Abstract
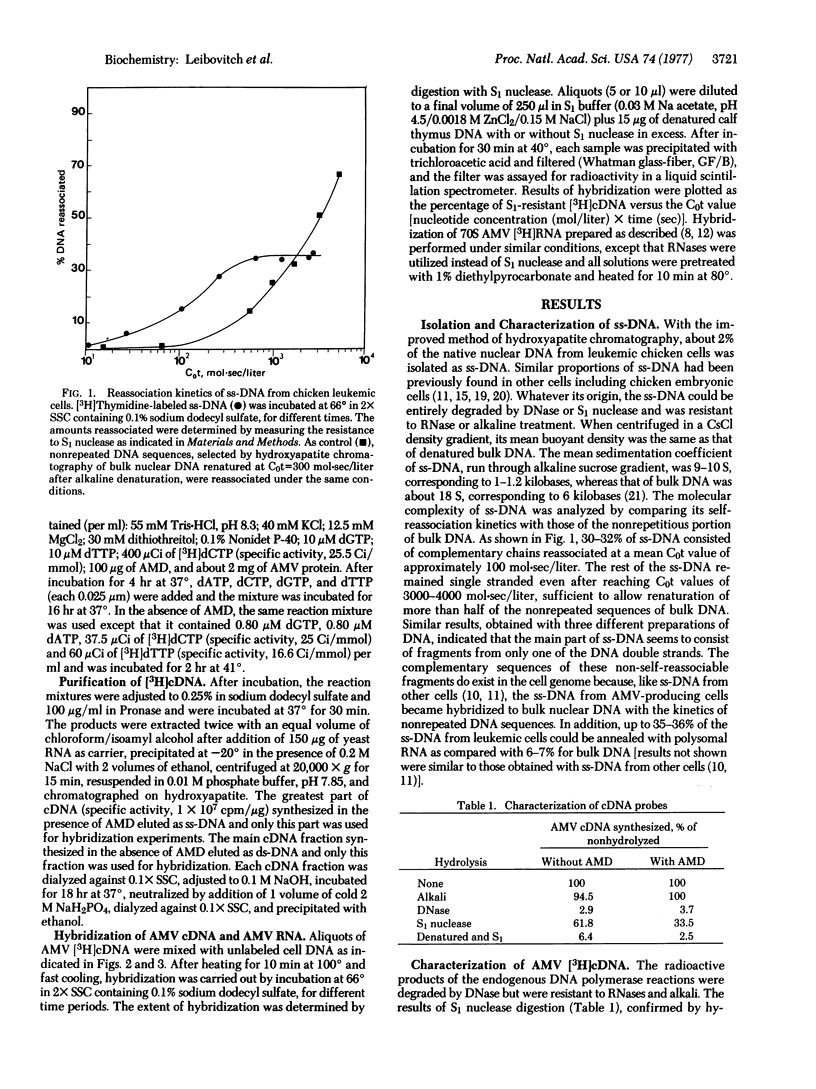
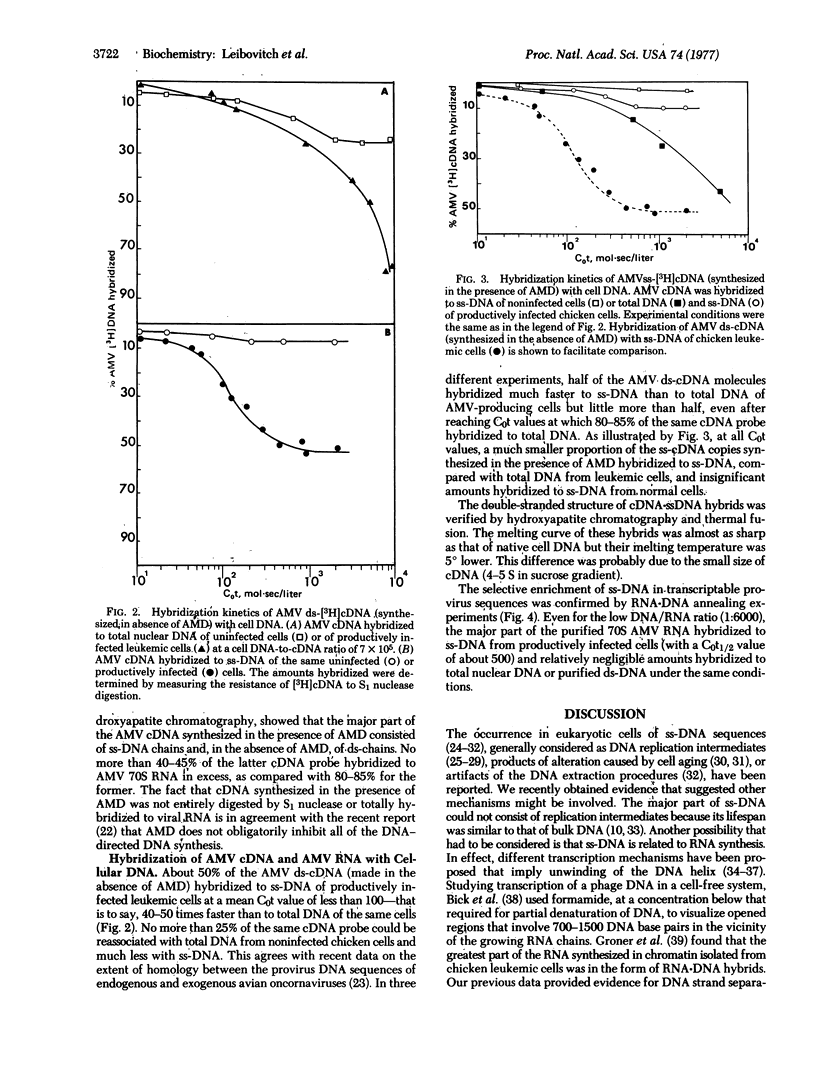
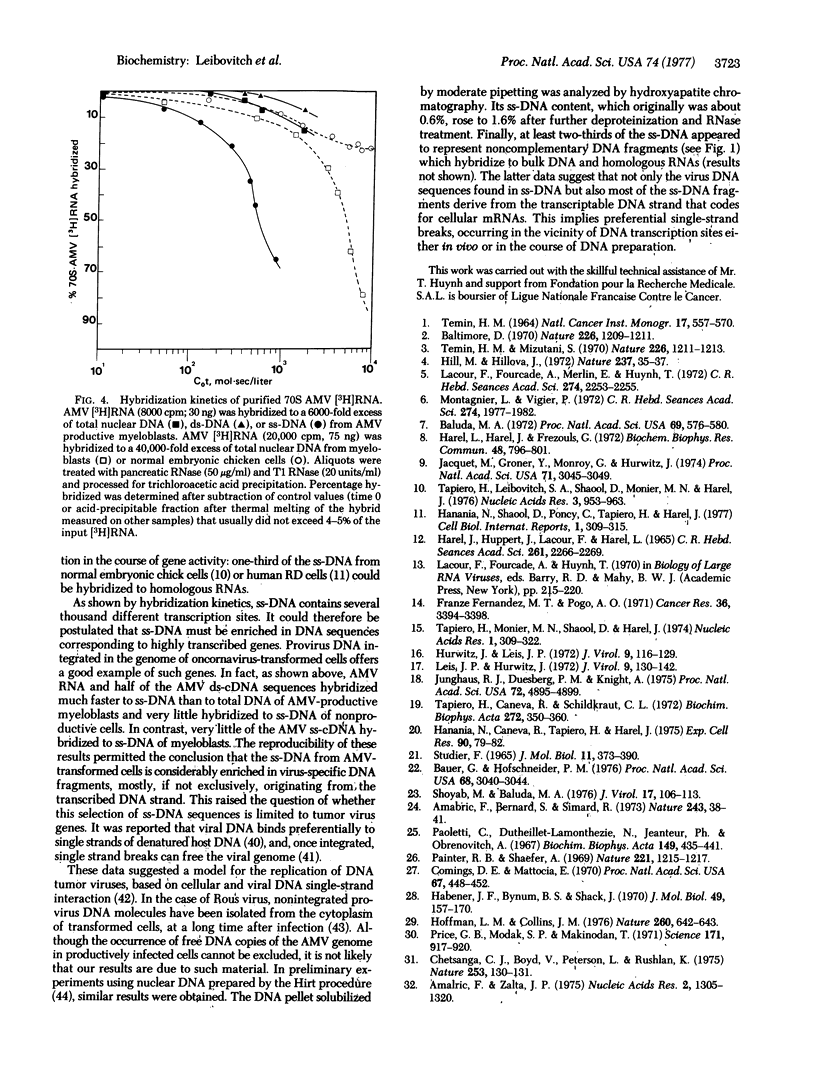
We previously found that a minor fraction of single-stranded DNA (ss-DNA) isolated from native nuclear DNA of normal chicken embryonic cells and cells of other species hybridized with bulk nuclear DNA or cellular RNA in great excess. At least one-third of ss-DNA belonging to the nonrepetitious part of the cell genome could be hybridized to homologous RNAs. In the present work, similar results were obtained with ss-DNA from cells of chickens infected by avian myeloblastosis virus (AMV). To investigate whether this enrichment of ss-DNA in transcribed DNA sequences involves provirus DNA, radioactive AMV RNA and cDNA copies of AMV RNA were used. Most of the 70S AMV RNA hybridized much faster to ss-DNA from productively infected leukemic cells than to bulk DNA. cDNA, either double-stranded or single-stranded, made in the presence of actinomycin D hybridized to total nuclear DNA with similar kinetics. In contrast, about half of the double-stranded cDNA molecules hybridized 40-50 times faster to ss-DNA than to total DNA, indicating that only one of the provirus DNA strands seems to be present in ss-DNA. This was confirmed by the fact that relatively insignificant amounts of the ss-cDNA molecules made in the presence of actinomycin D could be annealed to ss-DNA as compared with bulk DNA. These results indicate that actively transcribed DNA sequences can be selectively distributed in the ss-DNA fraction, probably because of single strand breaks in the vicinity of transcription sites.
Keywords: hydroxyapatite chromatography, avian myeloblastosis virus, molecular hybridization, provirus DNA transcription
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric F., Bernard S., Simard R. Detection of single-stranded DNA in the nucleolus. Nat New Biol. 1973 May 9;243(123):38–41. [PubMed] [Google Scholar]
- Amalric F., Zalta J. P. Rat hepatoma cells nucleolar DNA. 2. Analysis of nucleolar DNA. Nucleic Acids Res. 1975 Aug;2(8):1305–1320. doi: 10.1093/nar/2.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick M. D., Lee C. S., Thomas C. A., Jr Local destabilization of DNA during transcription. J Mol Biol. 1972 Oct 28;71(1):1–9. doi: 10.1016/0022-2836(72)90395-6. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BALDWIN R. L., BERG P. AN ENZYMICALLY SYNTHESIZED RNA OF ALTERNATING BASE SEQUENCE: PHYSICAL AND CHEMICAL CHARACTERIZATION. J Mol Biol. 1963 Oct;7:334–349. doi: 10.1016/s0022-2836(63)80028-5. [DOI] [PubMed] [Google Scholar]
- Chetsanga C. J., Boyd V., Peterson L., Rushlow K. Single-stranded regions in DNA of old mice. Nature. 1975 Jan 10;253(5487):130–131. doi: 10.1038/253130a0. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Mattoccia E. Replication of repetitious DNA and the S period. Proc Natl Acad Sci U S A. 1970 Sep;67(1):448–455. doi: 10.1073/pnas.67.1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenster J. H. Selective control of DNA helix openings during gene regulation. Cancer Res. 1976 Sep;36(9 Pt 2):3394–3398. [PubMed] [Google Scholar]
- Groner Y., Monroy G., Jacquet M., Hurwitz J. Chromatin as a template for RNA synthesis in vitro. Proc Natl Acad Sci U S A. 1975 Jan;72(1):194–199. doi: 10.1073/pnas.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Bynum B. S., Shack J. Destabilized secondary structure of newly replicated HeLa DNA. J Mol Biol. 1970 Apr 14;49(1):157–170. doi: 10.1016/0022-2836(70)90383-9. [DOI] [PubMed] [Google Scholar]
- Hanania N., Caneva R., Tapiero H., Harel J. Distribution of repetitious DNA in randomly growing and synchronized Chinese hamster cells. Exp Cell Res. 1975 Jan;90(1):79–86. doi: 10.1016/0014-4827(75)90359-6. [DOI] [PubMed] [Google Scholar]
- Hanania N., Schaool D., Poncy C., Tapiero H., Harel J. Isolation of single stranded transcription sites from human nuclear DNA. Cell Biol Int Rep. 1977 Jul;1(4):309–315. doi: 10.1016/0309-1651(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Harel J., Huppert J., Lacour F., Harel L. Mise en évidence d'un acide ribonucléique de très haut poids moléculaire dans le virus de la myéloblastose aviaire. C R Acad Sci Hebd Seances Acad Sci D. 1965 Sep 13;261(11):2266–2268. [PubMed] [Google Scholar]
- Harel L., Harel J., Frezouls G. DNA copies of viral RNA in rat cells transformed by Rous sarcoma virus (RSV). Biochem Biophys Res Commun. 1972 Aug 21;48(4):796–801. doi: 10.1016/0006-291x(72)90677-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hoffman L. M., Collins J. M. Single-stranded regions in regenerating rat liver DNA. Nature. 1976 Apr 15;260(5552):642–643. doi: 10.1038/260642a0. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet M., Groner Y., Monroy G., Hurwitz J. The in vitro synthesis of avian myeloblastosis viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3045–3049. doi: 10.1073/pnas.71.8.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaganov Iu N., Zarudnaia M. I., Lazurkin Iu S., Frank-Kamenetskii M. D., Beabealashvilli R. S., Savochkina L. P. Local unwinding of DNA during RNA synthesis in vitro. Nat New Biol. 1971 Jun 16;231(24):212–214. doi: 10.1038/newbio231212a0. [DOI] [PubMed] [Google Scholar]
- Lacour F., Fourcade A., Merlin E., Huynh T. Détection de virus de la myéloblastose aviaire dans des cultures de fibroblastes de poule traités par de l'ADN de cellules leucémiques productrices de virus. C R Acad Sci Hebd Seances Acad Sci D. 1972 Apr 10;274(15):2253–2255. [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell B., von Hippel P. H. Hydrogen exchange as a probe of the dynamic structure of DNA. I. General acid-base catalysis. J Mol Biol. 1970 Jun 14;50(2):297–316. doi: 10.1016/0022-2836(70)90194-4. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Vigier P. Un intermediaire ADN infectieux et transformant du virus du sarcome de Rous dans le cellules de poule transformees par ce virus. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 27;274(13):1977–1980. [PubMed] [Google Scholar]
- Painter R. B., Schaefer A. State of newly synthesized HeLa DNA. Nature. 1969 Mar 29;221(5187):1215–1217. doi: 10.1038/2211215a0. [DOI] [PubMed] [Google Scholar]
- Paoletti C., Dutheillet-Lamonthézie N., Jeanteur P., Obrenovitch A. Mise en évidence et étude cinétique des formes replicatives du DNA dans les cellules animales. Biochim Biophys Acta. 1967 Dec 19;149(2):435–450. [PubMed] [Google Scholar]
- Price G. B., Modak S. P., Makinodan T. Age-associated changes in the DNA of mouse tissue. Science. 1971 Mar 5;171(3974):917–920. doi: 10.1126/science.171.3974.917. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Wang J. C. Angular alteration of the DNA helix by E. coli RNA polymerase. Nat New Biol. 1972 Oct 11;239(93):167–170. doi: 10.1038/newbio239167a0. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Ribonucleotide sequence homology among avian oncornaviruses. J Virol. 1975 Jan;17(1):106–113. doi: 10.1128/jvi.17.1.106-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nonoyama M. Latent DNA of Epstein-Barr virus: separation from high-molecular-weight cell DNA in a neutral glycerol gradient. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4658–4661. doi: 10.1073/pnas.71.12.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H., Caneva R., Schildkraut C. L. Fractions of Chinese hamster DNA differing in their content of guanine+cytosine and evidence for the presence of single-stranded DNA. Biochim Biophys Acta. 1972 Jul 20;272(3):350–360. doi: 10.1016/0005-2787(72)90388-7. [DOI] [PubMed] [Google Scholar]
- Tapiero H., Leibowitch S. A., Shaool D., Monier M. N., Harel J. Isolation of single stranded DNA related to the transcriptional activity of animal cells. Nucleic Acids Res. 1976 Apr;3(4):953–963. doi: 10.1093/nar/3.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H., Monier M. N., Shaool D., Harel J. Distribution of repetitious sequences in chick nuclear DNA. Nucleic Acids Res. 1974 Feb;1(2):309–322. doi: 10.1093/nar/1.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H., Shaool D., Monier M. N., Harel J. Replication of repetitious DNA in synchronized chick fibroblast cells. Exp Cell Res. 1974 Nov;89(1):39–46. doi: 10.1016/0014-4827(74)90184-0. [DOI] [PubMed] [Google Scholar]
- Teich N., Lowy D. R., Hartley J. W., Rowe W. P. Studies of the mechanism of induction of infectious murine leukemia virus from AKR mouse embryo cell lines by 5-iododeoxyuridine and 5-bromodeoxyuridine. Virology. 1973 Jan;51(1):163–173. doi: 10.1016/0042-6822(73)90376-0. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Shank P. R. Unintegrated viral DNA is synthesized in the cytoplasm of avian sarcoma virus-transformed duck cells by viral DNA polymerase. J Virol. 1976 May;18(2):567–573. doi: 10.1128/jvi.18.2.567-573.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


