Background: We investigated murine salivary Muc19, in vivo and in vitro, to protect against dental infections.
Results: Muc19 attenuates dental infections by Streptococcus mutans and promotes its aggregation. Evidence for human salivary MUC19 is presented.
Conclusion: Murine Muc19 helps limit colonization via clearance of S. mutans.
Significance: A role for Muc19 in clearance expands upon acknowledged innate immune functions of salivary gel-forming mucins.
Keywords: Aggregation, Animal Model, Glycobiology, Infectious Disease, Innate Immunity, Microbiome, Mucin, Streptococcus
Abstract
Saliva functions in innate immunity of the oral cavity, protecting against demineralization of teeth (i.e. dental caries), a highly prevalent infectious disease associated with Streptococcus mutans, a pathogen also linked to endocarditis and atheromatous plaques. Gel-forming mucins are a major constituent of saliva. Because Muc19 is the dominant salivary gel-forming mucin in mice, we studied Muc19−/− mice for changes in innate immune functions of saliva in interactions with S. mutans. When challenged with S. mutans and a cariogenic diet, total smooth and sulcal surface lesions are more than 2- and 1.6-fold higher in Muc19−/− mice compared with wild type, whereas the severity of lesions are up to 6- and 10-fold higher, respectively. Furthermore, the oral microbiota of Muc19−/− mice display higher levels of indigenous streptococci. Results emphasize the importance of a single salivary constituent in the innate immune functions of saliva. In vitro studies of S. mutans and Muc19 interactions (i.e. adherence, aggregation, and biofilm formation) demonstrate Muc19 poorly aggregates S. mutans. Nonetheless, aggregation is enhanced upon adding Muc19 to saliva from Muc19−/− mice, indicating Muc19 assists in bacterial clearance through formation of heterotypic complexes with salivary constituents that bind S. mutans, thus representing a novel innate immune function for salivary gel-forming mucins. In humans, expression of salivary MUC19 is unclear. We find MUC19 transcripts in salivary glands of seven subjects and demonstrate MUC19 glycoproteins in glandular mucous cells and saliva. Similarities and differences between mice and humans in the expression and functions of salivary gel-forming mucins are discussed.
Introduction
Demineralization of teeth (i.e. dental caries) resulting from the production of organic acids by bacteria within overlying biofilms (i.e. dental plaque) continues to be among the most prevalent infectious diseases in developed as well as developing nations (1, 2), particularly among under-privileged groups (3). Streptococcus mutans is highly associated with dental caries worldwide, but it is also linked to bacterial endocarditis and to atheromatous plaques (4, 5), indicating an interconnection between oral health and cardiovascular diseases. Hence, efforts to understand and treat oral diseases have potential ramifications to the development and progression of serious systemic disease.
Through a combination of innate and adaptive immune mechanisms, the oral cavity establishes a homeostasis with the commensal microbiota that is subject to disruption by changes in diet or in immune status, thus allowing opportunistic pathogens to flourish. In particular, recurrent ingestion of fermentable carbohydrates promotes the selection of bacteria proficient at metabolizing carbohydrates to organic acids (i.e. acidogenic organisms) and that then thrive in the resulting low pH environment (i.e. aciduric).
Saliva plays a major role in innate immunity of the oral cavity, protecting hard oral surfaces through its flushing action, buffering of acids, promotion of re-mineralization, bacteriostatic and bactericidal activities, and functioning in selective adherence of commensals and in clearance of acidogenic microorganisms (6–8). Glantz (9) proposed saliva is organized into a continuous phase composed of electrolytes in water, less water-soluble proteins, salivary micelles, other heterotypic complexes, lipoid material, and cells (i.e. bacterial and released epithelial cells) all intertwined within a scaffold-like network structure. The network structure is due largely to intermolecular interactions of gel-forming mucins that impart saliva with its weak gel characteristics to function in lubrication and hydration (7, 10, 11). Intermolecular interactions between gel-forming mucins include calcium-mediated cross-links and disulfide-mediated oligomers (12). Mucin monomers as large as 2.9 × 106 Da are also present in whole saliva (13).
Gel-forming mucins are a major component of whole saliva (14), and in humans are the exocrine products of salivary mucous cells of the major submandibular and sublingual glands as well as numerous minor mucous glands lining the oral cavity (7, 8). Besides lubrication and hydration, these mucins function to control the initial selective adherence of bacteria to tooth surfaces and further provide bacteria with carbohydrate and amino acid nutrients (7, 8, 15).
In humans, MUC5B is well documented as a gel-forming mucin in saliva (16), However, MUC19 transcripts were detected in human submandibular and labial glands (17–19) and glycoproteins localized to salivary mucous cells (19). Conversely, MUC19 was undetected in human saliva in a recent proteomic study (20). It thus remains unclear whether MUC19 contributes to gel-forming mucins in human saliva.
In adult mice, Muc19 is the dominant gel-forming mucin and is synthesized and secreted exclusively by the major sublingual glands and minor mucous glands (21). Unlike humans, rodent submandibular glands produce a small secreted mucin, Muc10 (i.e. submandibular gland mucin), rather than gel-forming mucins (22). Muc5B transcripts are undetectable in the major salivary glands of mice, although the numerous minor mucous glands possess Muc5b, Muc5ac, and Muc2 transcripts in addition to Muc19 (21).
Despite the abundance of in vitro evidence supporting protective functions of individually isolated salivary components (6), the net effects of the loss of a specific salivary constituent on the innate immune functions of saliva in vivo have not been largely explored. Because of the dominant salivary expression of Muc19 compared with other gel-forming mucins in mice, Muc19 represents an attractive candidate to test the impact of deletion of a major gel-forming mucin on the innate immune functions of saliva. We therefore assessed whether the targeted deletion of Muc19 in mice significantly impacts the development of caries upon challenge with S. mutans combined with a highly cariogenic diet. Furthermore, we evaluated changes, in vitro, in innate protective mechanisms of saliva from Muc19 knock-out mice in interactions with S. mutans, and we explored changes in the oral microbiota. We further provide extensive evidence confirming expression of MUC19 transcripts in human salivary glands and further demonstrate MUC19 glycoproteins in stimulated human saliva. Evidence for MUC5AC in saliva is also given.
EXPERIMENTAL PROCEDURES
Materials
Unless indicated, all materials were from Invitrogen. All kits were used according to manufacturer's instructions.
Animals and Collection of Tissues
The University of Florida IACUC committee approved all animal procedures. Construction and genotyping of the Muc19−/− mouse model that targets the third through fourth exons encoding Muc19 have been described previously (23). For this study, heterozygous females were backcrossed five times to NFS/NCr males from our own breeding colony at the University of Florida, and heterozygous N5 progeny intercrossed to generate a colony of homozygous Muc19−/− mice.
Five mice of each sex and genotype were examined at 7 weeks of age for outward body characteristics (body shape and size; the number of digits and claws on each limb; fur color and texture on the back and belly; skin appearance; size and shape of the head; the position, size, and shape of ears; the size, position, clarity, and color of eyes; the number, position, size, and color of teeth; whiskers; the size and shape of limbs, genitals, anus, mammary glands; the color and hardness of feces; and the coloration of urine stains of the litter) and for any obvious differences in internal organs (palate, tongue, buccal mucosa, major salivary glands, esophagus, stomach, duodenum, pancreas, spleen, ileum, jejunum, cecum, colon, liver, gallbladder, kidney, adrenal glands, urinary bladder, tracheolarynx, thymus, lungs, heart, lymph nodes, mesentery, scrotum, testes, epididymis-seminal vesicle, bulbourethral gland, penis, ovaries, uterus, vagina, Harderian glands, lacrimal glands, and brain).
All animals were maintained under BSL2 conditions. Animals were euthanized by CO2 asphyxiation with confirmation of death via thoracotomy. Mice were genotyped as described previously (23). Unless indicated, tissues were excised, blotted on filter paper, flash-frozen in liquid nitrogen, and stored at −80 °C. Rat sublingual glands were from frozen stocks obtained previously as part of another study (24).
Caries Experimental Protocol and Scoring
The caries protocol was similar to that reported previously (25). Briefly, timed pregnancies were established using 20 breeding pairs each of homozygous NFS/NCr and Muc19−/− mice, and pups were inoculated at 16–17 days of age with ∼10 μl (108 CFU) of S. mutans UA159. The diet was converted to powdered Diet 2000 (56% sucrose) with 5% sucrose water. At 21 days of age, pups were weaned and caged in pairs with non-littermates of the same sex. Mice were weighed weekly and sacrificed 7 weeks after the initial inoculation. The left and right mandibles were removed aseptically and assayed for bacterial colonization of the molars. The buccal, lingual, and proximal surfaces of all mandibular and maxillary molars were scored for smooth surface and sulcal caries using Larson's modification of the Keyes' scoring system (26, 27). Scores were compared by analysis of variance with the Tukey-Kramer post hoc test at 99.9, 99.0, and 95% confidence.
Recoveries of S. mutans and Total Bacteria from Molars
Procedures were as described previously (25). Briefly, excised mandibles underwent dissection to isolate the molars. Sonicates of the molars from two non-cagemates were pooled, and serial dilutions were cultured on blood agar to estimate CFU of total bacteria. Recovery of S. mutans was determined from cultures on MSB agar (28).
Comparison of Indigenous Oral Bacteria
Procedures were as described previously (25). Oral swabs from mice (8–10 weeks of age) were streaked on sheep blood agar plates and incubated under aerobic and anaerobic conditions, and total recovered CFUs for each colony morphotype were determined. Colony isolates from each of three mice of each genotype were subjected to colony PCR using degenerate primers (29) complementary to the 5′- and 3′-ends of the 16 S rRNA gene. Consensus sequences were compared with reference sequences (version 13.2) in the Human Oral Microbiome Database (30) and the Ribosome Database Project, Release 10 (31). Alignments were performed via the BLAST server at the Human Oral Microbiome Database.
Collection of Stimulated Whole Saliva from Mice
Saliva collection was as described previously (25). Mice were anesthetized (ketamine, 75 mg/kg, plus xylazine, 8 mg/kg, i.p.); saliva flow was stimulated (subcutaneous 5 mg/kg pilocarpine, 0.5 mg/kg isoproterenol), and saliva was collected from the cheek pouch at 5-min intervals over a 20-min period.
Biofilms
For all in vitro assays with whole stimulated saliva, we used low-passage S. mutans UA159 containing the ΩKm element integrated into the gtfA gene with the vector pBGK (32), to confer kanamycin resistance (kindly provided by Dr. R. A. Burne). We thus avoided centrifugation of whole stimulated saliva, which promotes precipitation of a mucous gel in addition to removing small amounts of inherent microorganisms. The gtfA gene does not alter carbohydrate metabolism of S. mutans, except for melibiose and raffinose (33). Caries development by this strain is unaffected (34), and collected saliva produced no colonies under anaerobic conditions on blood agar containing 1 mg/ml kanamycin (data not shown).
Assays were carried out in 96-well plates containing hydroxyapatite discs (Clarkson Chromatography Products Inc.). Samples of mouse saliva were resuspended to a concentration of 63% (v/v) in saliva buffer (SB: 50 mm KCl, 1.0 mm KPO4, 1.0 mm CaCl2, and 0.1 mm MgCl2, pH 6.5) plus of 1 mg/ml kanamycin (SBK). Wells received 100 μl of saliva or SBK, and plates were incubated for 30 min at 37 °C. Kanamycin-resistant S. mutans UA159 cultured overnight in brain-heart infusion medium plus kanamycin (1 mg/ml) was diluted 1:10 in the same medium and cultured further at 37 °C in air, 5% CO2 to mid-log phase and then diluted 1:100 in pre-warmed semi-defined biofilm medium (BM: basic salts, vitamins, key amino acids, casamino acids) (35) containing 1% glucose plus 1 mg/ml kanamycin and incubated 4 h at 37 °C in air, 5% CO2. Approximately 106 cells in 150 μl were added to wells to give a final saliva concentration of 25%. After 24 h at 37 °C in air, 5% CO2, the medium was decanted, and biofilms were washed twice gently with 200 μl of sterile 120 mm NaCl to remove planktonic and loosely bound cells. Biofilms were stained with 200 μl of 5 μm Syto-13 in the dark for 25 min at room temperature, and fluorescence was determined. All experimental conditions were performed in triplicate.
Aggregation Assay
Kanamycin-resistant S. mutans UA159 were prepared as described above for biofilm assays. Cell suspensions were mixed with either samples of saliva diluted in SBK to give final concentrations as indicated (v/v) or increasing concentrations of rat Muc19, or a combination of saliva and Muc19. Initial cell suspensions (A600 ∼0.65) were transferred to a spectrophotometer with a temperature-controlled multicuvette positioner set at 37 °C. After 5 min the A600 of each sample was recorded at 10-min intervals over a 2-h period. Percent aggregation (percent decrease in A600) was calculated as (A600 at 0 min − A600 at 120 min)/(A600 at 0 min) × 100.
Adherence of S. mutans
Adherence assays were as described previously (25) using hydroxyapatite discs in 96-well plates and kanamycin-resistant S. mutans UA159. Briefly, wells received 100 μl of diluted saliva, Muc19, or buffer (background controls), and plates were incubated for 1 h at 37 °C with rotary shaking and washed twice with SBK. S. mutans in SBK (100 μl) was added to wells, incubated 1 h at 37 °C, and then washed with SB. Adherent cells were quantified using SYTO-13 and a standard curve. Values were normalized to the mean for cells bound to discs without saliva (100%). All experimental conditions were performed in triplicate.
Isolation of Rat Muc19 Glycoproteins
High density and high molecular weight glycoproteins were isolated from rat sublingual glands as part of a previous study (24). Of all gel-forming mucins (Muc2, Muc5ac, Muc5b, Muc6, and Muc19) only Muc19 was expressed in this gland by RT-PCR (data not shown), and the amino acid composition of high density and high molecular weight mucin glycoproteins isolated from rat sublingual glands are as predicted for rat Muc19 (36). Briefly, gland homogenates were pooled, and the cytosolic fraction was subjected to two rounds of cesium chloride density gradient fractionation under dissociative and reducing conditions. The resultant high density fractions were pooled and further fractionated by gel filtration under dissociative conditions. The high molecular weight material in the void volume and initial included fractions was pooled, dialyzed extensively, and lyophilized. The resultant material was free of lower molecular weight glycoproteins or proteins as assessed by SDS-PAGE (24).
Collection of Stimulated Human Whole Saliva and Human Salivary Glands
Stimulated whole saliva was collected from three healthy Caucasian female subjects, 41–57 years of age, with good oral health. Subjects refrained from oral hygiene procedures, smoking, eating, and drinking for 2 h prior to saliva collection. To collect stimulated whole saliva, subjects chewed a piece of paraffin (about 1.5 g) for 15 min, while refraining from swallowing. The first 30-s sample was discarded. At 30-s intervals, subjects spit into a 50-ml sterile plastic centrifuge tube kept on ice that contained 0.2 ml of protease inhibitor mixture (100-fold concentrated; Sigma). Collected saliva was assessed for volume (assuming a density of 1 g/ml) and resuspended by repeated pipetting with a 10-ml serological pipette, and 1-ml aliquots were frozen at −80 °C. Informed consent was obtained from all subjects. Human sublingual and palatal salivary gland tissues were obtained from human subjects within 36 h after death and immediately frozen in liquid nitrogen. An oral pathologist excised all tissues, and there were no signs of oral disease. Submandibular gland tissues were from pathologically normal tissue during surgery and obtained as frozen samples from the Cooperative Human Tissue Network, part of the Cancer Diagnosis Program of the NCI, National Institutes of Health. The seven samples were from four male and three female Caucasians, aged 40–76, mean age of 57. For immunofluorescence experiments, submandibular gland sections were from a 44-year-old Caucasian female, sublingual gland sections from a 60-year-old Caucasian female, and hard palate sections from a 52-year-old African-American female. Samples were obtained under protocols approved by the University of Florida Institutional Review Board.
RT-PCR
Procedures were as described previously (37). Briefly, RNA was isolated from frozen tissues or LS174T cells, and DNase I-treated RNA (5 μg) was reverse-transcribed with random primers. PCR conditions and specific primers used are shown in Table 1.
TABLE 1.
RT-PCR primers used to detect transcripts for gel-forming mucins in human submandibular glands
The positions of primers are based on reference sequences (GenBankTM accession numbers). PCRs incorporated 1 unit of AccuprimeTM Taq high fidelity DNA polymerase, 0.25 μm primers, and 20 ng of cDNA. Cycling conditions were as follows: 5 min at 94 °C; 35 cycles of 1 min at 94 °C, 1 min at 55 °C, and 2 min at 68 °C followed by 7 min at 68 °C.
| Transcript | Reference sequence | Primer sequence | Exons | Amp |
|---|---|---|---|---|
| bp | ||||
| MUC2 | NM_002457 | 5′-CAAGCACAGCACCGATTGCTGAGT-3′ | 22 | 441 |
| 5′-CACCTGGTGCGTAGTAGGTGTCGTT-3′ | 23 | |||
| MUC5AC | AJ001402 | 5′-GTTCTCCGGCCTCATCTTCTCC-3′ | 5 | 350 |
| 5′-GCTCAAAGACCTTGCTCAGAATCAG-3′ | 7 | |||
| MUC5B | NM_002458 | 5′-CAGGAGTCCATCTGCACCCAG-3′ | 42 | 294 |
| 5′-CCAGGTTTCATTCAGCTGGAC-3′ | 45/46 | |||
| MUC6 | U97698 | 5′-AGGAGGAGATCACGTTCAAG-3′ | 32 | 303 |
| 5′-TGTCATCTGCAGTCCTTCAG-3′ | 33 | |||
| MUC19 | AY236870 | 5′-GAGTGCCAACAAGACCACAAG-3′ | 26 | 820 |
| 5′-GGCCTGTTAAAACTGGGAAGG-3′ | 35 |
SDS-PAGE
Procedures were as described previously (21) with minor modifications. For MUC19 and MUC5B Western blots, stimulated whole human saliva was dialyzed (3,500 Mr cutoff) 48 h against water containing a mixture of protease inhibitors (Sigma), lyophilized, and resuspended in SDS-PAGE sample buffer. Samples were briefly sonicated to promote complete solubilization (two 10-s pulses at 30% amplitude with a 60-s interval). Gels were stained for proteins with Coomassie Blue or stained for highly glycosylated glycoproteins with Alcian blue, with or without subsequent silver enhancement (38).
Western Blots
Western blots were conducted as described previously (21) with the following modifications. To detect MUC5B, blots were probed overnight at 4 °C with rabbit anti-MUC5B raised against 118 residues just prior to the first cysteine-rich domain (0.05 μg/ml, Sigma). Nonimmune rabbit IgG (0.05 μg/ml) was used as control. Blots were also probed with chicken anti-MUC19 (1.5 μg/ml) raised against 24 gene-specific residues in the N terminus (kindly provided by Dr. Yin Chen) or with preimmune antibody (19). Samples of whole stimulated saliva were used directly to detect Muc10 using rabbit anti-Muc10 (39) at 1:1,000 (kindly provided by Dr. Paul Denny). To detect gp340, we used mouse gp340 goat polyclonal antibody (1 μg/ml, Santa Cruz Biotechnology). Blots were subsequently incubated for 30 min at room temperature with the appropriate secondary antibody and then processed using our published protocol.
Immunofluorescence
Immunofluorescence microscopy was carried out as described previously (23) except frozen tissue sections were fixed in acetone (−20 °C) and then rehydrated in graded ethanol solutions. Sections were probed with chicken anti-MUC19 (1.5 μg/ml) or with preimmune antibody (1.5 μg/ml). Immunodetection was with Alexa Fluor 568 goat anti-chicken IgY (1:500) and DAPI counterstaining.
Statistics
Comparisons between wild type and Muc19−/− mice were conducted with the paired or unpaired two-tailed t test or by analysis of variance with the Tukey-Kramer post hoc test using Prism 5.0 software (GraphPad Software, Inc., San Diego). Curve fitting was also with Prism 5.0.
RESULTS
Characterization of the Muc19−/− Phenotype
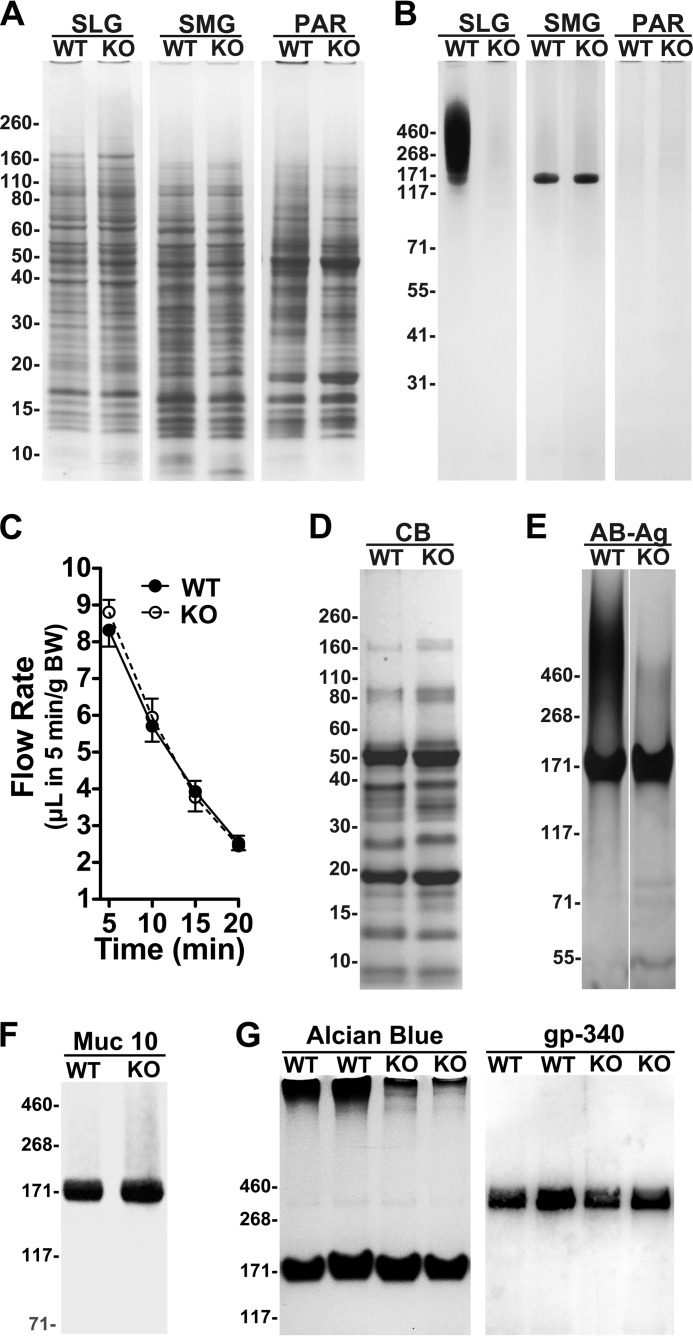
We recently described the targeting strategy to produce the Muc19−/− mouse model (23). For this study, we backcrossed Muc19+/− mice five times to NFS/NCr mice because this genetic background produces slightly higher smooth surface caries in control mice, thus permitting better delineation of an experimentally induced decrease in caries development (25, 40). We examined five mice of each sex and genotype at 7 weeks of age for outward body characteristics and for any obvious differences the size, shape, and color of internal organs. In all cases, no differences were noted between Muc19−/− and wild type mice. The number, position, size, and color of teeth were also very similar as were body weight and length (data not shown). Previously, we examined histologically multiple mucin-producing tissues in mice in which we deleted Muc19 expression but inserted a reporter GFP, and we found no abnormalities (21). No differences between the two genotypes were noted in protein profiles of parotid, submandibular, and sublingual glands (Fig. 1A). In rodents, these glands are distinct in secretory acinar cell types and in their major secretion products. Parotid glands contain serous cells with amylase as the major secretion product, and submandibular glands contain seromucous cells that produce the low molecular weight and non-gel-forming mucin, Muc10, and sublingual glands contain mucous cells that secrete Muc19. Sublingual glands of Muc19−/− mice do not express detectable Muc19, whereas expression of Muc10 in submandibular glands is unaffected (Fig. 1B). Previously, we demonstrated that sublingual glands of Muc19−/− mice appear normal histologically and ultrastructurally, except for the absence of large and electron translucent (i.e. mucous-like) secretion granules in acinar cells (23).
FIGURE 1.
Phenotypic characterizations of major salivary glands and saliva from wild type (WT) and Muc19−/− (KO) mice. A, no substantial differences are noted between wild type and Muc19−/− mice in protein profiles for each of the three major glands, parotid (PAR), submandibular (SMG), and sublingual (SLG), by SDS-PAGE in 10–20% gradient gels stained with Coomassie Blue. B, high molecular weight mucins, Muc19, are absent in sublingual glands of Muc19−/− mice, whereas expression of the lower molecular mass mucin, Muc10, in submandibular glands is unaffected in Muc19−/− mice. Mucin glycoproteins are undetected in parotid glands. SDS-PAGE 3–8% gradient gels were stained with Alcian blue with subsequent silver enhancement. Note that in A and B the glands were homogenized directly in SDS-PAGE sample buffer by sonication. C, saliva flow stimulated by subcutaneous injection of muscarinic cholinergic and β-adrenergic agonists measured at 5-min intervals after injection and volumes normalized to body weight (BW). Results are means ± S.E. from five mice of each genotype. There are no statistical differences between the two strains of mice (two-tailed unpaired t test, p > 0.05). D, whole stimulated saliva samples (6 μl) from wild type and Muc19−/− mice display equivalent protein expression profiles in 10–20% gradient SDS-polyacrylamide gels stained with Coomassie Blue (CB). E, whole stimulated saliva samples (10 μl) from wild type and Muc19−/− mice were briefly sonicated and run on 3–8% gradient SDS-polyacrylamide gels and stained for glycoproteins with Alcian blue (AB) and subsequent enhancement with silver (Ag). The prominent band at ∼175 kDa is Muc10. F, whole stimulated saliva samples (6 μl) from wild type and Muc19−/− mice run on a 3–8% gradient gel and probed for mouse Muc10. G, whole stimulated saliva samples (17 μl) from wild type and Muc19−/− mice run on a 3–8% gradient gel under nonreducing conditions and either stained for glycoproteins with Alcian blue alone or blotted and probed for mouse gp340. Note that gp340 (∼350 kDa) stains weakly with Alcian blue and that high molecular weight mucins barely enter the gel in the absence of prior brief sonication as used in E.
To evaluate the secretory function of the salivary system of Muc19−/− mice, we studied stimulated saliva flow. Stimulated flow was equivalent between wild type and Muc19−/− mice (Fig. 1C). As shown in Fig. 1D, no differences in protein profiles were noted in whole stimulated saliva from mice of both genotypes. Staining for highly glycosylated glycoproteins indicate a 175-kDa glycoprotein in both samples (Fig. 1E), representing Muc10, as observed in Western blot (Fig. 1F). Salivary agglutinin (i.e. gp340) is well expressed in whole stimulated saliva of wild type and Muc19−/− mice (Fig. 1G). From Fig. 1, E and G, it is apparent that high molecular weight glycoproteins are greatly attenuated in saliva from Muc19−/− mice. Residual glycoproteins in Muc19−/− saliva are due likely to contributions of Muc5b from minor mucous glands (see under “Discussion”). The absence of Muc19 in Muc19−/− mice combined with no other significant differences in phenotypic characteristics validates the use of this model system to assess the impact of salivary Muc19 on caries development.
Caries Development and Recovery of Bacteria
Muc19−/− and wild type mice were challenged with S. mutans strain UA159 and a highly cariogenic diet. Weight gains during this period between the two genotypes were very similar for each gender, although at weeks 5–7 Muc19−/− females were ∼4% lower in weight (data not shown). Both smooth and sulcal caries were assessed. Smooth surface caries is an evaluation of the proficiency of bacteria to adhere and colonize the smooth vertical tooth surfaces, whereas sulcal caries appraises colonization within the long angular depressions of horizontal surfaces where molars of the upper (maxilla) and lower (mandible) jaws meet, and in which food particles tend to become entrapped (41). As shown in Table 2, the mean of the incidence of total carious lesions to molar smooth surfaces (E score) is more than 2-fold higher in Muc19−/− mice compared with wild type. Furthermore, the proportional differences in caries scores further increases with the severity of lesions (i.e. Ds, Dm, and Dx scores), with up to 6-fold higher levels for the most severe type of lesion (Dx; progression into the entire dentin). The increase in smooth surface lesions occurred on surfaces juxtaposed to the tongue (lingual) and cheeks (buccal) but not to areas between teeth (proximal). In comparing these two sites, there is a trend for greater proportional increases in lesions on buccal versus lingual surfaces, although these differences are not significant (p > 0.05, analysis of variance).
TABLE 2.
Development of caries and their severities on molars of wild type and Muc19−/− mice
Values are means (S.E.) of Larson's modified Keyes' scores. 20 mice were in each group. Total smooth surface caries is the sum of buccal, lingual, and proximal caries. E is enamel affected; Ds is dentin exposed; Dm is ¾ of the dentin affected, and Dx is whole dentin affected.
| Wild type | Muc19 −/− | |
|---|---|---|
| Smooth surfaces | ||
| Total E | 8.90 (1.07) | 19.20 (1.74)a |
| Total Ds | 5.35 (1.02) | 15.05 (1.59)a |
| Total Dm | 1.40 (0.48) | 8.25 (1.27)a |
| Total Dx | 1.25 (0.46) | 7.55 (1.27)a |
| Buccal E | 2.20 (0.56) | 6.55 (0.83)a |
| Buccal Ds | 1.15 (0.36) | 5.50 (0.73)a |
| Buccal Dm | 0.25 (0.14) | 3.50 (0.59)a |
| Buccal Dx | 0.15 (0.11) | 3.40 (0.57)a |
| Lingual E | 5.10 (0.51) | 9.80 (0.87)b |
| Lingual Ds | 2.85 (0.55) | 7.40 (0.79)a |
| Lingual Dm | 0.75 (0.27) | 3.90 (0.58)a |
| Lingual Dx | 0.70 (0.27) | 3.45 (0.56)a |
| Proximal E | 1.60 (0.36) | 2.85 (0.53) |
| Proximal Ds | 1.35 (0.34) | 2.15 (0.42) |
| Proximal Dm | 0.40 (0.20) | 0.85 (0.23) |
| Proximal Dx | 0.40 (0.20) | 0.70 (0.22) |
| Sulcal surfaces | ||
| Total E | 8.50 (0.89) | 13.70 (0.89)b |
| Total Ds | 4.95 (0.79) | 10.55 (0.89)a |
| Total Dm | 1.50 (0.39) | 6.65 (0.73)a |
| Total Dx | 0.45 (0.15) | 4.65 (0.56)a |
a p < 0.001 versus wild type.
b p < 0.01 versus wild type.
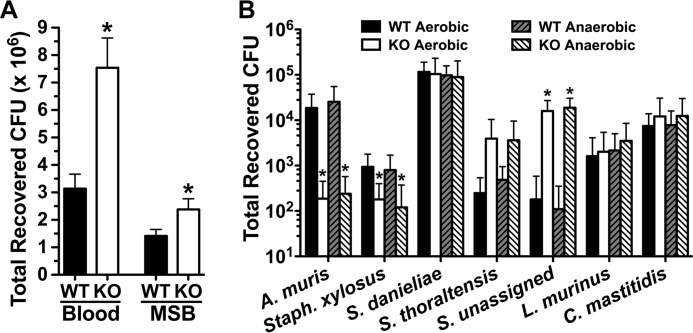
In comparing lesions on sulcal surfaces, total lesions in Muc19−/− mice are 1.6-fold higher than in wild type mice. As with smooth surface scores, the proportional differences in sulcal scores increases with lesion severity, to more than 10-fold for Dx scores. The higher levels of caries in Muc19−/− mice are consistent with recoveries from molars of S. mutans and total microbiota (Fig. 2A). Recovery of S. mutans is nearly 1.7-fold higher from Muc19−/− mice compared with wild type. For total cultivable bacteria, this difference is 2.4-fold.
FIGURE 2.
Cultivable microbiota from mice. A, recoveries by CFU of total bacteria on blood agar and of S. mutans on MSB agar from sonicates of mandibular molars. Values are means ± S.E. from molars of 20 mandibles prepared from each group. *, p < 0.05 for Muc19−/− mice (KO) compared with the wild type (WT). B, total recoveries for each of seven distinct colony morphotypes from the oral cavities of Muc19−/− and wild type mice. Oral swabs were vortexed in brain heart infusion medium and serial dilutions streaked onto duplicate blood agar plates cultured under aerobic or anaerobic conditions. Each colony morphotype is identified by its taxonomic species assignment from the RDP database as given in Table 3. Results are means ± S.E. from cultures of oral swabs from 10 mice in each group. *, p < 0.05 for Muc19−/− mice compared with the wild type.
Comparison of Indigenous Oral Bacteria
We determined whether deletion of Muc19 expression affects the indigenous oral microbiota. From oral swabs of uninfected mice of both genotypes, we consistently obtained seven distinct colony morphologies on blood agar under either aerobic or anaerobic culture conditions, whereas no colonies formed on MSB agar (selective for mutans streptococci). No additional colony morphologies were apparent with extended culture on blood agar. Given in Table 3 are the characteristics of each colony morphotype and their taxonomic assignments based on alignments of consensus 16 S rRNA sequences to the Ribosome Database Project. 16 S rRNA gene sequences from white colonies of cocci most closely align to uncultured bacteria assigned to the genus Streptococcus (designated S. unassigned). Five of the six species identified were shown previously to colonize mice (42–46), whereas Streptococcus thoraltensis has been found in rabbits (47). For comparisons with human oral microbiota, each consensus sequence was also aligned to the annotated Human Oral Microbiome Database 16 S rRNA reference sequences from the human oral microbiome.
TABLE 3.
Phenotypic characteristics and 16 S rRNA sequence alignments of colonies isolated on blood agar from oral swabs of wild type and Muc19−/− mice
Colony appearance on blood agar was identical under aerobic and anaerobic conditions. High quality consensus 16 S rRNA sequences from colony PCR of single isolates under aerobic conditions from three mice of each genotype were aligned by the BLAST server at Human Oral Microbiome Database (30) to two sets of 16 S rRNA reference sequences, Human Oral Microbiome Database (HOMD) 16 S rRNA RefSeq 13.2 and sequences of the Ribosome Database Project, release 10 (31). Highest scoring alignments were identical between consensus sequences from wild type and Muc19−/− mice. Percent identities are the total nucleotide mismatches per total matching nucleotides, including gaps.
| Colony appearance, cell appearance | Gram stain | Catalase activity | Reference sequences | Genus | Species | GenBank accession no. | Percent identities |
|---|---|---|---|---|---|---|---|
| Large gray, short thin rods | − | + | RDP | Actinobacillus | muris | AF024526 | 98.5 |
| HOMD | Haemophilus | haemolyticus | HM596277 | 93.9 | |||
| Large yellow, large cocci | + | + | RDP | Staphylococcus | xylosus | AB626129 | 99.9 |
| HOMD | Staphylococcus | warneri | L37603 | 98.3 | |||
| Medium gray (α-hemolytic), cocci, chains | + | − | RDP | Streptococcus | danieliae | GQ456229 | 98.7 |
| HOMD | Streptococcus | sanguinis | AF003928 | 97.2 | |||
| Large irregular gray, cocci, chains | + | − | RDP | Streptococcus | thoraltensis | Y09007 | 99.2 |
| HOMD | Streptococcus | salivarius | M58839 | 94.7 | |||
| Medium white, cocci, chains | + | − | RDP | Streptococcus | unassigned | AY538685 | 100 |
| HOMD | Streptococcus | cristatus | AB008313 | 95.2 | |||
| Medium white, medium to long rods | + | − | RDP | Lactobacillus | murinus | AF157049 | 99.9 |
| HOMD | Lactobacillus | salivarius | AF089108 | 95.0 | |||
| Small gray, short rods | + | + | RDP | Corynebacterium | mastitidis | AY34747 | 99.5 |
| HOMD | Corynebacterium | mucifaciens | X82059 | 95.4 |
We further determined total recoveries of each colony morphotype from Muc19−/− and wild type mice (see Fig. 2B). There were no differences in total recovered CFUs under aerobic and anaerobic culture conditions (about 1.4 × 105 CFUs total). The absence of Muc19 induced a shift in the relative proportions of Staphylococcus xylosus, Actinobacillus muris, and S. unassigned. Most noticeable was about a 100-fold decrease in A. muris and a 120-fold increase in S. unassigned, whereas S. xylosus decreased 5.8-fold. S. thoraltensis displayed a trend toward a 10-fold higher proportion in Muc19−/− mice (p = 0.10, anaerobic, and p = 0.09, aerobic). Unchanged in proportion were the species Corynebacterium mastitidis, Lactobacillus murinus, and Streptococcus danieliae, the latter representing about 75% of the total cultivable bacteria. Collectively, deletion of Muc19 expression increased the dominance of streptococcal species in the oral cavity from ∼77 to 89%.
In Vitro Assessments of Muc19 Protective Mechanisms
To interrogate possible mechanisms through which Muc19 may interact with S. mutans to limit its colonization under highly cariogenic conditions, we conducted a series of in vitro assays using either whole saliva from wild type and Muc19−/− mice or with Muc19 purified from rat sublingual glands (24).
Adherence of S. mutans to Muc19 and Saliva Pellicles on Hydroxyapatite Discs
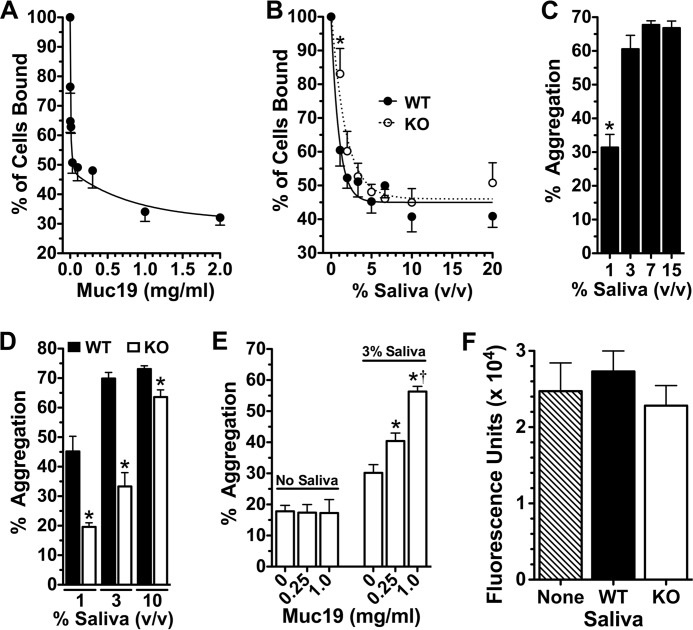
In humans, selective constituents of saliva interact rapidly with enamel surfaces to form an acquired enamel pellicle (48–51), which promotes initial adherence of Actinomyces species followed by acquisition and predominance of commensal streptococci such as Streptococcus sanguinis, Streptococcus gordonii, Streptococcus mitis, and Streptococcus oralis (48). In contrast, S. mutans adheres poorly to salivary pellicles formed in vitro (52). Because salivary gel-forming mucins display high affinity to naked hydroxyapatite (53–55) and are a component of the acquired enamel pellicle (50, 56–58), we determined whether Muc19 pellicles on hydroxyapatite influence S. mutans adherence. As shown in Fig. 3A, S. mutans adherence is reduced dramatically at low concentrations of Muc19, with near maximal inhibition of ∼70% at 2.0 mg/ml, and with 50% of maximal inhibition at 0.03 mg/ml. We next compared adherence after pretreatment of hydroxyapatite with increasing concentrations of saliva from wild type and Muc19−/− mice. At low concentrations, saliva from Muc19−/− mice tends to display less inhibition of adherence, with statistical significance at 1% saliva (see Fig. 3B).
FIGURE 3.
In vitro assays of the functional interactions of saliva from Muc19−/− (KO) and wild type (WT) mice with S. mutans. A, microtiter plate and fluorescent-based assay of adherence of S. mutans to hydroxyapatite discs pretreated with increasing concentrations (mg dry weight/ml) of Muc19. Adherence is normalized to the percent of cells bound in the absence of Muc19. Values are means ± S.E. from three separate experiments. B, adherence of S. mutans to hydroxyapatite discs pretreated with increasing concentrations of whole stimulated saliva from Muc19−/− (KO) and wild type (WT) mice. Adherence is normalized to the percent of cells bound in the absence of saliva. Values are means ± S.E. from five separate experiments. *, p < 0.05 for saliva from Muc19−/− versus wild type mice. C, percent aggregation of S. mutans with increasing concentrations of whole stimulated saliva from wild type mice. Values are means ± S.E. from 12 separate experiments. *, p < 0.05 versus all higher concentrations of saliva. D, aggregation of S. mutans with increasing concentrations of whole stimulated saliva from Muc19−/− (KO) and wild type (WT) mice. Values are means ± S.E. from 10 separate experiments. *, p < 0.05 for saliva from Muc19−/− versus wild type mice. E, aggregation of S. mutans with increasing Muc19 (mg dry weight/ml), in the presence or absence of 3% (v/v) whole stimulated saliva from Muc19−/− mice. Values are means ± S.E. from three separate experiments. *, p < 0.05 versus 3% saliva with no added Muc19. †, p < 0.05 for 1.0 mg/ml versus 0.25 mg/ml added Muc19. F, biofilm development of S. mutans cultured 24 h under aerobic conditions on hydroxyapatite discs in semi-defined biofilm medium containing 1% glucose and supplemented without or with 25% (v/v) whole stimulated saliva from Muc19−/− (KO) or wild type (WT) mice. After washing to remove planktonic and loosely bound cells, biofilms were quantified by fluorescent staining with Syto-13. Values are means ± S.E. from eight separate experiments. p > 0.16 in all cases.
Aggregation of S. mutans
Binding of bacteria to salivary constituents can lead to bacterial aggregation and subsequent clearance from the oral cavity. We therefore examined whether Muc19 functions to promote aggregation of S. mutans. As little as 3% saliva from wild type mice is sufficient for maximal aggregation of S. mutans (Fig. 3C). However, 1–3% saliva from Muc19−/− mice produces markedly reduced aggregation compared with the wild type and a small but significant decrease at 10% saliva (Fig. 3D). Although these results suggest Muc19 contributes to the aggregation properties of saliva, it is unclear whether Muc19 interacts with S. mutans directly or possibly indirectly through heterotypic complexation with other salivary constituents. We therefore assessed aggregation with increasing concentrations of Muc19 alone or when added to saliva from Muc19−/− mice. As shown in Fig. 3E, Muc19 alone at concentrations up to 1 mg dry weight/ml has no effect on aggregation. Conversely, aggregation is greatly enhanced with addition of Muc19 to 3% saliva from Muc19−/− mice, with aggregation approaching maximal levels observed with wild type saliva seen in Fig. 3C.
Development of Biofilms
We compared biofilm formation by S. mutans on hydroxyapatite discs in a semi-defined medium containing 1% glucose, without and with saliva from wild type and Muc19−/− mice. As shown in Fig. 3F, biofilm formation was not significantly different among all three conditions.
Detection of Transcripts of Gel-forming Mucins in Human Submandibular Glands
In light of the important role Muc19 plays in protection against caries in mice, we compared expression of MUC19 transcripts in submandibular glands from seven human subjects. Because we previously found expression of multiple gel-forming mucins in murine minor salivary glands (21), we further assayed for the other four gel-forming mucins. Transcripts for MUC19 and MUC5B were both readily detected in all seven samples (Fig. 4A). Interestingly, transcripts for MUC5AC and MUC6 were also detected, whereas MUC2 transcripts were barely detectable in only one sample.
FIGURE 4.
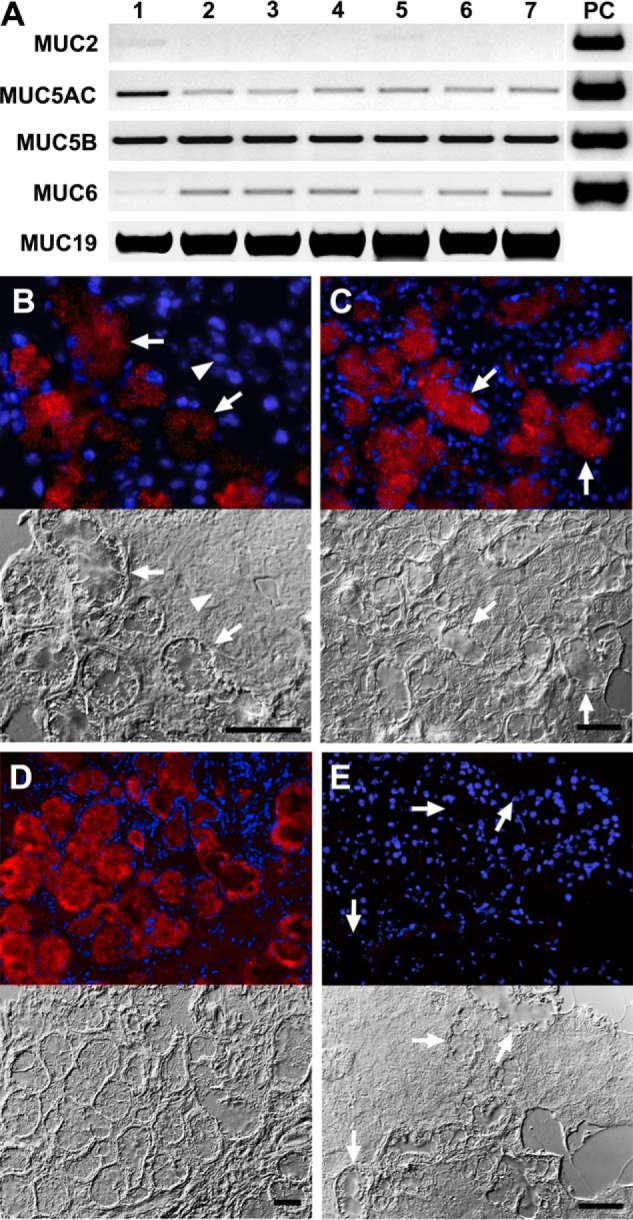
Expression of transcripts for gel-forming mucins in human submandibular glands and MUC19 immunolocalization in human salivary glands. A, expression of transcripts for gel-forming mucins MUC2, MUC5AC, MUC5B, MUC6, and MUC19 in submandibular gland tissues from seven healthy adults. Shown are RT-PCR products from 20 ng of cDNA. PC, positive controls for all transcripts but MUC19 are from the LS174T colon adenocarcinoma cell line. B, immunolocalization of MUC19 in a human submandibular gland. Chicken anti-MUC19 is detected with Alexa 568-labeled secondary antibody (red). Nuclei are stained blue (DAPI). Both fluorescence (upper image) and differential interference contrast images (lower image) are shown. Mucous cells within acini at the edge of a submandibular gland lobule stain for MUC19 (arrows). Under differential interference contrast optics, the mucous glands have a characteristic crater-like appearance, whereas individual serous acini are poorly distinguished in frozen sections (arrowhead). C, mucous cells within acini (arrows) of a sublingual gland stain with anti-MUC19. D, mucous glands of the hard palate also display anti-MUC19 staining. E, submandibular mucous glands (arrows) and other cellular elements are unstained by preimmune antibody. Bars, 50 μm.
Detection of Human MUC19 in Salivary Glands and Saliva
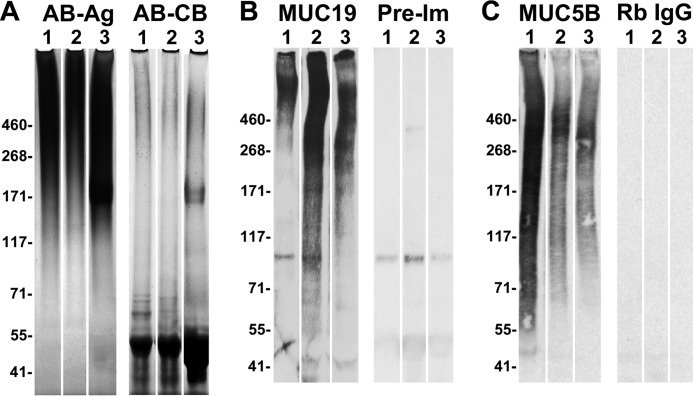
We localized MUC19 glycoproteins to mucous cells in submandibular (Fig. 4B), major sublingual (Fig. 4C), and minor glands of the hard palate (Fig. 4D) using an antibody raised against gene-specific residues in the N terminus. In examination of stimulated saliva from three human subjects by SDS-PAGE, we observed highly glycosylated glycoproteins at about 110 kDa or higher (Fig. 5A), whereas proteins were localized near the bottom of the gel (< 100 kDa). Interestingly, saliva from subject 3 displays a prominent glycoprotein near the 171-kDa marker, possibly due to high levels of secretion of the lower mass and non-gel-forming mucin, MUC7. We probed blots of all three saliva samples for MUC19 (Fig. 5B), and for comparison we probed for MUC5B (Fig. 5C). There are marked differences between subjects in reactivities of each antibody. For example, saliva from subject 1 displayed much less reactivity for MUC19, but more for MUC5B compared with the other two subjects. Furthermore, MUC19 mucins were more prominent in regions of lower mobilities than were MUC5B mucins.
FIGURE 5.
Probing human saliva for MUC19 and MUC5B. Stimulated saliva collected from three subjects was dialyzed and lyophilized and then run on 3–8% gradient SDS-polyacrylamide gels. Each lane contains resuspended dry weight material from 100 μl of saliva. A, gel stained first for highly glycosylated glycoproteins with Alcian blue with subsequent silver enhancement (AB-Ag), followed by destaining of the silver enhancement and Coomassie Blue staining for proteins (AB-CB). Proteins stained with Coomassie Blue are in the bottom third of the gel, and Alcian blue-stained glycoproteins are in the upper two-thirds. B, Western blots of identical aliquots of the samples in A probed with either chicken anti-MUC19 (MUC19) or with preimmune antibody (Pre-Im). C, samples identical to those in B probed with rabbit anti-MUC5B (MUC5B) or with nonimmune rabbit IgG.
We also surveyed results of the Human Salivary Proteome Project (59) and annotated the domain localization of peptides aligning to gel-forming mucins. In stimulated secretions from submandibular and sublingual glands, we find three peptides aligned to the very N terminus of human MUC19 (see Table 4), thus providing further evidence for MUC19 in stimulated saliva. We also found two peptides unique to MUC5AC, 30 peptides unique to MUC5B, and no peptides aligning to MUC6. One peptide is common to MUC2, MUC5AC, and MUC5B, whereas 18 peptides are common to MUC5AC and MUC5B (Table 4). The presence of multiple peptides shared between MUC5AC and MUC5B reflects the large degree of identity between the two apoproteins (37% by ClustalW alignment, not shown). All peptides are localized outside predicted glycosylated tandem repeat domains of each mucin apoprotein. For reference, a figure of the domain architecture for each mucin apoprotein is given in Fig. 6.
TABLE 4.
Gel-forming mucin peptides identified in stimulated submandibular-sublingual ductal saliva by the human salivary proteome project
NF means not found.
| Alignment of amino acids to each mucin apoprotein |
Mucin domain | |||||
|---|---|---|---|---|---|---|
| MUC2 NP_002448.3 | MUC5AC XP_006718462.1 | MUC5B NP_00244.2 | MUC6 NP_005952.2 | MUC19 NP_775871.2 | ||
| Unique to MUC19 | ||||||
| R.SGGFSYGSSSSGDLDR.K | NF | NF | NF | NF | 36–53 | 5′-End |
| R.KKPLFSLEFGSPGETEDK.S | NF | NF | NF | NF | 52–71 | 5′-End |
| K.KPLFSLEFGSPGETEDK.S | NF | NF | NF | NF | 53–71 | 5′-End |
| Unique to MUC5AC | ||||||
| R.FPGLCNYVFSEHC.G | NF | 98–112 | NF | NF | NF | VWD (1) |
| R.SVVGDVLEFGNSWK.L | NF | 1052–1067 | NF | NF | NF | Between VWD (3) and C8 (3) |
| Unique to MUC5B | ||||||
| R.AAYEDFNVQLR.R | NF | NF | 108–120 | NF | NF | VWD (1) |
| R.TGLLVEQSGDYIK.V | NF | NF | 161–175 | NF | NF | VWD (1) |
| R.LTPLQFGNLQK.L | NF | NF | 225–237 | NF | NF | Between VWD (1) and C8 (1) |
| K.LYDLHGDCSYVLSK.K | NF | NF | 439–454 | NF | NF | VWD (2) |
| K.AVTLSLDGGDTAIR.V | NF | NF | 479–494 | NF | NF | VWD (2) |
| R.NSFEDPCSLSVENENYAR.H | NF | NF | 601–620 | NF | NF | Between VWD (2) and C8 (2) |
| R.LTDPNSAFSR.C | NF | NF | 624–635 | NF | NF | C8 (2) |
| R.AAQLPDMPLEELGQQVDCDR.M | NF | NF | 686–707 | NF | NF | Between C8 (2) and TIL (2) |
| K.LSCLGASLQK.S | NF | NF | 802–813 | NF | NF | Between C8 (2) and TIL (2) |
| I.PCGTTGTTCSK.A | NF | NF | 964–976 | NF | NF | VWD (3) |
| R.GPGGDPPYK.I | NF | NF | 998–1008 | NF | NF | VWD (3) |
| R.KTSVFIR.L | NF | NF | 1028–1036 | NF | NF | VWD (3) |
| K.DGNYYDVGAR.V | NF | NF | 1245–1256 | NF | NF | Between C8 (3) and WxxW (1) |
| R.GYQVCPVLADIECR.A | NF | NF | 1389–1404 | NF | NF | WXXW (1) |
| R.AAQLPDMPLEELGQQVDCDR.M | NF | NF | 1403–1424 | NF | NF | WXXW (1) |
| K.SEQLGGDVESYDK.I | NF | NF | 1541–1555 | NF | NF | WXXW (2) |
| R.FNMCFNYNVR.V | NF | NF | 1887–1898 | NF | NF | WXXW (3) |
| R.AAGGAVCEQPLGLECR.A | NF | NF | 2367–2384 | NF | NF | WXXW (4) |
| R.AQAQPGVPLR.E | NF | NF | 2383–2394 | NF | NF | WXXW (4) |
| K.MCFNYEIR.V | NF | NF | 2419–2428 | NF | NF | WXXW (4) |
| R.AAGGAVCEQPLGLECR.A | NF | NF | 2924–2941 | NF | NF | WXXW (5) |
| R.AQAQPGVPLR.E | NF | NF | 2940–2951 | NF | NF | WXXW (5) |
| K.MCFNYEIR.V | NF | NF | 2976–2985 | NF | NF | WXXW (5) |
| R.AAGGAVCEQPLGLECR.A | NF | NF | 3624–3641 | NF | NF | WXXW (6) |
| R.AQAQPGVPLR.E | NF | NF | 3640–3651 | NF | NF | WXXW (6) |
| K.MCFNYEIR.V | NF | NF | 3676–3685 | NF | NF | WXXW (6) |
| R.AAGGAVCEQPLGLECR.A | NF | NF | 4181–4198 | NF | NF | WXXW (7) |
| K.MCFNYEIR.V | NF | NF | 4233–4242 | NF | NF | WXXW (7) |
| R.AGCHFYAVCNQHCDIDR.F | NF | NF | 4986–5004 | NF | NF | Between last tandem repeat and VWD (4) |
| K.SMDIVLTVTMVHGK.E | NF | NF | 5163–5178 | NF | NF | VWD (4) |
| Common to one or more mucins | ||||||
| K.TFDGDVFR.F | 46–55 | 90–99 | 86–95 | NF | NF | VWD (1) |
| R.FPGLCNYVFSEH.C | NF | 98–111 | 94–107 | NF | NF | VWD (1) |
| R.SEDCLCAALSSYVH.A | NF | 666–681 | 657–672 | NF | NF | C8 (2) |
| R.SEDCLCAALSSYVHAC.A | NF | 666–683 | 657–674 | NF | NF | C8 (2) |
| R.SEDCLCAALSSYVHACAAK.G | NF | 666–686 | 657–677 | NF | NF | C8 (2) |
| S.SYVHACAAK.G | NF | 676–686 | 667–677 | NF | NF | C8 (2) |
| W.KLSPSCPDALAPK.D | NF | 1065–1079 | 1077–1091 | NF | NF | Between VWD (3) and C8 (3) |
| W.KLSPSCPDALAPKDPCTANPFR.K | NF | 1065–1088 | 1077–1100 | NF | NF | Between VWD (3) and C8 (3) |
| K.LSPSCPDALAPK.D | NF | 1066–1079 | 1078–1091 | NF | NF | Between VWD (3) and C8 (3) |
| K.LSPSCPDALAPKDPCTANPFR.K | NF | 1066–1088 | 1078–1100 | NF | NF | Between VWD (3) and C8 (3) |
| K.LSPSCPDALAPKDPCTANPFRK.S | NF | 1066–1089 | 1078–1101 | NF | NF | Between VWD (3) and C8 (3) |
| S.PSCPDALAPK.D | NF | 1068–1079 | 1080–1091 | NF | NF | Between VWD (3) and C8 (3) |
| S.PSCPDALAPKDPCTANPFRK.S | NF | 1068–1089 | 1080–1101 | NF | NF | Between VWD (3) and C8 (3) |
| P.SCPDALAPKDPCTANPFRK.S | NF | 1069–1089 | 1081–1101 | NF | NF | Between VWD (3) and C8 (3) |
| C.PDALAPKDPCTANPFRK.S | NF | 1071–1089 | 1083–1101 | NF | NF | Between VWD (3) and C8 (3) |
| K.DPCTANPF.R | NF | 1078–1087 | 1090–1099 | NF | NF | Between VWD (3) and C8 (3) |
| K.DPCTANPFR.K | NF | 1078–1088 | 1090–1100 | NF | NF | Between VWD (3) and C8 (3) |
| K.DPCTANPFRK.S | NF | 1078–1089 | 1090–1101 | NF | NF | Between VWD (3) and C8 (3) |
| K.QCSILHGPTFAAC.R | NF | 1266–1280 | 1105–1119 | NF | NF | Between C8 (3) and WxxW (1); C8 (3) |
FIGURE 6.

Domain architecture of human gel-forming mucin apoproteins MUC2 (NP_002448.3), MUC5AC (XP_006718462.1), MUC5B (NP_002449.2), MUC6 (NP_005952.2), and MUC19 (NP_775871.2). All domain designations, except for the LAT domain, are from searches of the Conserved Domains Database (www.ncbi.nlm.nih.gov). Nonoverlapping domains with the lowest E-values are shown. Domains are as follows: SP, signal peptide; LAT, long amino terminus, containing serine-rich repeats predicted to be O-glycosylated; VWD, von Willebrand factor D, a Cys-containing domain involved in multimer formation; VWC, von Willebrand factor C, a Cys-containing domain involved in protein oligomerization; TR, tandem repeat region rich in serine and threonine residues predicted to be O-glycosylated; TIL, trypsin inhibitor like cysteine-rich domain, typically containing 10 Cys residues; WxxW, Mucin2_WXXW repeating region, containing at least six well conserved cysteine residues; C8, domain, containing 7 or 8 conserved Cys residues; CT, C-terminal cystine knot-like domain. O-Glycosylation predictions are from the NetOGlyc 4.0 Server.
DISCUSSION
Muc19 Protects against S. mutans Colonization and Development of Hard Surface Lesions
In a previous caries study with aquaporin 5−/− mice in which salivary flow is reduced 60%, smooth surface and sulcal caries were only marginally increased (51 and 26%, respectively) (40). In this study, absence of the predominant gel-forming mucin, Muc19, resulted in markedly greater increases in the incidence of total smooth surface and sulcal caries (116 and 61%, respectively). Correlated with increased caries were higher recoveries of S. mutans from molars of Muc19−/− mice. Under highly cariogenic conditions, Muc19 therefore contributes significantly to innate protection of hard oral surfaces against S. mutans by attenuating colonization and subsequent acid-induced lesions in mice. Inference for a negative correlation between caries and salivary gel-forming mucins in humans, as observed in our mouse model, was reported by Banderas-Tarabay et al. (60), in which the index scores in decayed, missing, and filled teeth in healthy human subjects were inversely related to the presence of high molecular mass glycoproteins in resting saliva.
Muc19 Influences the Commensal Oral Microbiota
Non-mutans streptococci represent the predominant commensals of the oral microbiota of wild type and Muc19−/− mice, consistent with previous observations in humans (61) as well as laboratory mice (62). Interestingly, expression of Muc19 promotes oral colonization of A. muris and S. xylosus, while attenuating colonization of S. unassigned. These results highlight the importance of individual salivary constituents to impact the oral microbiota (25) and the necessity for further investigations of homeostatic mechanisms between salivary components and the oral microbiome. Moreover, we acknowledge that differences in oral ecology may have impacted colonization by S. mutans in our caries study. For example, increased colonization by S. unassigned in Muc19−/− mice may present a more competitive environment for S. mutans (63, 64), but if S. unassigned is acidogenic, it may have facilitated S. mutans colonization (65). Additional investigations beyond the scope of this study are required to delineate the influence of ecological shifts on subsequent caries development.
Protective Mechanisms of Muc19 in Interactions with S. mutans
Pellicles formed with low concentrations of purified Muc19 markedly reduce adherence of S. mutans to hydroxyapatite, consistent with the results of Kishimoto et al. (66) in which pellicles of high mass glycoproteins from human submandibular-sublingual saliva diminish adherence of S. mutans. Interestingly, Nieuw Amerongen et al. (67) found that a pellicle of human salivary gel-forming mucins shields the underlying enamel surface from acid demineralization, suggesting a complete protective coating. Hence, the markedly reduced adherence of S. mutans to pellicles formed from saturating concentrations of Muc19 or from human high mass glycoproteins (66) indicates salivary gel-forming mucins are functionally analogous in humans and mice, either providing a relatively low density of binding sites for S. mutans or entrapping cells within the extended and branched mucin oligosaccharides (68).
Pellicles formed from whole mouse saliva hinder adherence of S. mutans onto hydroxyapatite up to 60%, comparable with inhibition observed previously with rat (69) and human saliva (52, 70). The absence of Muc19 increases adherence at only very low saliva concentrations, indicating other salivary constituents function in large part to shield hydroxyapatite-binding sites. In our assay, Muc19 may predominate in decreasing adherence at low saliva concentrations due to its selective absorbance to hydroxyapatite, a result of the abundance of gel-forming mucins in saliva (7, 8) and their high affinity for hydroxyapatite (53–55).
We find whole saliva (with or without Muc19) does not effect biofilm formation of S. mutans on hydroxyapatite when cultured in semi-defined medium containing vitamins, amino acids, and 1% glucose. To produce energy for growth and biofilm formation, S. mutans depends upon fermentation of dietary carbohydrates, and through carbohydrate catabolite repression it can elect to catabolize a preferred carbohydrate when other carbohydrates are available (71). Our results suggest S. mutans is unable to degrade salivary constituents, including gel-forming mucins, to provide dietary substrates more preferable to glucose in promoting biofilm formation. Similarly, human gel-forming mucins are unable to support growth of S. mutans (72) unless in a defined medium without amino acids (73) or in co-culture with other species that provide enzymes to sufficiently degrade mucins for catabolism (74, 75). Upon the consumption of sucrose by the host, S. mutans rapidly metabolizes this disaccharide to produce organic acid and also for production of a matrix of exopolysaccharides (primarily glucans) that serves as a supplemental source of carbohydrates during periods of fasting by the host, and it further promotes adherence of planktonic S. mutans expressing glucan-binding proteins (76). Because sucrose is highly preferred by S. mutans and readily available in the drinking water and diet of mice in our caries model, the absence of Muc19 likely had no significant direct effect on its survival and growth in supragingival plaque.
Saliva functions to aggregate and clear bacteria from the oral cavity via swallowing. Indeed, the level of aggregation of S. mutans by whole saliva from human subjects is inversely correlated with S. mutans numbers in plaque (77). Also, a greater capacity of saliva to aggregate S. mutans is associated with caries-resistant versus caries-susceptible individuals (78). We find maximal aggregation of S. mutans at about 3% saliva, whereas in the absence of Muc19, more than 3-fold higher saliva concentration is required for maximal aggregation. Early studies of aggregation of oral streptococci by crude high molecular weight fractions of saliva demonstrated association with constituents expressing blood group antigens (79), such as high molecular weight mucins (80). However, subsequent studies with highly enriched preparations of human gel-forming mucins found little bacterial binding or aggregating activities toward oral streptococci (81–83), similar to our results with murine Muc19.
Our collective results of murine saliva and purified Muc19 are analogous to previous results with human saliva and salivary gel-forming mucins in their interactions with S. mutans in aggregation, adherence to pellicles formed on hydroxyapatite, and support of growth and biofilm formation in monospecies culture. These similarities reinforce the mouse caries model as a tool to explore mechanisms of interactions between the host and S. mutans.
MUC5B and MUC19 in Human Salivary Glands and Saliva
In prior studies using antibodies to probe saliva or glandular secretions for gel-forming mucins, only MUC5B was identified, but not MUC2, MUC5AC, or MUC6 (84–87). Similarly, mass spectrometry of tryptic digests of isolated salivary gel-forming mucins identified peptides for MUC5B but not for MUC2, MUC5AC, or MUC6 (12, 84, 87). In another study, tryptic peptides from MUC5B were detected, whereas peptides predicted for MUC19 were absent in human resting saliva (20). These collective results argue for MUC5B as the sole gel-forming mucin in human saliva.
In contrast, there is evidence to suggest at least one additional gel-forming mucin in saliva. Troxler et al. (88) detected abundant MUC5B transcripts in sublingual glands by Northern analysis, whereas MUC5B transcripts were barely detected in submandibular glands, and transcripts for MUC2, MUC5AC, or MUC6 were absent in both glands. A subpopulation of unreactive sublingual mucous cells exhibited no immunoreactivity to MUC5B antibody (86). In sublingual gland secretions, one of two populations of mucins with different charge densities was unrecognized by MUC5B antibody (87). Labial gland secretions from 90 subjects were nearly all reactive to anti-MUC5B antibody, whereas secretions from buccal mucous glands (89) were reactive in only 11 subjects (16). MUC19 transcripts were detected in human submandibular and labial glands (17–19), and MUC19 glycoproteins immunolocalized to salivary gland mucous cells (19). We now confirm expression of MUC19 transcripts in submandibular glands from multiple human subjects as well as immunolocalization of MUC19 to mucous elements of major and minor salivary glands. More importantly, we detect MUC19 immunoreactivity to high molecular weight glycoproteins in saliva and show that mucins from the same subject display distinct patterns of MUC19 and MUC5B immunoreactivities. We also highlight peptides from MUC19 and MUC5AC in the salivary proteome database and identify MUC5AC peptides in acquired enamel pellicle (51, 57).
How Can These Two Conflicting Bodies of Results Be Reconciled?
Extensive glycosylation can hinder apomucin identification by blocking anti-peptide epitopes or the access of proteases to polypeptide domains (84, 85). For example, glycosylated tandem repeat sequences of mucins protect adjacent nonglycosylated regions from denaturation by acid and to proteolysis by pepsin (90) or cysteine protease (91). Also, a MUC5B antibody raised against a polypeptide sequence shared in four of the nonglycosylated WXXW domains displays markedly enhanced reactivity after reduction of mucins, suggesting the tertiary structure of these domains in nonreduced mucins shields antibody epitopes (86).
In the prior studies described above in which saliva or salivary glands were probed for gel-forming mucins, only one study probed for MUC19, as the gene was not yet identified, nor were antibodies available (10, 84, 86, 88, 92). In the one exception, Rousseau et al. (20), reported the absence of MUC19 in human saliva, as assessed by mass spectrometry, whereas Muc19 was identified in saliva of other mammalian species. Because the complete 5′-end of MUC19 had not been delineated, their model of the predicted N terminus encompassed the region containing three VWD2 domains but excluded the LAT domain (see Fig. 6), which appears to be unique to humans. MUC19 peptides identified in the human salivary proteome are near the 5′-end of the LAT domain, suggesting the more downstream region containing three VWD domains is protected from attack by trypsin. Within the LAT domain are predicted serine sites of O-glycosylation. Glycosylation within this domain and/or extensive glycosylation of the tandem repeat domain bordering the VWD region may therefore hinder access to trypsin. Moreover, in their assay of tryptic peptides from the VWD regions of Muc19 from other mammalian species, horse, rat, pig, and cow, Rousseau et al. (20) detected 25, 20, 8, and 3 peptides, respectively. Because this region shares 66% protein sequence identity between the four species (20), their results suggest species differences in steric hindrance to trypsin, possibly due to differences in the extent of glycosylation and/or oligosaccharide structures of the tandem repeat domain (93).
Differences and Similarities in Expression of Salivary Gel-forming Mucins between Mice and Humans
Although Muc19 and MUC5B appear to represent the predominant gel-forming mucin in murine and human saliva, respectively, we provide evidence for MUC19 expression in humans and previously detected Muc5b transcripts in murine minor mucous glands (21). Nevertheless, the relative expression of these mucins in saliva of each species remains unclear. In mice, Muc5b may account for the minor population of high molecular weight mucins observed in saliva from Muc19−/− mice. Decreased expression of human MUC19 may be related to decreased splicing efficiency of exons within the LAT domain, as splicing within this region is highly variable with some variants producing nonsense transcripts (19). Interestingly, this domain likely represents evolutionary remnants of a splice variant of the Muc19 gene encoding a small glycoprotein, Smgc, using exon 1 and Smgc-specific exons 2–18. Smgc is expressed in mucous cell precursors during rodent sublingual gland development prior to differentiation and concomitant switching to the Muc19 splice variant, utilizing exons 1 and 19–60 (19, 36, 37).
An exponential expansion of the S. mutans population occurred with the development of agriculture and the accompanying increase in dietary starch, with consequential positive selection in S. mutans for genes involved in sugar metabolism and acid tolerance (94). Assuming overlapping functions between MUC5B and MUC19 in controlling S. mutans colonization, the increase in dietary starch and associated caries pathogenesis of S. mutans may have favored increased MUC5B expression in the face of reduced MUC19 expression. Alternatively, other dietary, environmental, or microbial evolutionary pressures may have been better accommodated by MUC5B.
Previously, MUC5AC and MUC2 were immunolocalized to sporadic cells within glandular excretory ducts of human major and minor glands, whereas immunoreactivities were absent in acinar cells (95, 96). Immunoreactive ductal cells are likely goblet cells interspersed within glandular excretory ducts (97, 98). Our inability to detect MUC2 transcripts within intralobular tissue fragments of submandibular glands may be due to distribution of MUC2-positive cells to more distal extralobular excretory ducts (95, 96). In mice, we reported Muc2 transcripts are barely detectable in all minor mucous glands and the major salivary glands, including the serous parotid gland (21). We thus speculate Muc2 is restricted to goblet cells of extralobular excretory ducts, similar to humans. Our detection of MUC5AC transcripts in human submandibular glands, as well as Muc5ac transcripts in minor mucous glands of mice (21), may also represent expression in ductal goblet cells. However, low levels of expression in subpopulations of acinar mucous cells cannot be ruled out. MUC5AC and MUC2 in glandular ducts may function in localized innate and adaptive immune responses in response to bacterial invasion (99).
With respect to Muc6, we previously reported its absence in murine salivary glands (21), whereas evidence of MUC6 expression in human glands is conflicting. MUC6 immunoreactivity was reportedly absent in major or minor glands (95), although in another report a few submandibular mucous cells were immunoreactive in 10% of glands sampled (100). Our detection of MUC6 transcripts in human submandibular glands is more consistent with the latter study, suggesting that, unlike mice, MUC6 is a component of saliva, although likely a minor constituent.
Because of hindrance by glycosylation in identifying gel-forming mucins, additional studies are warranted that incorporate deglycosylation strategies to more clearly delineate the contribution of all gel-forming mucin species to human saliva, their distributions among the many different salivary mucous glands, variations in expression among individuals, interactions with S. mutans, and correlations with caries prevalence. Information from such studies may provide a framework for development of diagnostic markers or treatments for patients at a high risk for caries, such as those with hyposalivary function due either to autoimmune disease or irradiation of the head and neck region (101, 102).
Indirect Role for Muc19 in S. mutans Aggregation
Interestingly, we find that the addition of Muc19 to saliva from Muc19−/− mice restores aggregating activity to that of wild type saliva. Because Muc19 alone has very little aggregating activity, Muc19 must participate indirectly to aggregate S. mutans, likely through formation of heterotypic complexes with other salivary constituents that bind S. mutans. At least 38 human salivary proteins (those with a predicted signal peptide) have orthologs or paralogs in saliva of mice and/or rats (103). Unique to human saliva are additional proteins, including statherin-like proline-rich proteins, histatins, lysozyme, zinc α-glycoprotein, and the Ig saliva secretory complex (103). The 22 proteins unique to mice are thought to be involved mostly in wound healing, identification, or grooming (103).
In human saliva Wickstrom et al. (13) found oligomeric gel-forming mucins in noncovalent association with salivary sIgA, lactoferrin, lysozyme, MUC7, and GP340. Similarly, Raynal et al. (12) identified noncovalent association of high density mucins with cystatin, amylase, and GP340. MUC7 and GP340 are large glycoproteins that express blood group antigens (6–8, 104) and can bind and aggregate S. mutans (81–83). Therefore, MUC7 and GP340 likely contributed to the aggregating activity of crude high molecular weight fractions of saliva in early studies, as the fractions were prepared under nonreductive and nondissociative conditions.
In mice, gp340 binds S. mutans as well as other Gram-positive and Gram-negative bacteria (105). Additionally, Liu et al. (106) described mouse Muc10 and human MUC7 as homologues, as both are abundant in saliva, have protein cores of similar lengths, have N-terminal regions with similar compositions of basic amino acids, and contain two cysteines, which in MUC7 are within the binding domain of S. mutans (106). Both mucins also have a central region with serine- and threonine-rich tandem repeats, potential sites of O-glycosylation (22, 107). The two mucin genes further share equivalent positions within syntenic genomic regions (108). Importantly, saliva from Muc10−/− mice display decreased aggregation of S. mutans compared with the wild type, suggesting it also binds S. mutans.3
As described above, salivary gel-forming mucins of humans and mice exhibit similar characteristics in assays with S. mutans. Because Muc19 and MUC5B represent predominant gel-forming mucins in murine and human saliva, these two mucins apparently share many of the same functions in suppressing S. mutans colonization, suggesting at least some functional redundancies between different gel-forming mucins. We therefore hypothesize that gel-forming mucins participate in the aggregation of S. mutans by providing a scaffold for the association of salivary bacteria binding molecules (e.g. MUC7, GP340, and other constituents) as well as bacteriostatic or bactericidal proteins. These complexes thus help sequester, neutralize, and clear bacteria from the oral cavity, working in concert with constituents such as MUC7 and GP340 that may also act alone or within non-mucin-containing complexes to aggregate S. mutans. A role for gel-forming mucins in bacterial aggregation and clearance adds yet another protective role for these glycoproteins in the innate immune functions of saliva.
In light of species differences, mucin-protein interactions in mice may not necessarily be predictive of functional interactions in humans. It is therefore important to expand on our current limited information to fully delineate interactions between human gel-forming mucins and other salivary constituents and their role in bacterial aggregation and clearance.
Consideration of Mucin Glycoforms in Elucidation of Functions
Salivary gel-forming mucins are polydisperse in mass and charge densities, a result of multiple glycoforms that vary in either their extent of glycosylation and/or in their composition of several hundred unique oligosaccharides with different degrees of sialylation and sulfation (86, 109–111). Interestingly, human major and minor mucous glands display variations in mucin glycoforms (16, 87, 109), suggesting localized functional differences in anti-microbial actions and possibly formation of glycoform-specific heterotypic complexes. Moreover, chronic inflammatory conditions (i.e. ocular rosacea and rheumatoid arthritis) are associated with increased sulfation of salivary oligosaccharides (112, 113), likely due to expression of sulfotransferases in response to systemic inflammatory mediators (114). Mucin sulfation has been shown to promote colonic barrier function (115), adhesion of leukocytes and Helicobacter pylori (116), and resistance to bacterial mucin-degrading enzymes (117). Changes in mucin glycoforms in response to inflammation may therefore play a role in associated oral manifestations of systemic diseases (118). One may further postulate that inflammation accompanying severe caries affecting the tooth dentin and pulp may modify the profile of salivary mucin glycoforms and associated functions.
MUC19 and Systemic Diseases
In light of the association of S. mutans with blood-borne infections (4, 5), elucidation of innate mechanisms to control its oral colonization is relevant to systemic health. Recent genome-wide association studies identified MUC19 as a susceptibility gene of Crohn disease (119–121). Muc19 expression is absent in the gastrointestinal tract of mice (21, 122), as well as in cattle except for inconsistent and sparse expression in jejunum (123). MUC19 expression in the human gastrointestinal tract has not been studied extensively. We speculate that altered salivary expression of MUC19 affecting the oral microbiota may in turn influence the intestinal microbiota and immune homeostasis, through constant swallowing of bacteria aggregated within a complex containing acid-resistant gel-forming mucins. Interestingly, patients with Crohn disease reportedly have higher numbers of decayed tooth surfaces compared with controls (124). Studies of correlations between Crohn disease and salivary MUC19 may further add to the growing list of inter-relationships between the oral microbiota and both oral and systemic health (125, 126).
Acknowledgments
We acknowledge Robert Nguyen, Sarah L. Gullett, Richard L. Cannon, Pavandeep Kaur Birk, Helen N. Truong, Peter Habashy, Andrew V. Roman, Christopher S. Kuhns, Sneha Patel, Blair Cohen, Justin G. Myers, Lindsey Pikos, and Jillian Rose for excellent technical assistance. We thank Dr. Hardeep K. Chehal for assistance in obtaining human glandular tissues. We also thank Dr. Robert A. Burne for the UA159 strains of S. mutans.
This work was supported, in whole or in part, by National Institutes of Health Grants DE16362, DE014730, and DE020147 from NIDCR and Grant T90DE021990 (to G. C.). This work was also supported by University of Florida Research Opportunity Fund Award 83064 (to D. J. C.).
D. J. Culp, unpublished observations.
- VWD
- von Willebrand factor D.
REFERENCES
- 1. Marcenes W., Kassebaum N. J., Bernabé E., Flaxman A., Naghavi M., Lopez A., Murray C. J. (2013) Global burden of oral conditions in 1990–2010: a systematic analysis. J. Dent. Res. 92, 592–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Satcher D. (2000) Oral Health in America: A Report of the Surgeon General. United States Department of Health and Human Services, NIDCR, National Institutes of Health, Rockville, MD [Google Scholar]
- 3. Bowen W. H. (2002) Do we need to be concerned about dental caries in the coming millennium? Crit. Rev. Oral Biol. Med. 13, 126–131 [DOI] [PubMed] [Google Scholar]
- 4. Avilés-Reyes A., Miller J. H., Simpson-Haidaris P. J., Lemos J. A., Abranches J. (2014) Cnm is a major virulence factor of invasive Streptococcus mutans and part of a conserved three-gene locus. Mol. Oral Microbiol. 29, 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakano K., Nomura R., Matsumoto M., Ooshima T. (2010) Roles of oral bacteria in cardiovascular diseases–from molecular mechanisms to clinical cases: cell-surface structures of novel serotype k Streptococcus mutans strains and their correlation to virulence. J. Pharmacol. Sci. 113, 120–125 [DOI] [PubMed] [Google Scholar]
- 6. Scannapieco F. A. (1994) Saliva-bacterium interactions in oral microbial ecology. Crit. Rev. Oral Biol. Med. 5, 203–248 [DOI] [PubMed] [Google Scholar]
- 7. Tabak L. A. (1995) In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu. Rev. Physiol. 57, 547–564 [DOI] [PubMed] [Google Scholar]
- 8. Van Nieuw Amerongen A., Bolscher J. G., Veerman E. C. (2004) Salivary proteins: protective and diagnostic value in cariology? Caries Res. 38, 247–253 [DOI] [PubMed] [Google Scholar]
- 9. Glantz P. O. (1997) Interfacial phenomena in the oral cavity. Colloid Surface A 123, 657–670 [Google Scholar]
- 10. Offner G. D., Troxler R. F. (2000) Heterogeneity of high molecular-weight human salivary mucins. Adv. Dent Res. 14, 69–75 [DOI] [PubMed] [Google Scholar]
- 11. Schipper R. G., Silletti E., Vingerhoeds M. H. (2007) Saliva as research material: biochemical, physicochemical and practical aspects. Arch. Oral Biol. 52, 1114–1135 [DOI] [PubMed] [Google Scholar]
- 12. Raynal B. D., Hardingham T. E., Sheehan J. K., Thornton D. J. (2003) Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J. Biol. Chem. 278, 28703–28710 [DOI] [PubMed] [Google Scholar]
- 13. Wickström C., Christersson C., Davies J. R., Carlstedt I. (2000) Macromolecular organization of saliva: identification of ‘insoluble’ MUC5B assemblies and non-mucin proteins in the gel phase. Biochem. J. 351, 421–428 [PMC free article] [PubMed] [Google Scholar]
- 14. Rayment S. A., Liu B., Offner G. D., Oppenheim F. G., Troxler R. F. (2000) Immunoquantification of human salivary mucins MG1 and MG2 in stimulated whole saliva: factors influencing mucin levels. J. Dent. Res. 79, 1765–1772 [DOI] [PubMed] [Google Scholar]
- 15. Iontcheva I., Oppenheim F. G., Troxler R. F. (1997) Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich proteins, statherin, and histatins. J. Dent. Res. 76, 734–743 [DOI] [PubMed] [Google Scholar]
- 16. Sonesson M., Wickström C., Kinnby B., Ericson D., Matsson L. (2008) Mucins MUC5B and MUC7 in minor salivary gland secretion of children and adults. Arch. Oral Biol. 53, 523–527 [DOI] [PubMed] [Google Scholar]
- 17. Chen Y., Zhao Y. H., Kalaslavadi T. B., Hamati E., Nehrke K., Le A. D., Ann D. K., Wu R. (2004) Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am. J. Respir. Cell Mol. Biol. 30, 155–165 [DOI] [PubMed] [Google Scholar]
- 18. Kouznetsova I., Gerlach K. L., Zahl C., Hoffmann W. (2010) Expression analysis of human salivary glands by laser microdissection: differences between submandibular and labial glands. Cell. Physiol. Biochem. 26, 375–382 [DOI] [PubMed] [Google Scholar]
- 19. Zhu L., Lee P., Yu D., Tao S., Chen Y. (2011) Cloning and characterization of human MUC19 gene. Am. J. Respir. Cell Mol. Biol. 45, 348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rousseau K., Kirkham S., Johnson L., Fitzpatrick B., Howard M., Adams E. J., Rogers D. F., Knight D., Clegg P., Thornton D. J. (2008) Proteomic analysis of polymeric salivary mucins: no evidence for MUC19 in human saliva. Biochem. J. 413, 545–552 [DOI] [PubMed] [Google Scholar]
- 21. Das B., Cash M. N., Hand A. R., Shivazad A., Grieshaber S. S., Robinson B., Culp D. J. (2010) Tissue distibution of murine Muc19/Smgc gene products. J. Histochem. Cytochem. 58, 141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denny P. C., Mirels L., Denny P. A. (1996) Mouse submandibular gland salivary apomucin contains repeated N-glycosylation sites. Glycobiology 6, 43–50 [DOI] [PubMed] [Google Scholar]
- 23. Das B., Cash M. N., Robinson B., Kuhns C. S., Latchney L. R., Fallon M. A., Elliott R. W., Hand A. R., Culp D. J. (2013) The sld genetic defect: two intronic CA repeats promote insertion of the subsequent intron and mRNA decay. J. Biol. Chem. 288, 14742–14755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson G. E., Latchney L. R., Luo W., Hand A. R., Culp D. J. (1997) Biochemical and immunological studies and assay of rat sublingual mucins. Arch. Oral Biol. 42, 161–172 [DOI] [PubMed] [Google Scholar]
- 25. Culp D. J., Robinson B., Parkkila S., Pan P. W., Cash M. N., Truong H. N., Hussey T. W., Gullett S. L. (2011) Oral colonization by Streptococcus mutans and caries development is reduced upon deletion of carbonic anhydrase VI expression in saliva. Biochim. Biophys. Acta 1812, 1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keyes P. H. (1958) Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J. Dent. Res. 37, 1088–1099 [DOI] [PubMed] [Google Scholar]
- 27. Larson R. (1981) in Animal Models in Cariology (Tanzer J. M., ed) pp. 195–203, IRL Press, Oxford, UK [Google Scholar]
- 28. Emilson C. G., Bratthall D. (1976) Growth of Streptococcus mutans on various selective media. J. Clin. Microbiol. 4, 95–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paster B. J., Boches S. K., Galvin J. L., Ericson R. E., Lau C. N., Levanos V. A., Sahasrabudhe A., Dewhirst F. E. (2001) Bacterial diversity in human subgingival plaque. J. Bacteriol. 183, 3770–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen T., Yu W. H., Izard J., Baranova O. V., Lakshmanan A., Dewhirst F. E. (2010) The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010, baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cole J. R., Wang Q., Cardenas E., Fish J., Chai B., Farris R. J., Kulam-Syed-Mohideen A. S., McGarrell D. M., Marsh T., Garrity G. M., Tiedje J. M. (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, D141–D145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wen Z. T., Burne R. A. (2001) Construction of a new integration vector for use in Streptococcus mutans. Plasmid 45, 31–36 [DOI] [PubMed] [Google Scholar]
- 33. Barletta R. G., Curtiss R., 3rd (1989) Impairment of melibiose utilization in Streptococcus mutans serotype c gtfA mutants. Infect. Immun. 57, 992–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanzer J. M., Thompson A., Wen Z. T., Burne R. A. (2006) Streptococcus mutans: fructose transport, xylitol resistance, and virulence. J. Dent. Res 85, 369–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lemos J. A., Abranches J., Koo H., Marquis R. E., Burne R. A. (2010) Protocols to study the physiology of oral biofilms. Methods Mol. Biol. 666, 87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Culp D. J., Latchney L. R., Fallon M. A., Denny P. A., Denny P. C., Couwenhoven R. I., Chuang S. (2004) The gene encoding mouse Muc19: cDNA, genomic organization and relationship to Smgc. Physiol Genomics 19, 303–318 [DOI] [PubMed] [Google Scholar]
- 37. Das B., Cash M. N., Hand A. R., Shivazad A., Culp D. J. (2009) Expression of Muc19/Smgc gene products during murine sublingual gland development: cytodifferentiation and maturation of salivary mucous cells. J. Histochem. Cytochem. 57, 383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jay G. D., Culp D. J., Jahnke M. R. (1990) Silver staining of extensively glycosylated proteins on sodium dodecyl sulfate-polyacrylamide gels: enhancement by carbohydrate-binding dyes. Anal. Biochem. 185, 324–330 [DOI] [PubMed] [Google Scholar]
- 39. Denny P. A., Denny P. C. (1982) Localization of a mouse submandibular sialomucin by indirect immunofluorescence. Histochem. J. 14, 403–408 [DOI] [PubMed] [Google Scholar]
- 40. Culp D. J., Quivey R. Q., Bowen W. H., Fallon M. A., Pearson S. K., Faustoferri R. (2005) A mouse caries model and evaluation of aqp5−/− knockout mice. Caries Res. 39, 448–454 [DOI] [PubMed] [Google Scholar]
- 41. Barletta R. G., Michalek S. M., Curtiss R., 3rd (1988) Analysis of the virulence of Streptococcus mutans serotype c gtfA mutants in the rat model system. Infect. Immun. 56, 322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bisgaard M. (1986) Actinobacillus muris sp. nov. isolated from mice. Acta Pathol. Microbiol. Immunol. Scand. B Microbiol. 94, 1–8 [DOI] [PubMed] [Google Scholar]
- 43. Radaelli E., Manarolla G., Pisoni G., Balloi A., Aresu L., Sparaciari P., Maggi A., Caniatti M., Scanziani E. (2010) Suppurative adenitis of preputial glands associated with Corynebacterium mastitidis infection in mice. J. Am. Assoc. Lab. Anim. Sci. 49, 69–74 [PMC free article] [PubMed] [Google Scholar]
- 44. Gozalo A. S., Hoffmann V. J., Brinster L. R., Elkins W. R., Ding L., Holland S. M. (2010) Spontaneous Staphylococcus xylosus infection in mice deficient in NADPH oxidase and comparison with other laboratory mouse strains. J. Am. Assoc. Lab. Anim. Sci. 49, 480–486 [PMC free article] [PubMed] [Google Scholar]
- 45. Clavel T., Charrier C., Haller D. (2013) Streptococcus danieliae sp. nov., a novel bacterium isolated from the caecum of a mouse. Arch. Microbiol. 195, 43–49 [DOI] [PubMed] [Google Scholar]
- 46. Almirón M., Traglia G., Rubio A., Sanjuan N. (2013) Colonization of the mouse upper gastrointestinal tract by Lactobacillus murinus: a histological, immunocytochemical, and ultrastructural study. Curr. Microbiol. 67, 395–398 [DOI] [PubMed] [Google Scholar]
- 47. Borø S., McCartney C. A., Snelling T. J., Worgan H. J., McEwan N. R. (2010) Isolation of Streptococcus thoraltensis from rabbit faeces. Curr. Microbiol. 61, 357–360 [DOI] [PubMed] [Google Scholar]
- 48. Li J., Helmerhorst E. J., Leone C. W., Troxler R. F., Yaskell T., Haffajee A. D., Socransky S. S., Oppenheim F. G. (2004) Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 97, 1311–1318 [DOI] [PubMed] [Google Scholar]
- 49. Siqueira W. L., Custodio W., McDonald E. E. (2012) New insights into the composition and functions of the acquired enamel pellicle. J. Dent. Res. 91, 1110–1118 [DOI] [PubMed] [Google Scholar]
- 50. Vacca Smith A. M., Bowen W. H. (2000) In situ studies of pellicle formation on hydroxyapatite discs. Arch. Oral Biol. 45, 277–291 [DOI] [PubMed] [Google Scholar]
- 51. Zimmerman J. N., Custodio W., Hatibovic-Kofman S., Lee Y. H., Xiao Y., Siqueira W. L. (2013) Proteome and peptidome of human acquired enamel pellicle on deciduous teeth. Int. J. Mol. Sci. 14, 920–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clark W. B., Bammann L. L., Gibbons R. J. (1978) Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect. Immun. 19, 846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnsson M., Levine M. J., Nancollas G. H. (1993) Hydroxyapatite binding domains in salivary proteins. Crit. Rev. Oral Biol. Med. 4, 371–378 [DOI] [PubMed] [Google Scholar]
- 54. Nieuw Amerongen A. V., Oderkerk C. H., Veerman E. C. (1989) Interaction of human salivary mucins with hydroxyapatite. J. Biol. Buccale 17, 85–92 [PubMed] [Google Scholar]
- 55. Tabak L. A., Levine M. J., Jain N. K., Bryan A. R., Cohen R. E., Monte L. D., Zawacki S., Nancollas G. H., Slomiany A., Slomiany B. L. (1985) Adsorption of human salivary mucins to hydroxyapatite. Arch. Oral Biol. 30, 423–427 [DOI] [PubMed] [Google Scholar]
- 56. Al-Hashimi I., Levine M. J. (1989) Characterization of in vivo salivary-derived enamel pellicle. Arch. Oral Biol. 34, 289–295 [DOI] [PubMed] [Google Scholar]
- 57. Lee Y. H., Zimmerman J. N., Custodio W., Xiao Y., Basiri T., Hatibovic-Kofman S., Siqueira W. L. (2013) Proteomic evaluation of acquired enamel pellicle during in vivo formation. PLoS One 8, e67919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li J., Helmerhorst E. J., Corley R. B., Luus L. E., Troxler R. F., Oppenheim F. G. (2003) Characterization of the immunologic responses to human in vivo acquired enamel pellicle as a novel means to investigate its composition. Oral Microbiol. Immunol. 18, 183–191 [DOI] [PubMed] [Google Scholar]
- 59. Denny P., Hagen F. K., Hardt M., Liao L., Yan W., Arellanno M., Bassilian S., Bedi G. S., Boontheung P., Cociorva D., Delahunty C. M., Denny T., Dunsmore J., Faull K. F., Gilligan J., Gonzalez-Begne M., Halgand F., Hall S. C., Han X., Henson B., Hewel J., Hu S., Jeffrey S., Jiang J., Loo J. A., Ogorzalek Loo R. R., Malamud D., Melvin J. E., Miroshnychenko O., Navazesh M., Niles R., Park S. K., Prakobphol A., Ramachandran P., Richert M., Robinson S., Sondej M., Souda P., Sullivan M. A., Takashima J., Than S., Wang J., Whitelegge J. P., Witkowska H. E., Wolinsky L., Xie Y., Xu T., Yu W., Ytterberg J., Wong D. T., Yates J. R., 3rd, Fisher S. J. (2008) The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J. Proteome Res. 7, 1994–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Banderas-Tarabay J. A., Zacarías-D'Oleire I. G., Garduño-Estrada R., Aceves-Luna E., González-Begné M. (2002) Electrophoretic analysis of whole saliva and prevalence of dental caries. A study in Mexican dental students. Arch. Med. Res. 33, 499–505 [DOI] [PubMed] [Google Scholar]
- 61. Lazarevic V., Whiteson K., Huse S., Hernandez D., Farinelli L., Osterås M., Schrenzel J., François P. (2009) Metagenomic study of the oral microbiota by Illumina high throughput sequencing. J. Microbiol. Methods 79, 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chun J., Kim K. Y., Lee J. H., Choi Y. (2010) The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 10, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haukioja A., Loimaranta V., Tenovuo J. (2008) Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiol. Immunol. 23, 336–343 [DOI] [PubMed] [Google Scholar]
- 64. Kreth J., Merritt J., Qi F. (2009) Bacterial and host interactions of oral streptococci. DNA Cell Biol. 28, 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takahashi N., Nyvad B. (2008) Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 42, 409–418 [DOI] [PubMed] [Google Scholar]
- 66. Kishimoto E., Hay D. I., Gibbons R. J. (1989) A human salivary protein which promotes adhesion of Streptococcus mutans serotype c strains to hydroxyapatite. Infect. Immun. 57, 3702–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nieuw Amerongen A. V., Oderkerk C. H., Driessen A. A. (1987) Role of mucins from human whole saliva in the protection of tooth enamel against demineralization in vitro. Caries Res. 21, 297–309 [DOI] [PubMed] [Google Scholar]
- 68. Slomiany A., Slomiany B. L. (1978) Structures of the acidic oligosaccharides isolated from rat sublingual glycoprotein. J. Biol. Chem. 253, 7301–7306 [PubMed] [Google Scholar]
- 69. Kopec L. K., Bowen W. H. (1995) Adherence of microorganisms to rat salivary pellicles. Caries Res. 29, 507–512 [DOI] [PubMed] [Google Scholar]
- 70. Koo H., Nino de Guzman P., Schobel B. D., Vacca Smith A. V., Bowen W. H. (2006) Influence of cranberry juice on glucan-mediated processes involved in Streptococcus mutans biofilm development. Caries Res. 40, 20–27 [DOI] [PubMed] [Google Scholar]
- 71. Moye Z. D., Zeng L., Burne R. A. (2014) Modification of gene expression and virulence traits in Streptococcus mutans in response to carbohydrate availability. Appl. Environ. Microbiol. 80, 972–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Hoeven J. S., van den Kieboom C. W., Camp P. J. (1990) Utilization of mucin by oral Streptococcus species. Antonie Van Leeuwenhoek 57, 165–172 [DOI] [PubMed] [Google Scholar]
- 73. Mothey D., Buttaro B. A., Piggot P. J. (2014) Mucin can enhance growth, biofilm formation, and survival of Streptococcus mutans. FEMS Microbiol. Lett. 350, 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bradshaw D. J., Homer K. A., Marsh P. D., Beighton D. (1994) Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 140, 3407–3412 [DOI] [PubMed] [Google Scholar]
- 75. Wickström C., Svensäter G. (2008) Salivary gel-forming mucin MUC5B–a nutrient for dental plaque bacteria. Oral Microbiol. Immunol. 23, 177–182 [DOI] [PubMed] [Google Scholar]
- 76. Banas J. A. (2004) Virulence properties of Streptococcus mutans. Front. Biosci. 9, 1267–1277 [DOI] [PubMed] [Google Scholar]
- 77. Carlén A., Olsson J., Ramberg P. (1996) Saliva mediated adherence, aggregation and prevalence in dental plaque of Streptococcus mutans, Streptococcus sanguis and Actinomyces spp, in young and elderly humans. Arch. Oral Biol. 41, 1133–1140 [DOI] [PubMed] [Google Scholar]
- 78. Rosan B., Appelbaum B., Golub E., Malamud D., Mandel I. D. (1982) Enhanced saliva-mediated bacterial aggregation and decreased bacterial adhesion in caries-resistant versus caries-susceptible individuals. Infect. Immun. 38, 1056–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ligtenberg A. J., Veerman E. C., de Graaff J., Nieuw Amerongen A. V. (1990) Influence of the blood group reactive substances in saliva on the aggregation of Streptococcus rattus. Antonie Van Leeuwenhoek 57, 97–107 [DOI] [PubMed] [Google Scholar]
- 80. Prakobphol A., Leffler H., Fisher S. J. (1993) The high molecular-weight human mucin is the primary salivary carrier of ABH, Le(a), and Le(b) blood group antigens. Crit. Rev. Oral Biol. Med. 4, 325–333 [DOI] [PubMed] [Google Scholar]
- 81. Ligtenberg A. J., Walgreen-Weterings E., Veerman E. C., de Soet J. J., de Graaff J., Amerongen A. V. (1992) Influence of saliva on aggregation and adherence of Streptococcus gordonii HG 222. Infect. Immun. 60, 3878–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Murray P. A., Prakobphol A., Lee T., Hoover C. I., Fisher S. J. (1992) Adherence of oral streptococci to salivary glycoproteins. Infect. Immun. 60, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Slomiany B. L., Piotrowski J., Czajkowski A., Shovlin F. E., Slomiany A. (1993) Differential expression of salivary mucin bacterial aggregating activity with caries status. Int. J. Biochem. 25, 935–940 [DOI] [PubMed] [Google Scholar]
- 84. Issa S. M., Schulz B. L., Packer N. H., Karlsson N. G. (2011) Analysis of mucosal mucins separated by SDS-urea agarose polyacrylamide composite gel electrophoresis. Electrophoresis 32, 3554–3563 [DOI] [PubMed] [Google Scholar]
- 85. Thornton D. J., Howard M., Devine P. L., Sheehan J. K. (1995) Methods for separation and deglycosylation of mucin subunits. Anal. Biochem. 227, 162–167 [DOI] [PubMed] [Google Scholar]
- 86. Wickström C., Davies J. R., Eriksen G. V., Veerman E. C., Carlstedt I. (1998) MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem. J. 334, 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thornton D. J., Khan N., Mehrotra R., Howard M., Veerman E., Packer N. H., Sheehan J. K. (1999) Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology 9, 293–302 [DOI] [PubMed] [Google Scholar]
- 88. Troxler R. F., Iontcheva I., Oppenheim F. G., Nunes D. P., Offner G. D. (1997) Molecular characterization of a major high molecular weight mucin from human sublingual gland. Glycobiology 7, 965–973 [DOI] [PubMed] [Google Scholar]
- 89. Hand A. R., Pathmanathan D., Field R. B. (1999) Morphological features of the minor salivary glands. Arch. Oral Biol. 44, S3–S10 [DOI] [PubMed] [Google Scholar]
- 90. Loomes K. M., Senior H. E., West P. M., Roberton A. M. (1999) Functional protective role for mucin glycosylated repetitive domains. Eur. J. Biochem. 266, 105–111 [DOI] [PubMed] [Google Scholar]
- 91. van der Post S., Subramani D. B., Bäckström M., Johansson M. E., Vester-Christensen M. B., Mandel U., Bennett E. P., Clausen H., Dahlén G., Sroka A., Potempa J., Hansson G. C. (2013) Site-specific O-glycosylation on the MUC2 mucin protein inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB). J. Biol. Chem. 288, 14636–14646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nielsen P. A., Bennett E. P., Wandall H. H., Therkildsen M. H., Hannibal J., Clausen H. (1997) Identification of a major human high molecular weight salivary mucin (MG1) as tracheobronchial mucin MUC5B. Glycobiology 7, 413–419 [DOI] [PubMed] [Google Scholar]
- 93. Gerken T. A., Gupta R., Jentoft N. (1992) A novel approach for chemically deglycosylating O-linked glycoproteins. The deglycosylation of submaxillary and respiratory mucins. Biochemistry 31, 639–648 [DOI] [PubMed] [Google Scholar]
- 94. Cornejo O. E., Lefébure T., Bitar P. D., Lang P., Richards V. P., Eilertson K., Do T., Beighton D., Zeng L., Ahn S. J., Burne R. A., Siepel A., Bustamante C. D., Stanhope M. J. (2013) Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol. Biol. Evol. 30, 881–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Alos L., Lujan B., Castillo M., Nadal A., Carreras M., Caballero M., de Bolos C., Cardesa A. (2005) Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am. J. Surg. Pathol. 29, 806–813 [DOI] [PubMed] [Google Scholar]
- 96. Handra-Luca A., Lamas G., Bertrand J. C., Fouret P. (2005) MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications. Am. J. Surg. Pathol. 29, 881–889 [DOI] [PubMed] [Google Scholar]
- 97. Pinkstaff C. A. (1980) The cytology of salivary glands. Int. Rev. Cytol. 63, 141–261 [DOI] [PubMed] [Google Scholar]
- 98. Rother P. (1963) The secretory function of the parotid duct and the submandibular duct. Acta Histochem. 15, 325–346 [PubMed] [Google Scholar]
- 99. Brandtzaeg P. (2013) Secretory immunity with special reference to the oral cavity. J. Oral Microbiol. 10.3402/jom.v6.26102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nakajima K., Ota H., Zhang M. X., Sano K., Honda T., Ishii K., Nakayama J. (2003) Expression of gastric gland mucous cell-type mucin in normal and neoplastic human tissues. J. Histochem. Cytochem. 51, 1689–1698 [DOI] [PubMed] [Google Scholar]
- 101. Castro I., Sepúlveda D., Cortés J., Quest A. F., Barrera M. J., Bahamondes V., Aguilera S., Urzúa U., Alliende C., Molina C., González S., Hermoso M. A., Leyton C., González M. J. (2013) Oral dryness in Sjogren's syndrome patients. Not just a question of water. Autoimmun. Rev. 12, 567–574 [DOI] [PubMed] [Google Scholar]
- 102. Dijkema T., Terhaard C. H., Roesink J. M., Raaijmakers C. P., van den Keijbus P. A., Brand H. S., Veerman E. C. (2012) MUC5B levels in submandibular gland saliva of patients treated with radiotherapy for head-and-neck cancer: a pilot study. Radiat. Oncol. 7, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Karn R. C., Chung A. G., Laukaitis C. M. (2013) Shared and unique proteins in human, mouse and rat saliva proteomes: Footprints of functional adaptation. Proteomes 1, 275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ligtenberg A. J., Veerman E. C., Nieuw Amerongen A. V. (2000) A role for Lewis a antigens on salivary agglutinin in binding to Streptococcus mutans. Antonie Van Leeuwenhoek 77, 21–30 [DOI] [PubMed] [Google Scholar]
- 105. Madsen J., Tornøe I., Nielsen O., Lausen M., Krebs I., Mollenhauer J., Kollender G., Poustka A., Skjødt K., Holmskov U. (2003) CRP-ductin, the mouse homologue of gp-340/deleted in malignant brain tumors 1 (DMBT1), binds Gram-positive and Gram-negative bacteria and interacts with lung surfactant protein D. Eur. J. Immunol. 33, 2327–2336 [DOI] [PubMed] [Google Scholar]
- 106. Liu B., Rayment S. A., Gyurko C., Oppenheim F. G., Offner G. D., Troxler R. F. (2000) The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral streptococci and exhibits candidacidal activity. Biochem. J. 345, 557–564 [PMC free article] [PubMed] [Google Scholar]
- 107. Bobek L. A., Tsai H., Biesbrock A. R., Levine M. J. (1993) Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J. Biol. Chem. 268, 20563–20569 [PubMed] [Google Scholar]
- 108. Williams O. W., Sharafkhaneh A., Kim V., Dickey B. F., Evans C. M. (2006) Airway mucus: from production to secretion. Am. J. Respir. Cell Mol. Biol. 34, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bolscher J., Veerman E., Van Nieuw Amerongen A., Tulp A., Verwoerd D. (1995) Distinct populations of high M(r) mucins secreted by different human salivary glands discriminated by density-gradient electrophoresis. Biochem. J. 309, 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Thomsson K. A., Prakobphol A., Leffler H., Reddy M. S., Levine M. J., Fisher S. J., Hansson G. C. (2002) The salivary mucin MG1 (MUC5B) carries a repertoire of unique oligosaccharides that is large and diverse. Glycobiology 12, 1–14 [DOI] [PubMed] [Google Scholar]
- 111. Veerman E. C., van den Keijbus P. A., Nazmi K., Vos W., van der Wal J. E., Bloemena E., Bolscher J. G., Amerongen A. V. (2003) Distinct localization of MUC5B glycoforms in the human salivary glands. Glycobiology 13, 363–366 [DOI] [PubMed] [Google Scholar]
- 112. Ozcan S., An H. J., Vieira A. C., Park G. W., Kim J. H., Mannis M. J., Lebrilla C. B. (2013) Characterization of novel O-glycans isolated from tear and saliva of ocular rosacea patients. J. Proteome Res. 12, 1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Flowers S. A., Ali L., Lane C. S., Olin M., Karlsson N. G. (2013) Selected reaction monitoring to differentiate and relatively quantitate isomers of sulfated and unsulfated core 1 O-glycans from salivary MUC7 protein in rheumatoid arthritis. Mol. Cell. Proteomics 12, 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kreda S. M., Davis C. W., Rose M. C. (2012) CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb. Perspect. Med. 2, a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kawashima H. (2012) Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol. Pharm. Bull. 35, 1637–1641 [DOI] [PubMed] [Google Scholar]
- 116. Prakobphol A., Borén T., Ma W., Zhixiang P., Fisher S. J. (2005) Highly glycosylated human salivary molecules present oligosaccharides that mediate adhesion of leukocytes and Helicobacter pylori. Biochemistry 44, 2216–2224 [DOI] [PubMed] [Google Scholar]
- 117. Derrien M., van Passel M. W., van de Bovenkamp J. H., Schipper R. G., de Vos W. M., Dekker J. (2010) Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 1, 254–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chi A. C., Neville B. W., Krayer J. W., Gonsalves W. C. (2010) Oral manifestations of systemic disease. Am. Fam. Physician 82, 1381–1388 [PubMed] [Google Scholar]
- 119. Kumar V., Mack D. R., Marcil V., Israel D., Krupoves A., Costea I., Lambrette P., Grimard G., Dong J., Seidman E. G., Amre D. K., Levy E. (2013) Genome-wide association study signal at the 12q12 locus for Crohn's disease may represent associations with the MUC19 gene. Inflamm. Bowel Dis. 19, 1254–1259 [DOI] [PubMed] [Google Scholar]
- 120. Rivas M. A., Beaudoin M., Gardet A., Stevens C., Sharma Y., Zhang C. K., Boucher G., Ripke S., Ellinghaus D., Burtt N., Fennell T., Kirby A., Latiano A., Goyette P., Green T., Halfvarson J., Haritunians T., Korn J. M., Kuruvilla F., Lagacé C., Neale B., Lo K. S., Schumm P., Törkvist L., National Institute of Diabetes and Digestive Kidney Diseases Inflammatory Bowel Disease Genetics Consortium (NIDDK IBDGC), United Kingdom Inflammatory Bowel Disease Genetics Consortium, International Inflammatory Bowel Disease Genetics Consortium, Dubinsky M. C., Brant S. R., Silverberg M. S., Duerr R. H., Altshuler D., Gabriel S., Lettre G., Franke A., D'Amato M., McGovern D. P., Cho J. H., Rioux J. D., Xavier R. J., Daly M. J. (2011) Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 43, 1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Umeno J., Asano K., Matsushita T., Matsumoto T., Kiyohara Y., Iida M., Nakamura Y., Kamatani N., Kubo M. (2011) Meta-analysis of published studies identified eight additional common susceptibility loci for Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 17, 2407–2415 [DOI] [PubMed] [Google Scholar]
- 122. Kerschner J. E., Hong W., Taylor S. R., Kerschner J. A., Khampang P., Wrege K. C., North P. E. (2013) A novel model of spontaneous otitis media with effusion (OME) in the Oxgr1 knock-out mouse. Int. J. Pediatr. Otorhinolaryngol 77, 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hoorens P. R., Rinaldi M., Li R. W., Goddeeris B., Claerebout E., Vercruysse J., Geldhof P. (2011) Genome wide analysis of the bovine mucin genes and their gastrointestinal transcription profile. BMC Genomics 12, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sundh B., Johansson I., Emilson C. G., Nordgren S., Birkhed D. (1993) Salivary antimicrobial proteins in patients with Crohn's disease. Oral Surg. Oral Med. Oral Pathol. 76, 564–569 [DOI] [PubMed] [Google Scholar]
- 125. Aagaard K., Ma J., Antony K. M., Ganu R., Petrosino J., Versalovic J. (2014) The placenta harbors a unique microbiome. Sci. Transl. Med. 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Han Y. W., Wang X. (2013) Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 92, 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]