Abstract
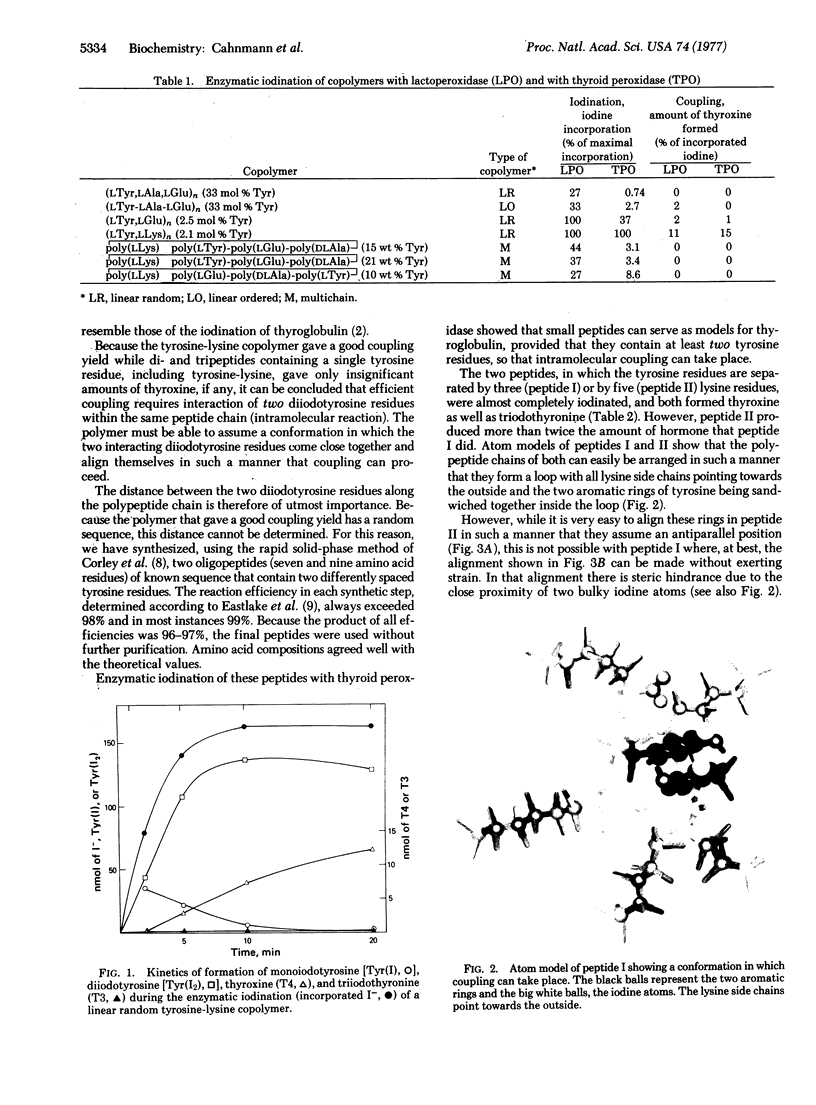
A linear random copolymer of tyrosine and lysine and two synthetic oligopeptides containing two tyrosine residues in addition to lysine residues give thyroid hormone (thyroxine and triodothyronine) residues in good yield upon enzymatic iodination with thyroid peroxidase. These synthetic peptides may serve as simple models for thyroglobulin, the protein in which biosynthesis of the thyroid hormone takes place. For the formation of significant amounts of hormone, such model compounds must contain at least two properly spaced tyrosine residues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corley L., Sachs D. H., Anfinsen C. B. Rapid solid-phase synthesis of bradykinin. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1353–1359. doi: 10.1016/0006-291x(72)90221-5. [DOI] [PubMed] [Google Scholar]
- Dème D., Fimiani E., Pommier J., Nunez J. Free diiodotyrosine effects on protein iodination and thyroid hormone synthesis catalyzed by thyroid peroxidase. Eur J Biochem. 1975 Feb 21;51(2):329–336. doi: 10.1111/j.1432-1033.1975.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Eastlake A., Curd J. G., Schechter A. N. The NH2-terminal region of the beta chain of sickle hemoglobin. I. Synthesis and purification of oligopeptides. J Biol Chem. 1976 Oct 25;251(20):6426–6430. [PubMed] [Google Scholar]
- Haeberli A., Bilstad J., Edelhoch H., Rall J. E. Elementary chain composition of guinea pig thyroglobulin. J Biol Chem. 1975 Sep 25;250(18):7294–7299. [PubMed] [Google Scholar]
- Lamas L., Dorris M. L., Taurog A. Evidence for a catalytic role for thyroid peroxidase in the conversion of diiodotyrosine to thyroxine. Endocrinology. 1972 Jun;90(6):1417–1426. doi: 10.1210/endo-90-6-1417. [DOI] [PubMed] [Google Scholar]
- Ogawara H., Bilstad J. M., Cahnmann H. J. Iodoamino acid distribution in thyroglobulin iodinated in vivo and in vitro. Biochim Biophys Acta. 1972 Feb 29;257(2):339–349. doi: 10.1016/0005-2795(72)90286-3. [DOI] [PubMed] [Google Scholar]
- Pommier J., Deme D., Nunez J. Effect of iodide concentration on thyroxine synthesis catalysed by thyroid peroxidase. Eur J Biochem. 1973 Sep 3;37(3):406–414. doi: 10.1111/j.1432-1033.1973.tb03000.x. [DOI] [PubMed] [Google Scholar]
- Pommier J., de Prailauné S., Nunez J. Peroxydase particulaire thyroïdienne. Biochimie. 1972;54(4):483–492. doi: 10.1016/s0300-9084(72)80233-5. [DOI] [PubMed] [Google Scholar]
- Vassart G., Refetoff S., Brocas H., Dinsart C., Dumont J. E. Translation of thyroglobulin 33S messenger RNA as a means of determining thyroglobulin quaternary structure. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3839–3843. doi: 10.1073/pnas.72.10.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]



