Abstract
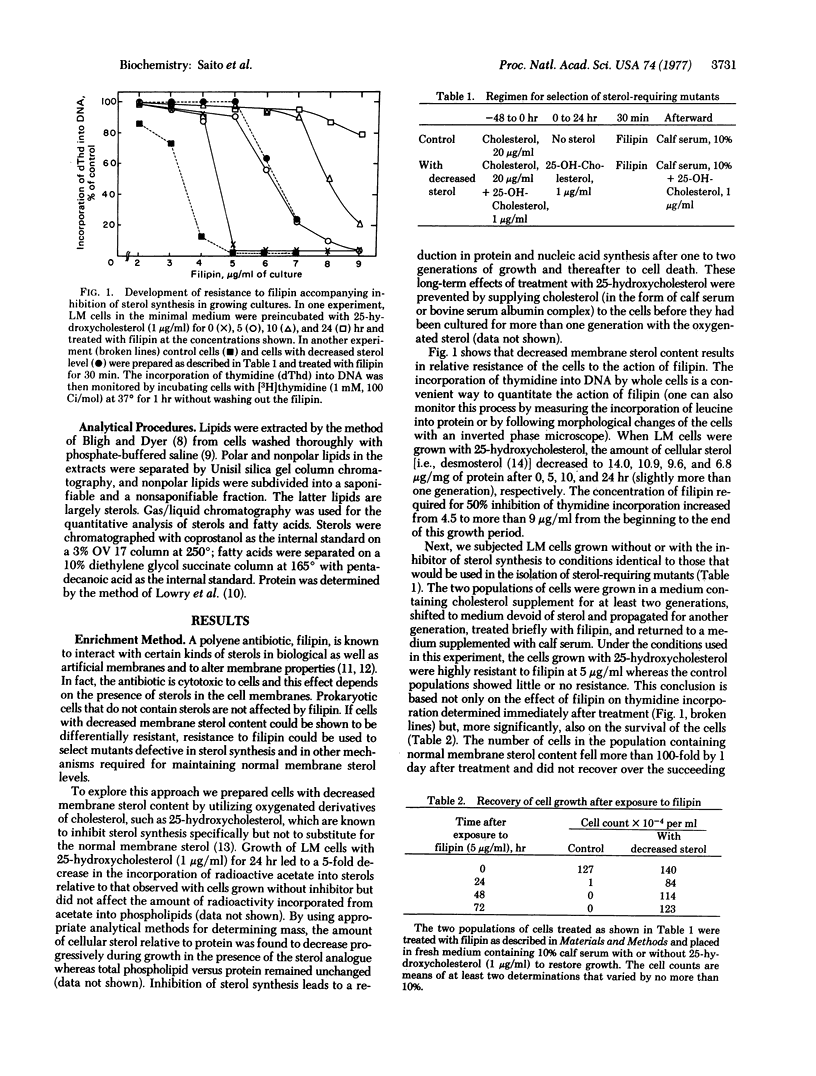
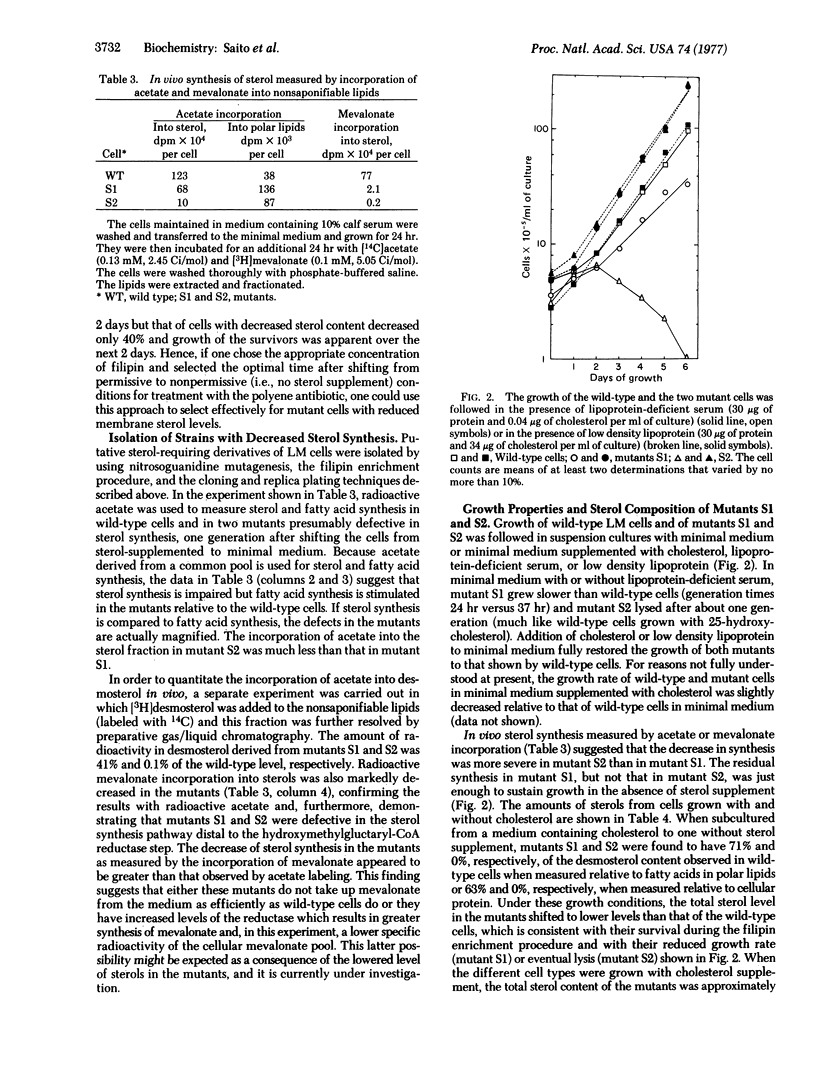
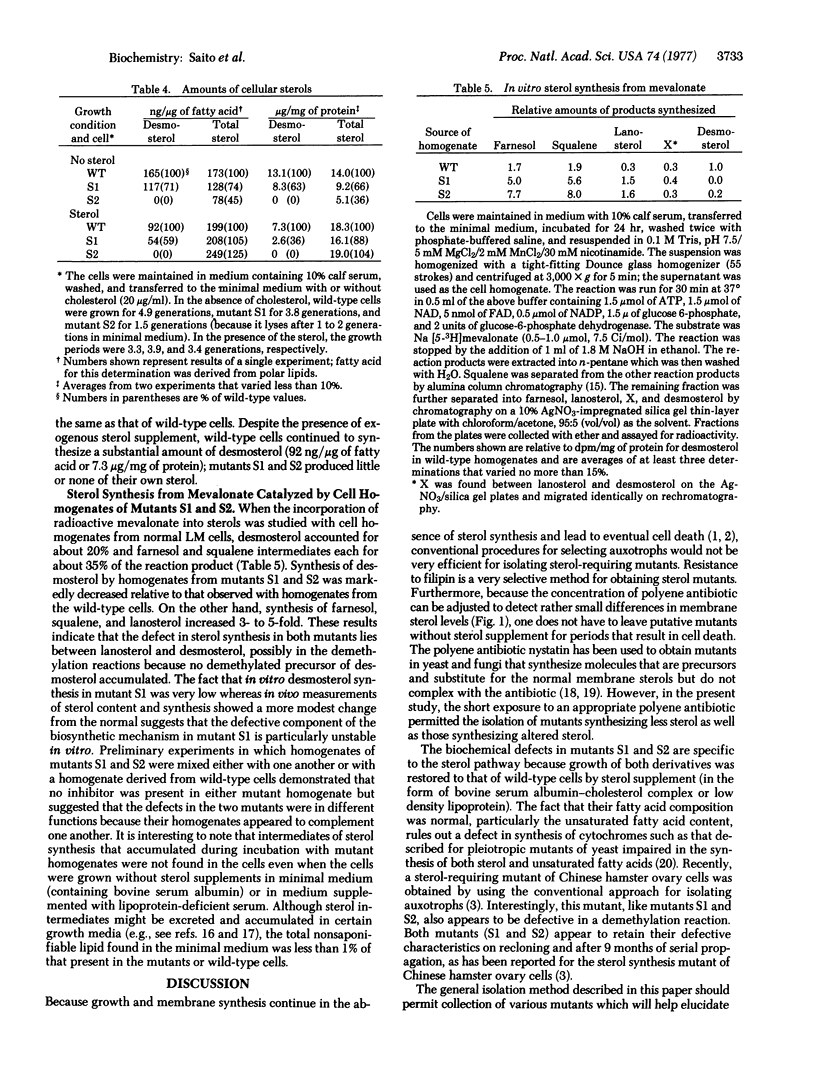
By using a chemically defined medium, a general and highly specific procedure was devised to select for mutant cells with less abundant or structurally altered sterol in their surface membranes. Within a certain concentration range, the polyene antibiotic filipin was shown to kill only cells with normal (as opposed to decreased) membrane sterol levels. Sterol-requiring derivatives of LM cells were isolated by chemical mutagenesis, filipin treatment, and cloning followed by replica plating in soft agar. Mutants (S1 and S2) are described which, when compared to normal cells, show decreased synthesis of demosterol in vivo from acetate and mevalonate relative to cell number or to fatty acid synthesis. When exogenous sterol is supplied, mutants S1 and S2 grow normally in suspension culture. However, when deprived of sterol supplement, mutant S1 grows slower than wild type cells and mutant S2 lyses within one to two generations. Gas/liquid chromatography revealed that the mutants contained a normal spectrum of fatty acids including unsaturated fatty acyl groups but, unlike wildtype cells, they have less abundant (mutant S1) or no (mutant S2) desmosterol in either the presence or absence of exogenous cholesterol. In vitro experiments with mevalonate as the substrate suggest that the defect in both mutants is in a demethylation reaction subsequent to lanosterol synthesis. The selection method developed here may permit the isolation of mutants with defective membrane incorporation of sterols and other polyisoprenoids as well as defective synthesis of these compounds.
Keywords: sterol biosynthesis, polyene antibiotic, membrane biogenesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bard M. Biochemical and genetic aspects of nystatin resistance in saccharomyces cerevisiae. J Bacteriol. 1972 Sep;111(3):649–657. doi: 10.1128/jb.111.3.649-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. Y., Telakowski C., Heuvel W. V., Alberts A. W., Vagelos P. R. Isolation and partial characterization of a cholesterol-requiring mutant of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):832–836. doi: 10.1073/pnas.74.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Kandutsch A. A., Waymouth C. Inhibition of cell growth by oxygenated derivatives of cholesterol. Nature. 1974 Oct 4;251(5474):419–421. doi: 10.1038/251419a0. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Fogelman A. M., Popják G. A direct relationship between the amount of sterol lost from rat hepatocytes and the increase in activity of HMG-CoA reductase. Biochem Biophys Res Commun. 1976 Jan 12;68(1):64–69. doi: 10.1016/0006-291x(76)90010-3. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Edwards P. A., Popják G. Mechanism of induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human leukocytes. J Biol Chem. 1977 Jan 25;252(2):644–651. [PubMed] [Google Scholar]
- Higuchi K. An improved chemically defined culture medium for strain L mouse cells based on growth responses to graded levels of nutrients including iron and zinc ions. J Cell Physiol. 1970 Feb;75(1):65–72. doi: 10.1002/jcp.1040750108. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Consequences of blocked sterol synthesis in cultured cells. DNA synthesis and membrane composition. J Biol Chem. 1977 Jan 25;252(2):409–415. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem. 1974 Oct 10;249(19):6057–6061. [PubMed] [Google Scholar]
- Karst F., Lacroute F. Isolation of pleiotropic yeast mutants requiring ergosterol for growth. Biochem Biophys Res Commun. 1973 Jun 8;52(3):741–747. doi: 10.1016/0006-291x(73)90999-6. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C. Antibiotic interaction with model membranes. Annu Rev Pharmacol. 1970;10:119–142. doi: 10.1146/annurev.pa.10.040170.001003. [DOI] [PubMed] [Google Scholar]
- Kuroki T. Agar plate cultrue and Lederberg-style replica plating of mammalian cells. Methods Cell Biol. 1975;9(0):157–178. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morris D. C., Safe S., Subden R. E. Detection of the ergosterol and episterol isomers lichesterol and fecosterol in nystatin-resistant mutants of Neurospora crassa. Biochem Genet. 1974 Dec;12(6):459–466. doi: 10.1007/BF00486063. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Demel R. A., de Kruyff B., van Deenen L. L. Studies on the biological properties of polyene antibiotics. Evidence for the direct interaction of filipin with cholesterol. J Biol Chem. 1972 Mar 25;247(6):1918–1929. [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., Demel R. A., van Deenen L. L. The effect of cholesterol and epicholesterol incorporation on the permeability and on the phase transition of intact Acholeplasma laidlawii cell membranes and derived liposomes. Biochim Biophys Acta. 1972 Jan 17;255(1):331–347. doi: 10.1016/0005-2736(72)90032-6. [DOI] [PubMed] [Google Scholar]


