Abstract
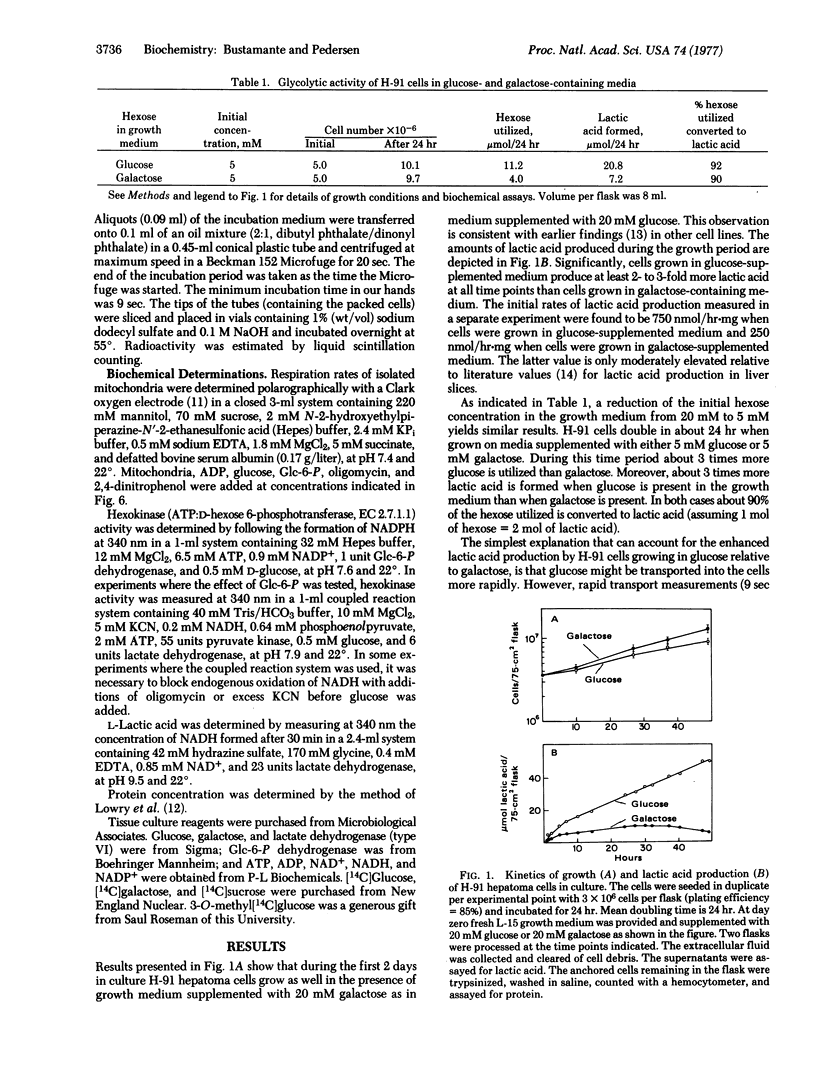
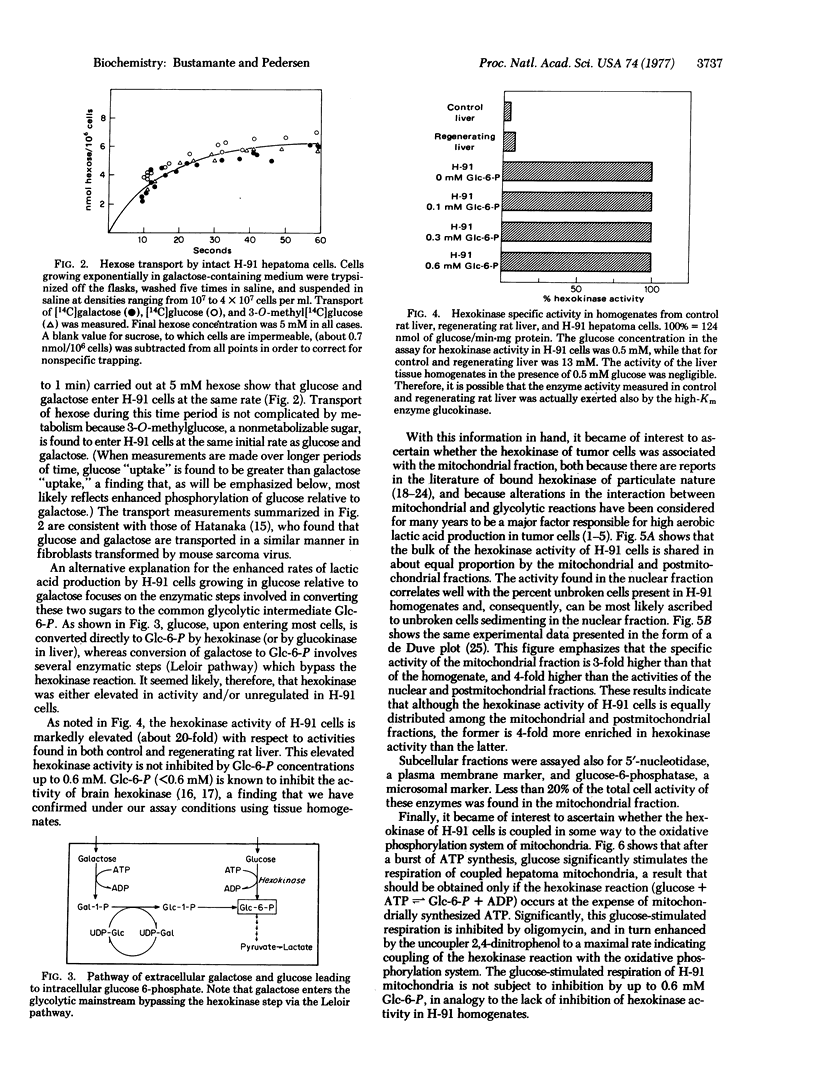
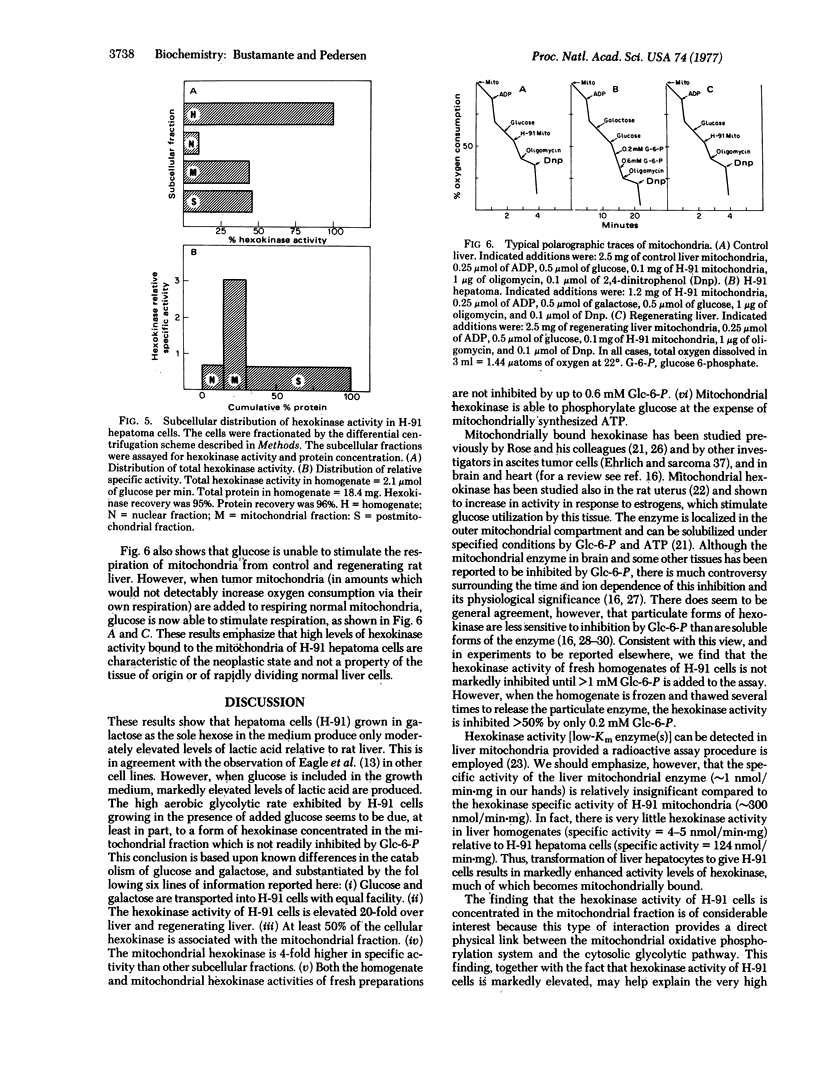
A tumorigenic anchorage-dependent cell line (H-91) was established in culture from an azo-dye-induced rat ascites hepatoma. When grown in a glucose-containing medium the cells exhibit high rates of lactic acid production characteristic of rapidly growing tumor cells. However, when glucose is replaced with galactose the cells grow equally well but exhibit only moderately elevated rates of lactic acid production. The molecular basis for this observation cannot be attributed to differences in permeability because initial rates of glucose and galactose entry into hepatoma cells are identical. Rather, the activity of hexokinase (ATP:D-hexose 6-phosphotransferase, EC 2.7.1.1) is found to be high in hepatoma cells, about 20-fold higher than that of control and regenerating rat liver. Moreover, tumor hexokinase activity is not inhibited by low concentrations (<0.6 mM) of the reaction product glucose 6-phosphate. Additionally, 50% of the hexokinase activity of hepatoma cells is found associated with the mitochondrial fraction. This fraction is 3-fold enriched in hexokinase activity relative to the homogenate and 4-fold enriched relative to the nuclear and postmitochondrial fractions. Tumor mitochondrial hexokinase appears to be coupled directly to oxidative phosphorylation, because addition of glucose to respiring hepatoma mitochondria (after a burst of ATP synthesis) results in stimulation of respiration. In contrast, glucose has no effect on the respiration of mitochondria from control and regenerating liver. These results suggest that the high glycolytic capacity of H-91 hepatoma cells is due, at least in part, to an elevated form of hexokinase concentrated in the mitochondrial fraction of the cell.
Keywords: L-lactic acid, D-glucose, D-galactose, liver, neoplasia
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquer N. A., McLean P. The effect of oestrogen on the activity and binding of multiple forms of hexokinase in the rat uterus. Biochem Biophys Res Commun. 1969 Sep 24;37(1):158–164. doi: 10.1016/0006-291x(69)90894-8. [DOI] [PubMed] [Google Scholar]
- Bartley J. C., Barber S., Abraham S. Identity, release, and binding of mitochondrial-bound hexokinases in mammary glands and adenocarcinomas of lactating mice. Cancer Res. 1975 Jul;35(7):1649–1653. [PubMed] [Google Scholar]
- Burk D., Woods M., Hunter J. On the significance of glucolysis for cancer growth, with special reference to Morris rat hepatomas. J Natl Cancer Inst. 1967 Jun;38(6):839–863. [PubMed] [Google Scholar]
- Bustamante E., Soper J. W., Pedersen P. L. A high-yield preparative method for isolation of rat liver mitochondria. Anal Biochem. 1977 Jun;80(2):401–408. doi: 10.1016/0003-2697(77)90661-3. [DOI] [PubMed] [Google Scholar]
- EAGLE H., BARBAN S., LEVY M., SCHULZE H. O. The utilization of carbohydrates by human cell cultures. J Biol Chem. 1958 Sep;233(3):551–558. [PubMed] [Google Scholar]
- Garfinkel L. Computer simulation study of hexokinase II from Ehrlich ascites cells. Eur J Biochem. 1975 Feb 21;51(2):437–447. doi: 10.1111/j.1432-1033.1975.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Gosalvez M., Pérez-García J., Weinhouse S. Competition for ADP between pyruvate kinase and mitochondrial oxidative phosphorylation as a control mechanism in glycolysis. Eur J Biochem. 1974 Jul 1;46(1):133–140. doi: 10.1111/j.1432-1033.1974.tb03605.x. [DOI] [PubMed] [Google Scholar]
- Gots R. E., Bessman S. P. The functional compartmentation of mitochondrial hexokinase. Arch Biochem Biophys. 1974 Jul;163(1):7–14. doi: 10.1016/0003-9861(74)90448-2. [DOI] [PubMed] [Google Scholar]
- Graziani Y. Bioflavonoid regulation of ATPase and hexokinase activity in Ehrlich ascites cell mitochondria. Biochim Biophys Acta. 1977 May 11;460(2):364–373. doi: 10.1016/0005-2728(77)90221-3. [DOI] [PubMed] [Google Scholar]
- Hatanaka M. Saturable and nonsaturable process of sugar uptake: effect of oncogenic transformation in transport and uptake of nutrients. J Cell Physiol. 1976 Dec;89(4):745–749. doi: 10.1002/jcp.1040890435. [DOI] [PubMed] [Google Scholar]
- Knull H. R., Taylor W. F., Wells W. W. Insulin effects on brain energy metabolism and the related hexokinase distribution. J Biol Chem. 1974 Nov 10;249(21):6930–6935. [PubMed] [Google Scholar]
- Koobs D. H. Phosphate mediation of the Crabtree and Pasteur effects. Science. 1972 Oct 13;178(4057):127–133. doi: 10.1126/science.178.4057.127. [DOI] [PubMed] [Google Scholar]
- Kosow D. P., Rose I. A. Ascites tumor mitochondrial hexokinase II. Effect of binding on kinetic properties. J Biol Chem. 1968 Jul 10;243(13):3623–3630. [PubMed] [Google Scholar]
- Kosow D. P., Rose I. A. Origin of the delayed feedback control of glucose utilization in ascites tumor cells. Biochem Biophys Res Commun. 1972 Jul 25;48(2):376–383. doi: 10.1016/s0006-291x(72)80061-5. [DOI] [PubMed] [Google Scholar]
- LEIBOVITZ A. THE GROWTH AND MAINTENANCE OF TISSUE-CELL CULTURES IN FREE GAS EXCHANGE WITH THE ATMOSPHERE. Am J Hyg. 1963 Sep;78:173–180. doi: 10.1093/oxfordjournals.aje.a120336. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Racker E. Bioenergetics and the problem of tumor growth. Am Sci. 1972 Jan-Feb;60(1):56–63. [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Mitochondrial hexokinase. Release, rebinding, and location. J Biol Chem. 1967 Apr 10;242(7):1635–1645. [PubMed] [Google Scholar]
- SWEENEY M. J., ASHMORE J., MORRIS H. P., WEBER G. COMPARATIVE BIOCHEMISTRY HEPATOMAS. IV. ISOTOPE STUDIES OF GLUCOSE AND FRUCTOSE METABOLISM IN LIVER TUMORS OF DIFFERENT GROWTH RATES. Cancer Res. 1963 Aug;23:995–1002. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick P., Lang D., Racker E. Regulatory mechanisms in carbohydrate metabolism. IX. Stimulation of aerobic glycolysis by energy-linked ion transport and inhibition by dextran sulfate. J Biol Chem. 1973 Jul 25;248(14):5175–5175. [PubMed] [Google Scholar]
- Singh M., Singh V. N., August J. T., Horecker B. L. Alterations in glucose metabolism in chick embryo cells transformed by Rous sarcoma virus. Transformation-specific changes in the activities of key enzymes of the glycolytic and hexose monophosphate shunt pathways. Arch Biochem Biophys. 1974 Nov;165(1):240–246. doi: 10.1016/0003-9861(74)90160-x. [DOI] [PubMed] [Google Scholar]
- Singh V. N., Singh M., August J. T., Horecker B. L. Alterations in glucose metabolism in chick-embryo cells transformed by Rous sarcoma virus: intracellular levels of glycolytic intermediates. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4129–4132. doi: 10.1073/pnas.71.10.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Walborg E. F., Jr, Chang J. P. Establishment of a transplantable ascites variant of a rat hepatoma induced by 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1970 Sep;30(9):2306–2309. [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J Biol Chem. 1968 Jul 10;243(13):3640–3647. [PubMed] [Google Scholar]


