Abstract
Notwithstanding the advances in tumor research, diagnosis, and treatment, breast cancer is still a challenge worldwide. This global burden of disease has been associated with population aging and the persistence of cancer-related behaviors. The number of women diagnosed with breast cancer has been estimated as increasing, especially in middle-income countries such as Brazil. Estimates from the Instituto Nacional de Câncer (INCA) point to breast cancer as the major malignant neoplasia in Brazilian women and the main cause of death from cancer in the country. This fact has been associated with increased life expectancy, urbanization, and cancer-related behaviors. Given this scenario, it is clear that there is a need for identifying and discussing which factors have substantially contributed to this growing number of cases in Brazil, including access to treatment, prevention and early diagnosis, weaknesses of the local health policy, and intrinsic genetic peculiarities of the Brazilian population. This review aims to address the role of such factors.
Keywords: breast cancer, treatment, prevention, epidemiology, Brazil, cancer screening, mammograms, health policies
Breast cancer epidemiology in Brazil
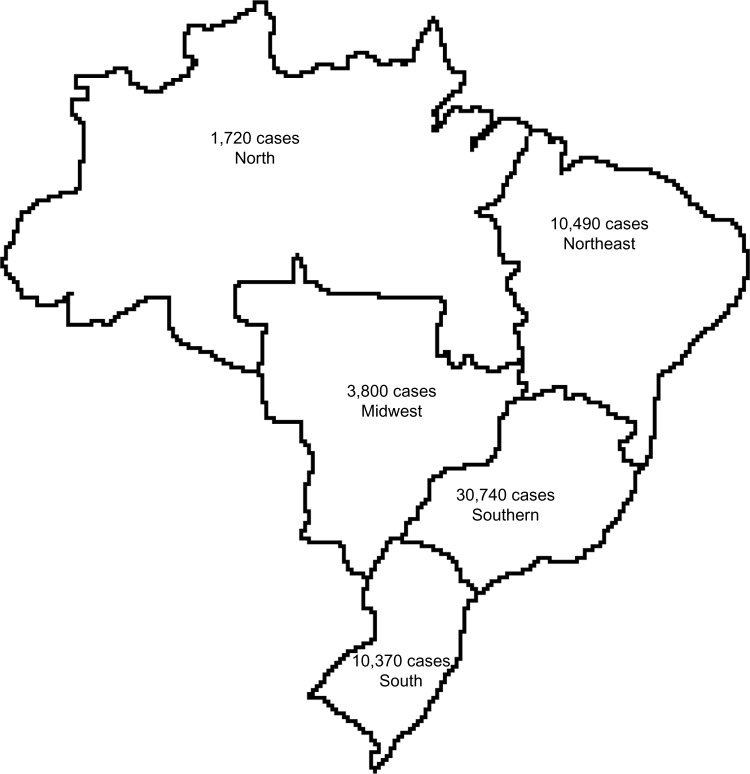
Brazil currently has a population of about 200 million people, with a variety of ethnicities distributed in a wide territory that is divided into five geographic regions (Figure 1). In 2010, the Brazilian population was composed of 48% Caucasians, 44% multiracial, and 7% African descent.1 The health system is organized as two types: the public health system (called Sistema Único de Saúde [SUS]) and the private system, supported individually by the population. It is estimated that about 75% of the population are currently users of the public health system.
Figure 1.
Representative geographic distribution of Brazilian macroregions and their respective cancer estimates for the year 2014.
Notes: The main population agglomerates are the South and Southern Regions. Data were obtained from the Instituto Nacional de Câncer (INCA) (2014).23
SUS was created in 1988, during the reformulation of the Brazilian Federal Constitution, through a law known as Organic Law of SUS (Lei Orgânica do SUS 8.080), aiming to ensure the complete access of all Brazilians to a public and unpaid health system. Thenceforth, the SUS has become one of the most important social achievements known in the world to aim to ensure free access to health care. The principles that guide this initiative are based on the decentralization and universalization of health services.2
In spite of the efforts to improve diagnosis and treatment, breast cancer is one of the main challenges faced by the Brazilian government. Global statistics demonstrated that 60% of deaths related to breast cancer occur in economically developing countries such as Brazil.3 The Instituto Nacional de Câncer (INCA) is responsible for providing the epidemiological data regarding cancer statistics in the country, and registers data from different regions of Brazil (Figure 1). The southern region has the highest incidence rates of breast cancer.
It was estimated that about 13,000 women died from breast cancer in 2010.4 Over the last few decades, breast cancer has been pointed to as the leading cause of death from cancer among Brazilian women, as well as the main malignant cancer that affects the female population. Azevedo and Mendonça5 analyzed the breast cancer statistics for 1986, before the implementation of SUS. They showed that 12.8% of Brazilian women died in that year due to cancer causes, and breast cancer was the leading cause of those deaths. Surprisingly, the authors described that the incidence rates of breast cancer were 176.9 cases per 100,000 women in the south region, reaching 277.3 cases per 100,000 women in Sao Paulo in the early 1980s.
Another analysis, published by Moraes in 1998,6 after SUS policy implementation and initial consolidation, revealed that the total number of breast cancer cases was about 32,000 – of which 6.6% were deaths from cancer in that year. Additionally, the author highlighted the huge heterogeneity observed throughout the different geographic regions of the country, in which the rates of disease could vary from 29.15 cases per 100,000 women in the north region to 66.12 cases per 100,000 women in the South Region. Importantly, since that time, the staging profile of women with breast cancer in Brazil was characterized by advanced stages of the disease, evidencing the late diagnosis as a national public health issue. It is important to highlight that Brazil has a diverse ethnic population.
Interestingly, we can observe that the incidence of breast cancer in South American countries is half that of European countries (about 44 cases per 100,000 Latin women versus 84 cases per 100,000 women in Northern Europe). On the other hand, although there is a substantial difference in the number of cases, the mortality rates are similar.7 This reinforces the fact that developing countries such as Brazil are subject to severe problems regarding the access to health services, diagnosis, and modern treatments.
Mortality rates of breast cancer in Brazil
Overview
Figure 2 shows the profile of deaths by breast cancer in Brazil according to the DATASUS online database.8 During the period ranging from 1992–2012, a progressive increase in the mortality rates due to breast cancer disease in Brazil was observed. Furthermore, Table 1 shows the number of deaths associated with breast cancer and specific age groups in the period 1992–2012.8 The highest number of deaths is observed in the age group ranging from 50–69 years (45.5% of the cases). In this context, several factors must be considered, especially regarding the increased number of death notifications related to this disease. Concerning this issue, INCA and DATASUS implemented the SISMAMA, an online tool that registers information about breast and gynecological cancer screening.9 In the period from 2009–2013, the SISMAMA registered more than 12 million mammograms nationwide. About 1.4% of these cases indicated a high cancer risk, corresponding to approximately 168,000 cases. An important set of mammograms report pathological alterations in women aged over 40 years, which may be related to aging.10,11
Figure 2.
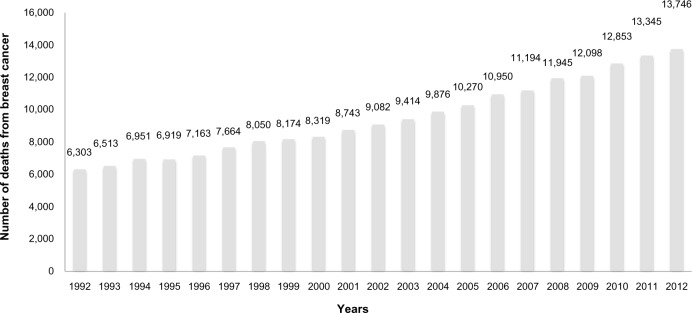
Breast cancer mortality rates in Brazil in the period 1992–2012.
Note: Data were collected from the DATASUS online database (DATASUS, 2011).8
Table 1.
Number of deaths associated with breast cancer and specific age groups in the period 1992–2012
| Age group (years) | Number of deaths from breast cancer | % |
|---|---|---|
| 30–39 | 14,374 | 7.1 |
| 40–49 | 37,279 | 18.6 |
| 50–59 | 48,910 | 24.5 |
| 60–69 | 41,975 | 21.0 |
| 70–79 | 32,346 | 16.7 |
| Over 80 | 22,825 | 11.4 |
Note: Data were collected from the DATASUS online database (DATASUS, 2014).8
The contributions of specific risk factors for explaining breast cancer rates in Brazilian women are considered and discussed as follows.
Biological features
In part, the deaths associated with breast cancer may be associated with the aggressive biological profile of tumors. The determination of the molecular cancer subtype is globally achieved by immunohistochemical analysis. In Brazil, testing for the molecular markers of breast cancer is not standardized, and it is not present in all cancer centers.4 Data concerning the immunohistochemical profiling of breast cancer in patients attended by SUS have demonstrated that the Brazilian women with breast cancer presents a similar molecular pattern of disease when compared to other regions of the globe, with predominance of the luminal subtype among the affected population,12–16 which represents a good prognosis for treatment response and disease survival. Endocrine and genetic factors are among the main prognostic determinants for breast cancer.17
Molecular studies have shown the presence of pivotal known gene modifications in breast tumors, such as BRCA mutations, especially in Brazil. BRCA is the main known gene that confers breast cancer susceptibility, and one of the first studies reporting such mutations in Brazilian women with familial history was reported by Dufloth et al in 2005.18 The authors reported that the prevalence of BRCA1/BRCA2 mutations in Brazilian women in the southern region diagnosed with breast cancer was 13%. Other studies have been conducted to investigate the prevalence of BRCA-related mutations in Brazil. An investigation aiming to investigate the risk factors associated with hereditary breast and ovarian cancer syndrome was conducted by Ewald et al.19 The authors identified a high frequency of BRCA founder mutation (c.5266dup) in Brazilian women diagnosed with bilateral breast cancer (5% of 137 investigated women). It is important to highlight that this prevalence is similar to that found in other populations.20 Finally, as is happening worldwide, the aging of the Brazilian population is increasing year by year.21
Population screening and early diagnosis
The current methods available for breast cancer screening and diagnosis are able to detect the disease at early stages. Among the variety of factors that contribute to the high mortality of breast cancer worldwide, the lack of availability of early detection services is a global problem for developing countries.3 Although mammograms are encouraged by the SUS and offered without any cost to the target female population, the screenings often fail to provide accurate results. Undoubtedly, the delay that occurs between the initial suspected lesion and the confirmation of diagnosis affects the outcome profiling of breast cancer21 and is still a problem in Brazil.
TNM (tumor, node, metastasis) staging of women diagnosed with breast cancer in Brazil is presented predominantly as advanced disease. A prospective study from the Grupo Brasileiro de Estudos do Câncer de Mama (GBECAM) assessed epidemiological data from women diagnosed with breast cancer in all Brazilian regions and showed that about 40% of diagnosed women were staged at TNM III–IV. The results revealed that the median age at diagnosis of such patients is about 58 years, and indicated that these women with breast cancer assisted by SUS presented advanced disease at diagnosis and poor prognosis, probably due to reduced access to modern therapies.22
Brazil uses the Breast Image Reporting and Data System (BI-RADS) to categorize the results obtained from mammogram screenings.9 An overview of the BI-RADS classification of mammograms in Brazilian women for breast cancer screening is presented in Table 2. In accordance with Ministry of Health policies, mammograms are encouraged by SUS, targeting the female population over 50 years of age and being performed every 2 years, or annually in the case of altered clinical exams. Women presenting with familial history are encouraged to undergo annual screening of the breasts.23 It is estimated that 50% of Brazilian women older than 50 years have had at least one mammography in their life,24 but there are no reliable data regarding posterior follow-up by a new mammogram. The International Agency for Research on Cancer25 recommends that at least 70% of the target female population should be screened with mammograms.26 Following the recommendations from the International Agency for Research on Cancer could further substantially contribute to understanding the mortality rates observed in the last 20 years, which are represented in Figure 2. Educational programs are strongly encouraged by the government, since, unfortunately, there is a lack of awareness among Brazilian women about the relevance of early detection via annual mammograms after the age of 50 years.
Table 2.
Distribution according to the BI-RADS of mammograms performed in the period 2009–2013
| Age group (years) | BI-RADS 0, n | BI-RADS 4, n | BI-RADS 5, n | BI-RADS 6, n | Total, n |
|---|---|---|---|---|---|
| 35–39 | 66,386 | 6,166 | 744 | 474 | 73,770 |
| 40–44 | 239,799 | 20,152 | 1,716 | 852 | 262,519 |
| 45–49 | 296,264 | 27,979 | 2,526 | 1,228 | 327,997 |
| 50–54 | 269,948 | 28,595 | 3,076 | 1,299 | 302,918 |
| 55–59 | 198,735 | 22,964 | 3,033 | 1,155 | 225,887 |
| 60–64 | 137,011 | 17,479 | 2,773 | 900 | 158,163 |
| 65–69 | 80,756 | 12,056 | 2,164 | 678 | 95,654 |
| Over 70 | 69,893 | 14,480 | 3,697 | 1,101 | 89,171 |
Note: Data were collected from the DATASUS online database (DATASUS, 2014).8
Abbreviation: BI-RADS, Breast Image Reporting and Data System.
Screening policies have to be urgently expanded, since it is estimated that only 60% of the target population had mammograms in 2008.27 A survey conducted by the Brazilian Society of Mastology (Sociedade Brasileira de Mastologia [SBM]) indicated that there were 4,228 mammogram devices in Brazil in 2013, with more than one-half available under SUS, totaling 2,226 devices.28 The Ministry of Health recommends one piece of equipment per 240,000 habitants; therefore, this number is more than sufficient.29 Therefore, the problem is not due to a lack of equipment, but to the geographic concentration of these devices in the more developed areas.1
Lifestyle
Globally, we have observed continuous increasing changes in lifestyle are observable among developing countries. In the past, Brazil was an essentially agricultural country, whose growing industrial and economic development began after the end of the military political regime (early 1990s), when the economic opening of the country toward purchase of imported products occurred. The increased purchasing power subsequently changed the eating habits of the population toward the attainment of manufactured products. Since then, a gradual increase in the weight of the population has been observed; currently, about 30% of individuals are pre-obese/obese.25 This lifestyle modification, in association with increased physical inactivity, extensive working hours, and intensive exposure to carcinogens, helps to explain the cumulative growing incidence of cancer in the Brazilian population year after year.30,31 In addition, the increasing incidences of hormonal contraceptive use, postmenopausal hormone replacement therapy, nulliparity, late age at first birth, and enhanced alcohol consumption by women have increased the risk for breast cancer development in all countries.3
Challenges in therapeutic approaches
At the end of the 1980s, Brazil experienced a political movement toward economic opening of its borders and the entry of new technologies, as well as access to imported drugs. At that time, there was little research regarding the development of new drugs in Brazil, and this fact strongly limited access to the chemotherapeutic drugs available in developed countries. Thus, the chemotherapeutic regimens in Brazil could not follow the international guidelines of the early 1990s, because the necessary drugs for breast cancer treatment were not available. Therefore, oncologists adapted such protocols according to the availability of such drugs in the country (the main drugs are shown in Figure 3). Furthermore, scientific publications regarding this theme at that time are scarce.
Figure 3.
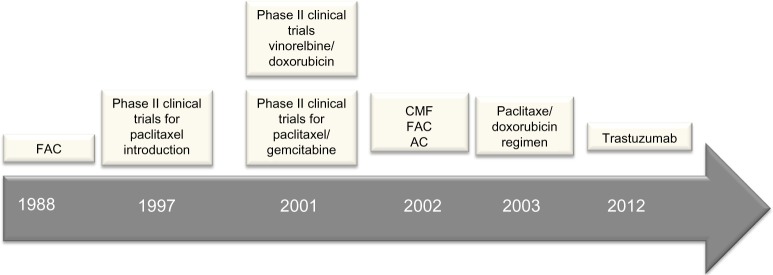
Timeline representing the main treatment options employed under Brazil’s public health system (SUS) for treating breast cancer patients.
Abbreviations: AC, adriamycin, cyclophosphamide; CMF, cyclophosphamide, methotrexate, 5-fluorouracil; FAC, 5-fluorouracil, adriamycin, cyclophosphamide; SUS, Sistema Único de Saúde.
Historically, the first report after SUS implementation in Brazil was published by Baracat et al,32 a reference group until nowadays in the field of breast cancer chemotherapy. The group reported the use of the CAF protocol (cyclophosphamide, doxorubicin, fluorouracil) for treating Brazilian women diagnosed with inflammatory breast cancer. A subsequent study was published in 1997, and described the Phase II clinical evaluation of doxorubicin/paclitaxel plus G-CSF for metastatic breast cancer in patients attending the Hospital das Clínicas de Porto Alegre.33 In the same year, Murad et al34 published the Phase II clinical trial for paclitaxel and ifosfamide testing in Brazil, intended for treating women with metastatic disease.
The 2000s were years of ongoing chemotherapy clinical trials in Brazil, and several international publications reported the advances. In 2001, Murad et al tested paclitaxel/gemcitabine in a Phase II clinical trial for metastatic breast cancer treatment.35 Hegg et al,36 in the same year, published the Phase II clinical trial for fractionated vinorelbine/doxorubicin as first-line therapy for advanced breast cancer. Another study, conducted by Costa et al in 2002,37 described advances occurring in the field of chemotherapy, most of them based on anthracycline-based regimens. The authors reported the use of several protocols for breast cancer treatment, such as the CMF protocol (cyclophosphamide, methotrexate, 5-fluorouracil), the FAC protocol (5-fluorouracil, adriamycin, cyclophosphamide), and the FEC (5-fluorouracil, epirubicin, and cyclophosphamide) and AC protocols (adriamycin, cyclophosphamide).
The current SUS protocol, based on paclitaxel (175 mg/m2) and doxorubicin (60 mg/m2), began last decade in Brazil, in about the year 2003.38 Also in 2003, publications reported the use of the sentinel lymph node biopsy in a pilot study in Brazil.39 In 2012, trastuzumab-based chemotherapy was introduced in SUS. A study regarding the use of trastuzumab in the Brazilian population was published in the same year.40
When establishing a comparative timeline, there is a clear delay between the introduction of chemotherapeutic drugs in developed countries and its usage in the Brazilian population. Nevertheless, considering that SUS is a public health system that offers prevention, diagnosis, and treatment for breast cancer at no cost, it is important to emphasize that, despite its limitations, the system has achieved important gains for the population. Breast cancer chemotherapy is a high-cost treatment, which most of the Brazilian population would not be able to afford. In this context, SUS policies have worked to ensure free and fast access to oncologic treatments, as well as to support all the phases of diagnosis and follow-up. To regulate these actions, the National Policy of Oncologic Attention (Política Nacional de Atenção Oncológica) was recently created, which aims to develop actions to ensure that all oncologic patients have full access to the public health system.41,42
Perspectives and conclusion
Brazil is a model example of a country that has developed a successful public health system. The SUS faces a wide range of challenges concerning full access, early diagnosis, and modern therapeutics, as well expansion of their coverage area across the country. Pivotal advances have been achieved, which resulted in the National Policy of Oncologic Attention. Investment in local research must be strongly expanded, and should serve as the basis for the development of targeted strategies directed to the particularities of the Brazilian population. This concentration of effort could also facilitate the implementation of more expensive treatments against breast cancer under the SUS and means that high-cost/better quality treatments would be more accessible to the Brazilian population.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Instituto Brasileiro de Geografia e Estatística (IBGE) Escassez e Fartura: Distribuição da Oferta de Equipamentos de Diagnóstico por Imagem no Brasil [Scarcity and abundance: distribution of supply diagnostic imaging equipment in Brazil] Rio de Janeiro: IBGE; 2009. [Accessed August 2, 2014]. [cited February 14, 2013]. Available from: http://www.ibge.gov.br/home/estatistica/populacao/indic_sociosaude/2009/com_esca.pdf. Portuguese. [Google Scholar]
- 2.Ministério da Saúde. Secretaria Executiva . SUS: Princípios e Conquistas [SUS: Principles and Achievements] Brasília: Ministério da Saúde; 2000. [Accessed August 4, 2014]. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/sus_principios.pdf. Portuguese. [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Lee BL, Liedke PE, Barrios CH, Simon SD, Finkelstein DM, Goss PE. Breast cancer in Brazil: present status and future goals. Lancet Oncol. 2012;13(3):e95–e102. doi: 10.1016/S1470-2045(11)70323-0. [DOI] [PubMed] [Google Scholar]
- 5.Azevedo G, Mendonça S. Cancer in the female population in Brazil. Rev Saude Publica. 1993;27(1):68–75. Portuguese. [PubMed] [Google Scholar]
- 6.Moraes MF. A mortalidade por câncer de mama no Brasil [Mortality from breast cancer in Brazil] Revista Brasileira de Cancerologia. 1998;44:2. Portuguese. [Google Scholar]
- 7.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 8.DATASUS [database on the Internet] Ministério da Saúde; 2008. [Accessed July 28, 2014]. Available from: http://www2.datasus.gov.br/DATASUS/index.php?area=01. Portuguese. [Google Scholar]
- 9.SISMAMA . Sistema de Informação do Controle do Câncer de Mama – manual gerencial [Information Systems for Control of Breast Cancer – Manual Management]. Equipe de Elaboração: Maria Beatriz Kneipp Dias. Rio de Janeiro: Brazillian Ministry of Health; [Accessed July 28, 2014]. Available from: http://www2.inca.gov.br/wps/wcm/connect/cancermama/site/home/sismama. Portuguese. [Google Scholar]
- 10.Prata PR. A transição epidemiológica no Brasil [The epidemiologic transition in Brazil] Cad Saude Publica. 1992;8:168–175. Portuguese. [Google Scholar]
- 11.Doll R. Are we winning the fight against cancer? An epidemiological assessment. EACR – Mühlbock memorial lecture. Eur J Cancer. 1990;26:500–508. doi: 10.1016/0277-5379(90)90025-o. [DOI] [PubMed] [Google Scholar]
- 12.Herrera AC, Panis C, Victorino VJ, et al. Molecular subtype is determinant on inflammatory status and immunological profile from invasive breast cancer patients. Cancer Immunol Immunother. 2012;61:2193–2201. doi: 10.1007/s00262-012-1283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panis C, Victorino VJ, Herrera AC, et al. Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat. 2012;133:881–888. doi: 10.1007/s10549-011-1851-1. [DOI] [PubMed] [Google Scholar]
- 14.Panis C, Lemos LG, Victorino VJ, et al. Immunological effects of taxol and adryamicin in breast cancer patients. Cancer Immunol Immunother. 2012;61:481–488. doi: 10.1007/s00262-011-1117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panis C, Herrera AC, Victorino VJ, et al. Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res Treat. 2012;133:89–97. doi: 10.1007/s10549-011-1693-x. [DOI] [PubMed] [Google Scholar]
- 16.Panis C, Herrera AC, Victorino VJ, Aranome A, Cecchini R. Screening of circulating TGF-β levels and its clinicopathological significance in human breast cancer. Anticancer Res. 2013;33:737–742. [PubMed] [Google Scholar]
- 17.Adami HO, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. 2nd ed. Oxford: Oxford University Press; 2008. [Google Scholar]
- 18.Dufloth RM, Carvalho S, Heinrich JK, et al. Analysis of BRCA1 and BRCA2 mutations in Brazilian breast cancer patients with positive family history. Sao Paulo Med J. 2005;123(4):192–197. doi: 10.1590/S1516-31802005000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewald IP, Izetti P, Vargas FR, et al. Prevalence of the BRCA1 founder mutation c.5266dupin Brazilian individuals at-risk for the hereditary breast and ovarian cancer syndrome. Hered Cancer Clin Pract. 2011;9:12. doi: 10.1186/1897-4287-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koifman S, Koifman RJ. Environment and cancer in Brazil: an overview from a public health perspective. Mutat Res. 2003;544(2–3):305–311. doi: 10.1016/j.mrrev.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon SD, Bines J, Barrios CH, et al. Clinical characteristics and outcome of treatment of Brazilian women with breast cancer treated at public and private institutions – the AMAZONE project of the Brazilian breast cancer study group (GBECAM); Abstracts of the 2009 CTRC-AACR San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, TX. Suppl to volume 69, Issue 24 of Cancer Research. [Google Scholar]
- 23.Instituto Nacional de Câncer . Controle do Câncer de Mama: fatores de risco [Breast cancer control: risk factors] [webpage on the Internet] Rio de Janeiro: Ministério da Saúde; [Accessed July 28, 2014]. Available from: http://www2.inca.gov.br/wps/wcm/connect/acoes_programas/site/home/nobrasil/programa_controle_cancer_mama/fatores_risco. Portuguese. [Google Scholar]
- 24.Lima-Costa MF, Matos DL. Prevalence and factors associated with mammograms in the 50–69-year age group: a study based on the Brazilian National Household Sample Survey (PNAD-2003) Cad Saude Publica. 2007;23:1665–1673. doi: 10.1590/s0102-311x2007000700018. Portuguese. [DOI] [PubMed] [Google Scholar]
- 25.Boyle P, Levin B. World Cancer Report 2008. Lyon: International Agency for Research on Cancer; 2008. [Accessed August 15th, 2014]. Available from: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/ [Google Scholar]
- 26.Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12:387–398. doi: 10.1016/S1470-2045(11)70031-6. [DOI] [PubMed] [Google Scholar]
- 27.FIOCRUZ – Cobertura de mamografia – PROADESSII – Fiocruz. 2011. [Accessed August 2, 2014]. Available from: http://www.proadess.icict.fiocruz.br/index.php?pag=fic&cod=A07&tab=1. Portuguese.
- 28.Freitas RF, Rahal RMS, Rodrigues DCN. Dados sobre produção de mamografias no Brasil, em 2012 e 2013 e número de mamógrafos em uso em 2013 [Data regarding mammograms in Brazil, between 2012 and 2013, and the number of mammographs]. Fevereiro de 2014. [Accessed July 28, 2014]. Available from: http://www.sbmastologia.com.br/index/index.php/component/content/article/50-destaque-medico/321-dados-sobre-producao. Portuguese.
- 29.Portaria n.° 1101/GM [Ordinance No 1101 / GM] Brasília: Ministério da Saúde; 2002. [Accessed November 19, 2014]. Available from: http://dtr2001.saude.gov.br/sas/PORTARIAS/Port2002/Gm/GM-1101.htm. Portuguese. [Google Scholar]
- 30.Laurenti R. Transição demográfica e transição epidemiológica [Demographic and epidemiologic transition]; Anais do 1° Congresso Brasileiro de Epidemiologia; 1990 set 2–6; Campinas, Brasil. Rio de Janeiro: Abrasco; 1990. pp. 143–165. Portuguese. [Google Scholar]
- 31.Albala C, Vio F, Yanez M. Transición epidemiológica en América Latina: comparación de cuatro países [Epidemiological transition in Latin America: comparison of 4 countries] Rev Med Chil. 1997;125(6):719–727. Portuguese. [PubMed] [Google Scholar]
- 32.Baracat FF, de Lima GR, de Oliveira AB, Baracat F, Baracat F. Inflammatory carcinoma of the breast. Experience of 43 cases at the Instituto do Câncer Arnaldo Vieira de Carvalho. Rev Paul Med. 1988;106(1):14–20. Portuguese. [PubMed] [Google Scholar]
- 33.Schwartsmann G, Mans DR, Menke CH, et al. A phase II study of doxorubicin/paclitaxel plus G-CSF for metastatic breast cancer. Oncology (Williston Park) 1997;11(4 Suppl 3):24–29. [PubMed] [Google Scholar]
- 34.Murad AM, Guimaraes RC, Amorim WC, Morici AC, Ferreira-Filho AF, Schwartsmann G. Phase II trial of the use of paclitaxel and gemcitabine as a salvage treatment in metastatic breast cancer. Breast Cancer Res Treat. 1997;45(1):47–53. doi: 10.1023/a:1005882314735. [DOI] [PubMed] [Google Scholar]
- 35.Murad AM, Guimarães RC, Aragão BC, Scalabrini’Neto AO, Rodrigues VH, Garcia R. Gemcitabine and paclitaxel as salvage therapy in metastatic breast cancer. Oncology (Williston Park) 2001;15(2 Suppl 3):25–27. [PubMed] [Google Scholar]
- 36.Hegg R, Costa MA, Perdicaris M, et al. A phase II trial of fractionated vinorelbine/doxorubicin as first line therapy for advanced breast cancer. Curr Med Res Opin. 2001;16(4):225–234. doi: 10.1185/030079901750176726. [DOI] [PubMed] [Google Scholar]
- 37.Costa LJ, Varella PC, del Giglio A. Weight changes during chemotherapy for breast cancer. Sao Paulo Med J. 2002;120(4):113–117. doi: 10.1590/S1516-31802002000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anelli A, Brentani RR, Gadelha AP, Amorim De Albuquerque A, Soares F. Correlation of p53 status with outcome of neoadjuvant chemotherapy using paclitaxel and doxorubicin in stage IIIB breast cancer. Ann Oncol. 2003;14(3):428–432. doi: 10.1093/annonc/mdg104. [DOI] [PubMed] [Google Scholar]
- 39.Piato JR, Barros AC, Pincerato KM, Sampaio AP, Pinotti JA. Sentinel lymph node biopsy in breast cancer after neoadjuvant chemotherapy. A pilot study. Eur J Surg Oncol. 2003;29(2):118–120. doi: 10.1053/ejso.2002.1349. [DOI] [PubMed] [Google Scholar]
- 40.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 41.Ministério da Saúde (Br). Secretaria de Atenção à Saúde . Controle dos Cânceres do Colo do Útero e da Mama [Control of breast and uterine cancers] Brasília: Ministério da Saúde; 2014. Portuguese. [Google Scholar]
- 42.Ministério da Saúde (Br). Secretaria de Atenção à Saúde . Controle dos Cânceres do Colo do Útero e da Mama [Control of breast and uterine cancers] Brasília: Ministério da Saúde; 2006. Portuguese. [Google Scholar]