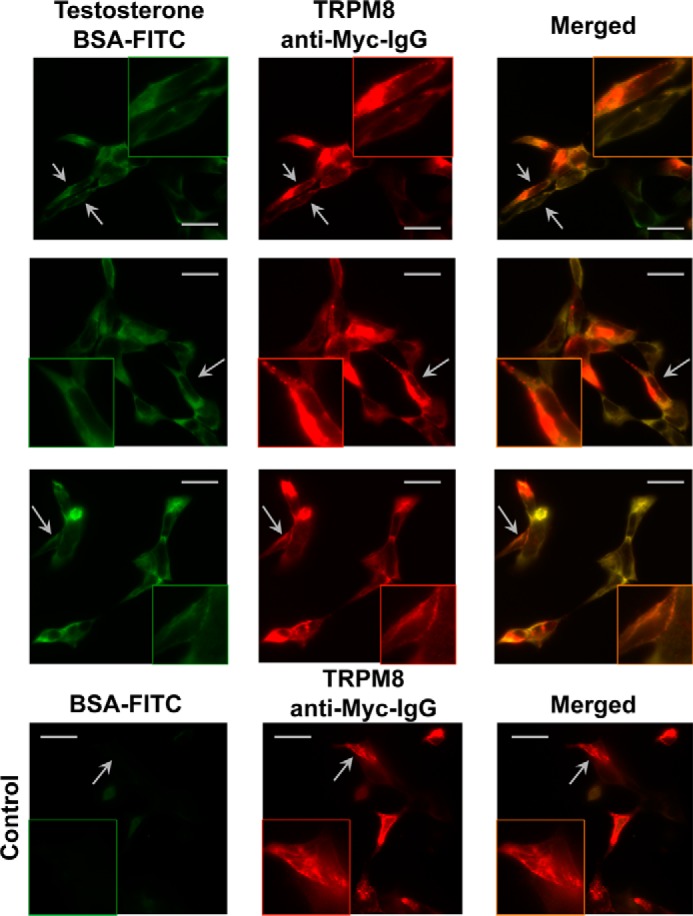
Immunocytochemistry experiments demonstrate TRPM8-testosterone interaction on HEK-293 cells. The top three rows show HEK-293 cells transiently expressing Myc-tagged TRPM8 treated with testosterone/BSA/FITC (testosterone, 10 nm; BSA/FITC, 1 nm). Cells expressing TRPM8 also show staining corresponding to testosterone/BSA/FITC and suggest TRPM8-testosterone interaction. BSA/FITC (1 nm) (bottom row) was used as a control and demonstrates no staining on TRPM8. The images were obtained with an Olympus BX61 confocal microscope (Minneapolis, MN) (×60 objective). Scale bars, 10 μm.
Testosterone Activates TRPM8 Channels in the Primary Human Prostate Cells
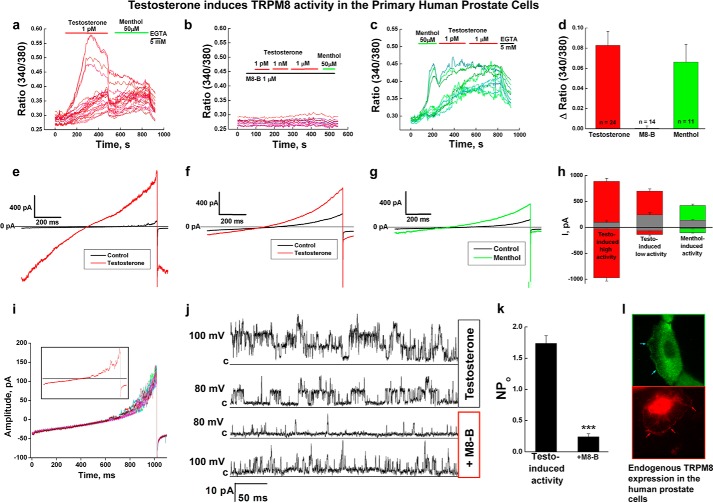
Direct testosterone binding to TRPM8 led us to investigate what effect this steroid hormone exerts on the channel function. To evaluate this interaction, we tested endogenous TRPM8 responses upon acute testosterone application to the primary human prostate epithelial cells.
Initially, the cells were recovered from cryopreservation by culturing in the prostate epithelial basal medium. During the first week of cultivation, the cells exhibited low basal Ca2+ levels and were irresponsive to the TRPM8 agonist menthol, as judged by the Ca2+-imaging experiments (data not shown). In the second week of cultivation, the primary prostate cells developed a consistent phenotype and showed Ca2+ uptake upon induction with both testosterone and menthol, which was inhibited by preapplication of TRPM8 inhibitor, N-(2-aminoethyl)-N-(4-(benzyloxy)-3-methoxybenzyl)thiophene-2-carboxamide hydrochloride, M8-B (1 μm) (Fig. 6, a–d).
FIGURE 6.
TRPM8 channel activity in the primary human prostate epithelial cells. a, testosterone-induced (1 pm) Ca2+ uptake observed in the primary human prostate cells (n = 5). b, preapplication of the TRPM8 antagonist M8-B (1 μm) inhibits testosterone- and menthol-induced activation (n = 8). c, menthol-induced (50 μm) Ca2+ uptake. d, summary of the testosterone- and menthol-induced responses of endogenous TRPM8 channels in the prostate cells. e–i, whole-cell patch clamp experiments. e, testosterone-induced (50 nm) high amplitude channel activity, voltage ramp from −100 to 100 mV (n = 3); f, testosterone-induced (50 nm) low amplitude channel activity, voltage ramp from −100 to 100 mV (n = 4); g, menthol-induced (500 μm) channel activity, voltage ramp from −100 to 100 mV (n = 3); h, bar graph summary of the testosterone- and menthol-induced responses (values of currents are taken at −100 and 100 mV); i, basal channel activity observed in the voltage ramp recordings obtained from −100 to 100 mV. j and k, whole-cell configuration recording where single channel events were observed in the presence of testosterone (50 nm) at 100 and 80 mV. The subsequent addition of TRPM8 antagonist M8-B (8 μm) inhibited the channel openings. j, representative current traces; k, summary of the NPo before (number of events analyzed = 17,456) and after the addition of M8-B (number of events analyzed = 5204) (error bars, S.E.). l, immunocytochemistry experiments show endogenous TRPM8 channel expression on the plasma and endoplasmic reticulum membranes. The protein was detected with TRPM8 antibody and with secondary antibody Alexa-488 (green) or Alexa-594 (red) to choose better resolution. The images were obtained using an Olympus-BX61 confocal microscope with a ×60 objective. Error bars, S.E.
Similar to Ca2+ imaging, whole-cell patch clamp experiments demonstrated testosterone- and menthol-induced channel activities (Fig. 6, e–i). In the absence of the agonists, we observed basal current, which is characteristic of TRPM8 channel activity as seen in whole-cell patch clamp experiments with transfected model cells (22). Specifically, the channel was closed at negative voltages and was activated at positive voltages (see black traces in Fig. 6, e–g and i). The typical maximal integral conductance measured at 100 mV varied from 50 pA to several hundred pA and probably reflects heterogeneity of the cell population. We observed two different patterns of activation with testosterone. One type of activation exhibited high current amplitude and lack of rectification, when the channel was active at all voltages (Fig. 6e). Another type exhibited outwardly rectifying currents of lower magnitude; however, their basal current was notably larger, in comparison with the first type (Fig. 6, f and h). Menthol-induced currents demonstrated typical for TRPM8 outward rectification, further confirming that the channel activity observed in our experiments is related to TRPM8. In some experiments, the current density was sufficiently low to allow us to resolve single channel events even in the whole-cell configuration (Fig. 6, i and j). This testosterone-evoked channel activity was inhibited with TRPM8 inhibitor M8-B (Fig. 6, j and k). TRPM8 channel localization was evident in the plasma membrane and endoplasmic reticulum membranes of the primary prostate cells (Fig. 6l).
Despite the fact that the basal channel behavior was similar in all experiments, the channel activation evoked by testosterone or menthol was detected only in about half of the cases. As detailed in the accompanying study (25), we hypothesize that this is due to complex regulation of TRPM8 activity in cellular systems.
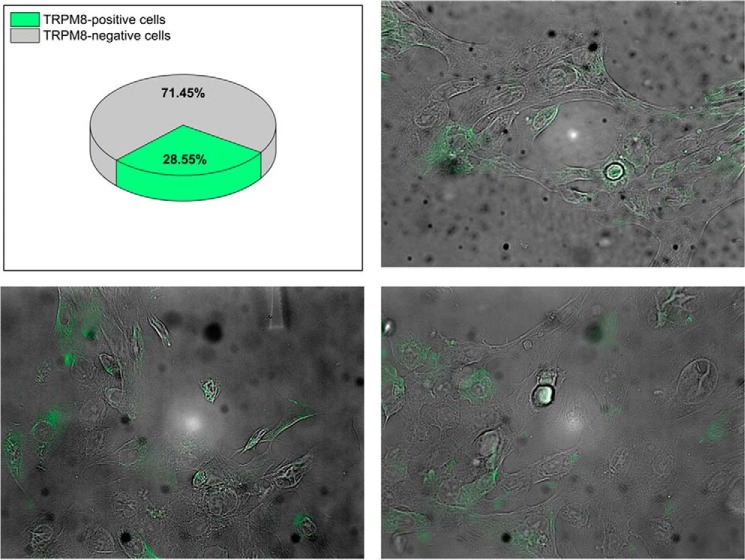
Furthermore, we performed immunocytochemistry analysis to estimate what fraction of the primary prostate cells expressed TRPM8. We found that TRPM8 was expressed in about 29% of the primary prostate cells on the second week of cultivation after the recovery from cryopreservation (Fig. 7). This observation indicates that the channel expression is only partially recovered and may explain the lack or the low current density in some cells tested by patch clamp experiments. Together, these experiments indicate that testosterone exerts an ionotropic effect on the endogenous TRPM8 channels.
FIGURE 7.
Endogenous TRPM8 expression in the primary human prostate cells. Immunocytochemistry experiments demonstrate expression of the endogenous TRPM8 protein in about 29% of the cells in the second week of cultivation after the recovery from cryopreservation. The protein was detected with anti-TRPM8 antibody.
DISCUSSION
TRPM8 has initially been found and cloned from human prostate cells. This channel is highly expressed in the prostate tissues of healthy individuals and in prostate cancer patients (7). It was previously demonstrated that during prostate cancer, levels of TRPM8 mRNA are elevated, and those correlated with various tumor stages; thus, TRPM8 protein was considered as a diagnostic marker for the disease (23, 24). However, the role of this channel in the prostate gland and in prostate cancer remained unclear.
Here we studied TRPM8 cellular patterns and distribution in the human prostate cells. The immunohistochemistry experiments resulted in detecting similar localization patterns for TRPM8 and endogenous androgens. High levels of colocalization, observed in human prostate tissues, indicated that TRPM8 and testosterone are probably engaged in interactions, whether they take place on the plasma membrane of the prostate periphery or on the endoplasmic reticulum membranes in the lumen cells (Fig. 1, a–c). The IP experiments further supported our hypothesis for direct molecular interaction between TRPM8 and testosterone (Fig. 3), which was further confirmed by the ELISA showing that testosterone directly binds to the purified TRPM8 protein (Fig. 4a).
A novel role of TRPM8 as a membrane testosterone receptor could have several physiological implications. As seen from the cellular responses, observed in the primary human epithelial cells endogenously expressing TRPM8, testosterone elicits Ca2+ uptake and induces channel current (Fig. 6). This indicates that testosterone exerts an ionotropic effect on TRPM8 channels (also see the accompanying article (25)). Because TRPM8 channels have relatively high selectivity for Ca2+ and little selectivity among monovalent cations (5), our finding suggests that testosterone-induced TRPM8 could be an important regulator of Ca2+ homeostasis in prostate cells. In this view, TRPM8 would be an important channel to control cell cycling, including growth, proliferation, and apoptosis. Endogenous androgen level adaptation, desensitization phenomena, and competitive binding to other androgen-related proteins (25) could trigger the TRPM8 channel activation/inactivation mechanism. Furthermore, down-regulation of plasma membrane TRPM8 activity could have an impact on cancer development. Further analysis of testosterone-dependent TRPM8 regulation may reveal valuable information and strategies for the development of novel therapeutic agents and treatment of prostate cancer.
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM098052 (to E. Z.). This work was also supported by Heart and Stroke Foundation of Canada Grant G-13-0003008 (to E. P.) and by the pilot project support from the National Institutes of Health Grant 8P20GM103432-12 (to B. T.).
- AR
- androgen receptor
- LMNG
- lauryl maltose neopentyl glycol
- IP
- immunoprecipitation
- DHT
- 5-α-dihydrotestosterone
- M8-B
- N-(2-aminoethyl)-N-(4-(benzyloxy)-3-methoxybenzyl)thiophene-2-carboxamide hydrochloride.
REFERENCES
- 1. Bautista D. M., Siemens J., Glazer J. M., Tsuruda P. R., Basbaum A. I., Stucky C. L., Jordt S.-E., Julius D. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 [DOI] [PubMed] [Google Scholar]
- 2. Dhaka A., Murray A. N., Mathur J., Earley T. J., Petrus M. J., Patapoutian A. (2007) TRPM8 is required for cold sensation in mice. Neuron 54, 371–378 [DOI] [PubMed] [Google Scholar]
- 3. Colburn R. W., Lubin M. L., Stone D. J., Jr., Wang Y., Lawrence D., D'Andrea M. R., Brandt M. R., Liu Y., Flores C. M., Qin N. (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386 [DOI] [PubMed] [Google Scholar]
- 4. Behrendt H. J., Germann T., Gillen C., Hatt H., Jostock R. (2004) Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 141, 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKemy D. D., Neuhausser W. M., Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 [DOI] [PubMed] [Google Scholar]
- 6. Peier A. M., Moqrich A., Hergarden A. C., Reeve A. J., Andersson D. A., Story G. M., Earley T. J., Dragoni I., McIntyre P., Bevan S., Patapoutian A. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 [DOI] [PubMed] [Google Scholar]
- 7. Tsavaler L., Shapero M. H., Morkowski S., Laus R. (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 61, 3760–3769 [PubMed] [Google Scholar]
- 8. Bidaux G., Flourakis M., Thebault S., Zholos A., Beck B., Gkika D., Roudbaraki M., Bonnal J.-L., Mauroy B., Shuba Y., Skryma R., Prevarskaya N. (2007) Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J. Clin. Invest. 117, 1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgentaler A. (2006) Testosterone and prostate cancer: a historical perspective on a modern myth. Eur. Urol. 50, 935–939 [DOI] [PubMed] [Google Scholar]
- 10. Beato M., Chávez S., Truss M. (1996) Transcriptional regulation by steroid hormones. Steroids 61, 240–251 [DOI] [PubMed] [Google Scholar]
- 11. Bruck N., Bastien J., Bour G., Tarrade A., Plassat J. L., Bauer A., Adam-Stitah S., Rochette-Egly C. (2005) Phosphorylation of the retinoid X receptor at the ω loop, modulates the expression of retinoic-acid-target genes with a promoter context specificity. Cell. Signal. 17, 1229–1239 [DOI] [PubMed] [Google Scholar]
- 12. Wierman M. E. (2007) Sex steroid effects at target tissues: mechanisms of action. Adv. Physiol. Educ. 31, 26–33 [DOI] [PubMed] [Google Scholar]
- 13. Zakharian E., Thyagarajan B., French R. J., Pavlov E., Rohacs T. (2009) Inorganic polyphosphate modulates TRPM8 channels. PLoS One 4, e5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lukacs V., Rives J. M., Sun X., Zakharian E., Rohacs T. (2013) Promiscuous activation of transient receptor potential vanilloid 1 (TRPV1) channels by negatively charged intracellular lipids: the key role of endogenous phosphoinositides in maintaining channel activity. J. Biol. Chem. 288, 35003–35013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asuthkar S., Gondi C. S., Nalla A. K., Velpula K. K., Gorantla B., Rao J. S. (2012) Urokinase-type plasminogen activator receptor (uPAR)-mediated regulation of WNT/β-catenin signaling is enhanced in irradiated medulloblastoma cells. J. Biol. Chem. 287, 20576–20589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asuthkar S., Velpula K. K., Nalla A. K., Gogineni V. R., Gondi C. S., Rao J. S. (2014) Irradiation-induced angiogenesis is associated with an MMP-9-miR-494-syndecan-1 regulatory loop in medulloblastoma cells. Oncogene 33, 1922–1933 [DOI] [PubMed] [Google Scholar]
- 17. Yudin Y., Lukacs V., Cao C., Rohacs T. (2011) Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J. Physiol. 589, 6007–6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bidaux G., Roudbaraki M., Merle C., Crépin A., Delcourt P., Slomianny C., Thebault S., Bonnal J. L., Benahmed M., Cabon F., Mauroy B., Prevarskaya N. (2005) Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr. Relat. Cancer 12, 367–382 [DOI] [PubMed] [Google Scholar]
- 19. Henshall S. M., Afar D. E., Hiller J., Horvath L. G., Quinn D. I., Rasiah K. K., Gish K., Willhite D., Kench J. G., Gardiner-Garden M., Stricker P. D., Scher H. I., Grygiel J. J., Agus D. B., Mack D. H., Sutherland R. L. (2003) Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 63, 4196–4203 [PubMed] [Google Scholar]
- 20. Zhang L., Barritt G. J. (2004) Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 64, 8365–8373 [DOI] [PubMed] [Google Scholar]
- 21. Zakharian E., Cao C., Rohacs T. (2010) Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 30, 12526–12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao C., Yudin Y., Bikard Y., Chen W., Liu T., Li H., Jendrossek D., Cohen A., Pavlov E., Rohacs T., Zakharian E. (2013) Polyester modification of the mammalian TRPM8 channel protein: implications for structure and function. Cell Rep. 4, 302–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuessel S., Sickert D., Meye A., Klenk U., Schmidt U., Schmitz M., Rost A. K., Weigle B., Kiessling A., Wirth M. P. (2003) Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int. J. Oncol. 23, 221–228 [PubMed] [Google Scholar]
- 24. Kiessling A., Füssel S., Schmitz M., Stevanovic S., Meye A., Weigle B., Klenk U., Wirth M. P., Rieber E. P. (2003) Identification of an HLA-A*0201-restricted T-cell epitope derived from the prostate cancer-associated protein trp-p8. Prostate 56, 270–279 [DOI] [PubMed] [Google Scholar]
- 25. Asuthkar S., Demirkhanyan L., Sun X., Elustondo P., Krishnan V., Baskaran P., Velpula K. K., Thyagarajan B., Pavlov E., Zakarian E. (December 5, 2015) The TRPM8 protein Is a testosterone receptor. II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 290, 2670–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]