Abstract
Background
Weekly maternal intravenous immunoglobulin (IVIG) is the cornerstone of antenatal treatment of foetal and neonatal alloimmune thrombocytopenia (FNAIT). The aim of this study was to describe the neonatal outcome and management in neonates with FNAIT treated antenatally with IVIG.
Materials and methods
All neonates treated antenatally and delivered at our centre between 2006 and 2012 were included in the study. We assessed the neonatal outcome and management, including the occurrence of intracranial haemorrhage, platelet count at birth and need for postnatal platelet transfusions or postnatal IVIG treatment.
Results
A total of 22 neonates were included of whom 12 (55%) had severe thrombocytopenia at birth (platelet count ≤50×109/L). Most neonates (67%, 8/12) with severe thrombocytopenia received a platelet transfusion after birth. None of the neonates required postnatal treatment with IVIG. Three neonates had petechiae and haematomas, without clinical consequences. One foetus suffered from intracranial haemorrhage, which was detected just before the planned start of antenatal IVIG at 28 weeks’ gestation.
Discussion
Our results suggest that antenatal maternal IVIG and, if necessary, postnatal matched platelet transfusions, are effective and safe for the treatment of FNAIT.
Keywords: alloimmune thrombocytopenia, neonatal, intravenous immunoglobulin, intracranial haemorrhage
Introduction
Foetal and neonatal alloimmune thrombocytopenia (FNAIT) is the most common cause of isolated severe thrombocytopenia in the foetus and neonate1–3. It is caused by maternal allo-antibodies against antigens of paternal origin on foetal platelets, resulting in platelet destruction and severe foetal and neonatal thrombocytopenia4. In 80–95% of the affected cases, FNAIT is caused by foetomaternal incompatibility for human platelet antigen 1a (HPA-1a)5–8. Approximately 2% of the Caucasian women are HPA-1a-negative (HPA-1bb), of whom only 8–12% will become immunised and produce allo-antibodies9–14. The most feared complication of severe neonatal thrombocytopenia is intracranial haemorrhage (ICH), which occurs with an incidence of 14–20% in untreated first pregnancies affected by FNAIT5,15–17. The majority of these haemorrhages occur at the end of the second trimester and clinical outcomes are devastating in most cases18,19.
The main treatment goal in FNAIT is to prevent ICH occurring either antenatally or during delivery. A non-invasive strategy with antenatal weekly injections of intravenous immunoglobulin (IVIG) given to the mother is nowadays considered the optimal antenatal management in FNAIT. A few studies have also suggested a possible effect of oral steroids7,20,21.
Invasive strategies with repeated foetal blood sampling and intrauterine platelet transfusions are associated with procedure-related complications such as rupture of membranes, bleeding from the puncture site and acute foetal distress and are no longer regarded as primary management options6,7,22,23.
Our centre, the Leiden University Medical Centre, is the national referral centre for cases of FNAIT in the Netherlands. Our management changed over time from an invasive to a non-invasive strategy6. Although antenatal IVIG administration has become the cornerstone of current treatment, questions remain about the optimal dose, best gestational age to start and the exact mechanisms of action24. In addition, little is known about the optimal postnatal management. The treatment of first choice after birth is transfusion of matched platelets, but in cases of emergency without immediate availability, random platelets are also given. Postnatal IVIG is effective in increasing platelet counts, but the response is much slower than that after platelet transfusions and the risk of destruction of transfused random platelets exists15,16,25–27.
The aim of this study was to evaluate the neonatal outcome and management in all FNAIT cases treated antenatally with IVIG during a 6-year period.
Materials and methods
Study population
All infants with FNAIT treated with antenatal maternal IVIG (Nanogam®, Sanquin, Amsterdam, The Netherlands) at our centre between January 2006 and January 2012 were included in this study. All these cases were already known due to a previously affected pregnancy and were treated according to a completely non-invasive protocol6. Pregnancies were divided into high-risk and standard-risk groups, based on whether a previous sibling had or had not suffered an ICH, respectively. In the standard-risk group (no sibling with ICH), antenatal IVIG was started at a gestational age of 28 weeks. In the high-risk group (previous sibling with ICH), IVIG treatment was started earlier, at 16 weeks’ gestation. Between 2005 and 2008 the Leiden University Medical Centre participated in the NOICH-trial, in which mothers were randomised to a weekly dose of 0.5 or 1 g/kg maternal weight. The trial was prematurely stopped in 2008 due to shortage of inclusions; however, all FNAIT cases are still prospectively collected in an international web-based registry (www.medscinet.com/noich/).
Since 2008, the weekly dose of IVIG in patients with standard-risk pregnancies has varied between 0.5 g/kg and 1 g/kg maternal weight, while that in high-risk pregnancies is 1 g/kg maternal weight.
At birth, platelet counts were determined from the umbilical cord using a standardised flow cytometric method. In the case of thrombocytopenia <100×109/L, the platelet count was repeated manually. Neonates were examined to rule out the presence of haematomas and petechiae. A cranial ultrasound examination was performed in all neonates within 24 hours after delivery. Matched platelet transfusions were to be administered if the platelet count was ≤50×109/L in bleeding neonates (with petechiae, haematomas or ICH) or <30×109/L in non-bleeding neonates. In 2010, the transfusion trigger for non-bleeding neonates was lowered to 20×109/L. HPA-1bb/5aa-typed platelet concentrates were available 24 hours a day. In case of emergency (i.e. clinical bleeding) and no immediate availability of matched platelets, random platelets were to be transfused. If multiple matched platelet transfusions did not result in a sufficient rise of platelet count, treatment with IVIG could be considered. No strict criterion for the start of IVIG was maintained. Platelet counts were determined at least daily during the first days of life, until a spontaneous rise or stable level was observed. The postnatal protocol was independent of inclusion in the NOICH-study.
Data collection
Data were collected retrospectively and entered in a database, which also included information about previous pregnancies. Antenatal and postnatal baseline characteristics were collected and included HPA incompatibility type, dosage of IVIG, gestational age at the start of IVIG, ICH in previous pregnancy, mode of delivery, gestational age at birth, birth weight and Apgar score ≤7 at 5 minutes.
Outcome measures were platelet count at birth, presence of haematomas and/or petechiae, occurrence of ICH, number of postnatal platelet transfusions needed (matched or random), postnatal use of IVIG, course of platelet counts over time and neonatal outcome.
Statistics
Data are reported as mean values±standard deviation, minimum and maximum, numerical values or categories. Analyses were performed with IMB SPSS Statistics version 20 (SPSS Inc., Chicago, IL, USA). Categorical data were analysed using a Fisher’s exact test.
Results
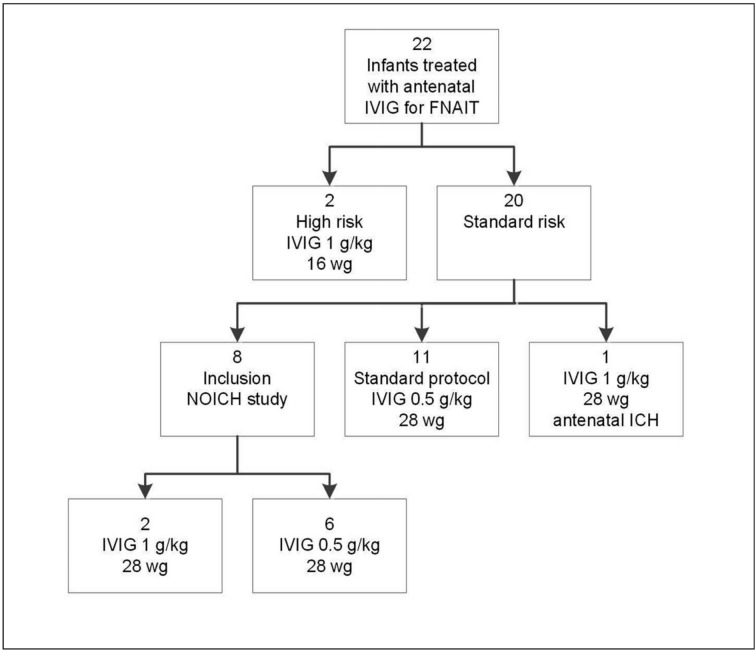
During the 6-year study period, 22 neonates with FNAIT treated with antenatal IVIG were included. Two pregnancies (9%) were considered high risk because a sibling had had an ICH; the IVIG dosage in these cases was set at 1 g/kg starting at 16 weeks’ gestation. Twenty (91%) pregnancies were considered as standard risk, of which 20 were treated from 28 weeks’ gestation onward with 0.5 g/kg or 1 g/kg. Eight pregnancies were included in the NOICH trial and were randomised to receive either 1 g/kg (n=2) or 0.5 g/kg (n=6). One pregnancy in the standard-risk population started with 1 g/kg IVIG at 28 weeks’ gestation, because of a detected antenatal ICH. No adverse effects of maternal IVIG therapy were reported. A flowchart of all included patients is shown in Figure 1. The baseline characteristics of the patients included in the study are shown in Table I.
Figure 1.
Flowchart of included patients.
IVIG: intravenous immunoglobulin; ICH: intracranial haemorrhage; wg: weeks of gestation.
Table I.
Baseline characteristics of the patients included (n=22).
| HPA type | |
| 1a, n. (%) | 18 (82) |
| 5b, n. (%) | 2 (9) |
| 15a, n. (%) | 1 (3,5) |
| 1a and 5b, n. (%) | 1 (3,5) |
|
| |
| ICH in previous pregnancy, n. (%) | 2 (9) |
|
| |
| Gestational age at start of IVIG | |
| 16 weeks, n. (%) | 2 (9) |
| 28 weeks, n. (%) | 20 (91) |
|
| |
| Dose of IVIG | |
| 0.5 g/kg, n. (%) | 17 (77) |
| 1 g/kg, n. (%) | 5 (23) |
|
| |
| Caesarean delivery, n. (%) | 8 (36) |
|
| |
| Neonates from mothers with miscarriages/spontaneous abortionsb | 3 |
|
| |
| Gravidity | 2.5±1.1 (2–6) |
|
| |
| Gestational age at birth, weeksa | 37.3±1.6 (33–39) |
|
| |
| Birth weight, gramsa | 2,917±538 (1,855–3,730) |
|
| |
| Apgar score <7 at 5 minutes, n (%) | 0 (0) |
IVIG: intravenous immunoglobulin; HPA: human platelet antigen; ICH: intracranial haemorrhage.
Value given as mean±SD (range);
Two included neonates were siblings, so in total two mothers in the population had miscarriages/spontaneous abortions.
The mean platelet count at birth was 95±89×109/L (range: 6–277×109/L) and severe thrombocytopenia (platelet count ≤50×109/L) was detected in 12 (55%) neonates. The mean platelet count at birth in neonates who received 0.5 g/kg IVIG antenatally was 104±89×109/L while that in neonates who received 1 g/kg IVIG was 63±91×109/L.
In the group of 12 neonates with severe thrombocytopenia, eight required one matched platelet transfusion, whereas in four neonates the platelet count increased spontaneously. None of the neonates received postnatal IVIG. The mean age at the time the platelet count achieved a safe level above 150×109/L was 3.0±1.6 (range, 1–7) days. Five infants were discharged with a stable platelet count between 100 and 150×109/L.
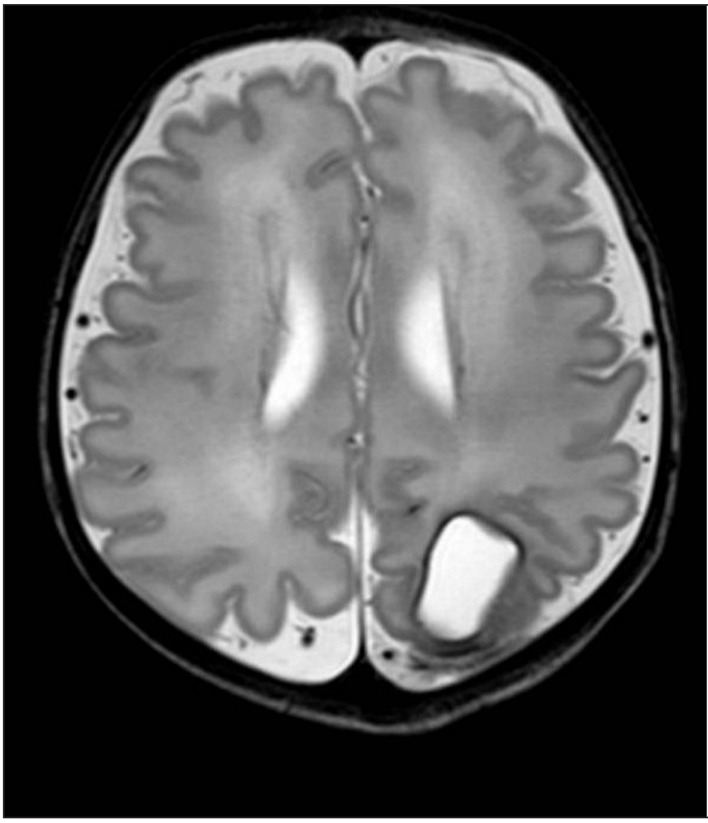
Routine cranial ultrasound was performed in all neonates. One foetus had an ICH, which was detected by coincidence during a routine foetal ultrasound at 27 weeks’ gestation, just 1 day before the planned start of IVIG treatment. She was treated according to the standard-risk protocol, because her sibling had not had an ICH. Her platelet count at birth was 6×109/L and she had several petechiae and haematomas. One matched platelet transfusion was administered at birth which provided a good platelet count increment. On the second day of life, magnetic resonance imaging of the brain showed a left occipital porencephalic cyst, remnant of the antenatal ICH (Figure 2). At 2 years of age, a physical and neurodevelopment assessment (using the Bayley Scales of Infant Development III)28 was performed showing normal growth and neurodevelopment outcome. Petechiae were detected in two other neonates with platelet counts of 29×109/L and 26×109/L at birth; both had good responses to the transfusion of one matched platelet unit each.
Figure 2.
Neonatal cerebral magnetic resonance image showing a large porencephalic cyst in the left occipital lobe. The cyst is a remnant of a large antenatal ICH, detected at 27 weeks of gestation.
Five neonates with severe thrombocytopenia were born after 2010, when the platelet transfusion trigger in non-bleeding neonates was lowered from 30×109/L to 20×109/L platelets. One of them (with a platelet count of 22×109/L without clinical bleeding) had a spontaneous rise and did not receive a platelet transfusion according to the new protocol. The other four infants had platelet counts <20×109/L (n=2) or petechiae or haematomas (n=2) as the indication for a platelet transfusion. Table II provides an overview of the neonatal outcome, including statistical analysis for dosage of antenatal IVIG and platelet count at birth.
Table II.
Neonatal outcome in the 22 cases.
| Outcome | p-value | |
|---|---|---|
| Platelet count at birth, ×109/La | 95±89 (6–277) | |
|
| ||
| Platelet count ≤50×109/L, n. (%) | 12 (55) | 0.323 |
| IVIG 0.5 g/kg | 8 (67) | |
| IVIG 1.0 g/kg | 4 (33) | |
|
| ||
| Platelet count ≤30×109/L, n. (%) | 8 (36) | 0.309 |
| IVIG 0.5 g/kg | 5 (63) | |
| IVIG 1.0 g/kg | 3 (37) | |
|
| ||
| Platelet count ≤20×109/L, n (%) | 3 (14) | 1.000 |
| IVIG 0.5 g/kg | 2 (67) | |
| IVIG 1.0 g/kg | 1 (33) | |
|
| ||
| Lowest platelet count, daysa | 1.6±1.0 (1–4) | |
|
| ||
| Petechiae and/or hematomas, n. (%) | 3 (14) | |
|
| ||
| ICH, n. (%) | 1 (5) | |
|
| ||
| Neonates receiving postnatal platelet transfusions, n. (%) | 8 (36) | |
|
| ||
| Postnatal age when receiving platelet transfusions, daysa | 1.3±0.7 (1–3) | |
|
| ||
| Postnatal age at which platelet count >150×109/L, daysa | 3.0±1.6 (1–7) | |
IVIG: intravenous immunoglobulin; ICH: intracranial haemorrhage.
Value given as mean±SD (range);
Neonates with non-HPA-1a immunisation were not included in this analysis.
Discussion
This cohort study shows a favourable neonatal outcome in FNAIT after non-invasive antenatal maternal treatment with IVIG. Approximately one third (8/23) of the neonates required matched platelet transfusions at birth with a rise in platelet counts within a few hours and postnatal IVIG was not necessary. An antenatal ICH was detected in only one infant at 27 weeks of gestation, just before the start of antenatal IVIG. Despite the cerebral injury, the infant showed a normal neurobehavioral outcome at 2 years of age. Non-invasive management of FNAIT with antenatal maternal IVIG and postnatal matched platelet transfusions seems to be effective and safe.
In this study, approximately one half (12/23) of the neonates had a platelet count at birth ≤50×109/L. The incidence of severe thrombocytopenia found in this study is similar to the incidence reported in several other cohorts (range, 34 to 61%)16,29,30. Berkowitz et al. reported a lower incidence of severe thrombocytopenia (14%), but they included only infants without siblings with severe thrombocytopenia or ICH20. A sibling with ICH or severe thrombocytopenia is one of the most important risk factors for recurrence of severe thrombocytopenia31. Besides neonates with HPA-1a-incompatability, we also included cases with HPA-5b-incompatibility (n=2) and HPA-15a-incompatibility (n=1). The incidence of severe thrombocytopenia reported in this study may be influenced by the fact that HPA-5b incompatibility is associated with less severe thrombocytopenia18,32.
The incidence of ICH in our study was 4% (1/23) which is consistent with the incidence reported by others (range, 0–10%)5–7,16,20,29,30,33. The only infant with ICH in our study did not have a sibling with ICH and was therefore planned to start with IVIG at 28 weeks of gestation (standard-risk group). However an ICH was detected just 1 day before the planned start of IVIG. Whether starting IVIG before 28 weeks of gestation would have prevented the ICH is not known. Consensus on the optimal timing of starting treatment with IVIG is currently lacking. In our study, severe thrombocytopenia at birth was not associated with an increased rate of ICH. Our study confirms the previously suggested possible protective effect of IVIG for ICH even without an increase in foetal platelet counts30,34,35. In addition, all infants had an adequate and quick response to postnatal matched platelet transfusions and postnatal IVIG was not necessary. This positive effect of antenatal IVIG in combination with postnatal matched platelet transfusions was also reported in earlier studies16,25,27. In contrast to our observations in human beings, a reduction of bleeding complications in mouse studies with IVIG was accompanied by an increase of platelet counts36.
Several questions on the optimal IVIG treatment remain unanswered, including the optimal dose (0.5, 1 or 2 g/kg), schedule (weekly or more frequently), gestational age at which to initiate IVIG and the additional value of antenatal oral steroids. Our study was neither designed nor powered to analyse these issues and was primarily focused on postnatal management. A large international web-based registry of all FNAIT cases (prospective continuation after the NOICH-trial) may shed more light on this subject in the near future. Data on the timing of antenatal ICH with suggestions for the gestational age at which to initiate antenatal IVIG have already been published19.
In unknown first cases of FNAIT, ICH may be prevented by antenatal screening for HPA-1a negative genotypes in all pregnant women. Studies suggest that the maternal antibody titre during pregnancy is a possible predictive factor for severe thrombocytopenia, although the diagnostic value is not clear as reliable cut-off levels have not yet been repeatedly demonstrated9,13,16. Kjeldsen et al. suggested a policy of antenatal screening for immunised HPA-1a-negative pregnant women and performing a Caesarean section at a gestational age of 36–38 weeks. Using this policy, there were two cases of ICH among 170 immunised HPA-1a-negative women, although in the absence of a control group of vaginal delivery at term no definitive conclusions can be drawn from this study37. More research is needed to identify women at greatest risk in order to investigate the advantages of offering antenatal treatment with maternal IVIG in a more targeted way.
The retrospective design of this study is a limiting factor; we tried to minimise possible bias by using strict definitions and cut-off values. Another limitation is the relatively small sample size, due to the rarity of this disease. However, sufficient retrospective evidence is needed to design and perform ethically justified randomised controlled trials.
In conclusion, our study results suggest that non-invasive antenatal treatment of FNAIT with weekly maternal IVIG and, if necessary, postnatal transfusion of matched platelets is safe and effective. Based on our experience, our policy is to give matched platelets to non-bleeding neonates with a platelet count of <20×109/L and to bleeding neonates with a platelet count <50×109/L. In general, postnatal administration of IVIG is not recommended as first choice therapy, but it can be useful in addition to random platelet transfusions (in case that matched platelets are not available). More research is needed to optimise the dose and schedule of antenatal IVIG treatment and to detect more factors predictive of severe foetal or neonatal thrombocytopenia.
Acknowledgements
Margreth N. van der Lugt collected the data, performed the statistical analysis and drafted the manuscript. Anouk Figee helped with the collection and interpretation of the data. Marije M. Kamphuis, Noortje P.M. Paridaans, Frans J. Walther and Dick Oepkes helped with the interpretation of the data and revised the manuscript critically. Enrico Lopriore conceived the study, participated in its design and coordination and helped to draft and revise the manuscript. All authors read and approved the final manuscript.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Bussel JB, Zacharoulis S, Kramer K, et al. Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatr Blood Cancer. 2005;45:176–83. doi: 10.1002/pbc.20282. [DOI] [PubMed] [Google Scholar]
- 2.Burrows RF, Kelton JG. Fetal thrombocytopenia and its relation to maternal thrombocytopenia. N Engl J Med. 1993;329:1463–66. doi: 10.1056/NEJM199311113292005. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfus M, Kaplan C, Verdy E, et al. Frequency of immune thrombocytopenia in newborns: a prospective study. Immune Thrombocytopenia Working Group. Blood. 1997;89:4402–6. [PubMed] [Google Scholar]
- 4.Pearson HA, Shulman NR, Marder VJ, et al. Isoimmune neonatal thrombocytopenic purpura. Clinical and therapeutic considerations. Blood. 1964;23:154–77. [PubMed] [Google Scholar]
- 5.Knight M, Pierce M, Allen D, et al. The incidence and outcomes of fetomaternal alloimmune thrombocytopenia: a UK national study using three data sources. Br J Haematol. 2011;152:460–8. doi: 10.1111/j.1365-2141.2010.08540.x. [DOI] [PubMed] [Google Scholar]
- 6.van den Akker ES, Oepkes D, Lopriore E, et al. Noninvasive antenatal management of fetal and neonatal alloimmune thrombocytopenia: safe and effective. BJOG. 2007;114:469–73. doi: 10.1111/j.1471-0528.2007.01244.x. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz RL, Kolb EA, McFarland JG, et al. Parallel randomized trials of risk-based therapy for fetal alloimmune thrombocytopenia. Obstet Gynecol. 2006;107:91–6. doi: 10.1097/01.AOG.0000192404.25780.68. [DOI] [PubMed] [Google Scholar]
- 8.Kamphuis MM, Paridaans N, Porcelijn L, et al. Screening in pregnancy for fetal or neonatal alloimmune thrombocytopenia: systematic review. BJOG. 2010;117:1335–43. doi: 10.1111/j.1471-0528.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 9.Williamson LM, Hackett G, Rennie J, et al. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–7. [PubMed] [Google Scholar]
- 10.Turner ML, Bessos H, Fagge T, et al. Prospective epidemiologic study of the outcome and cost-effectiveness of antenatal screening to detect neonatal alloimmune thrombocytopenia due to anti-HPA-1a. Transfusion. 2005;45:1945–56. doi: 10.1111/j.1537-2995.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 11.Davoren A, McParland P, Crowley J, et al. Antenatal screening for human platelet antigen-1a: results of a prospective study at a large maternity hospital in Ireland. BJOG. 2003;110:492–6. [PubMed] [Google Scholar]
- 12.Durand-Zaleski I, Schlegel N, Blum-Boisgard C, et al. Screening primiparous women and newborns for fetal/neonatal alloimmune thrombocytopenia: a prospective comparison of effectiveness and costs. Immune Thrombocytopenia Working Group. Am J Perinatol. 1996;13:423–31. doi: 10.1055/s-2007-994382. [DOI] [PubMed] [Google Scholar]
- 13.Killie MK, Husebekk A, Kjeldsen-Kragh J, et al. A prospective study of maternal anti-HPA 1a antibody level as a potential predictor of alloimmune thrombocytopenia in the newborn. Haematologica. 2008;93:870–7. doi: 10.3324/haematol.12515. [DOI] [PubMed] [Google Scholar]
- 14.Tiller H, Killie MK, Skogen B, et al. Neonatal alloimmune thrombocytopenia in Norway: poor detection rate with nonscreening versus a general screening programme. BJOG. 2009;116:594–8. doi: 10.1111/j.1471-0528.2008.02068.x. [DOI] [PubMed] [Google Scholar]
- 15.Mueller-Eckhardt C, Kiefel V, Grubert A, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989;1:363–6. doi: 10.1016/s0140-6736(89)91733-9. [DOI] [PubMed] [Google Scholar]
- 16.Bertrand G, Drame M, Martageix C, et al. Prediction of the fetal status in noninvasive management of alloimmune thrombocytopenia. Blood. 2011;117:3209–13. doi: 10.1182/blood-2010-08-302463. [DOI] [PubMed] [Google Scholar]
- 17.Bussel JB, Zabusky MR, Berkowitz RL, et al. Fetal alloimmune thrombocytopenia. N Engl J Med. 1997;337:22–6. doi: 10.1056/NEJM199707033370104. [DOI] [PubMed] [Google Scholar]
- 18.Spencer JA, Burrows RF. Feto-maternal alloimmune thrombocytopenia: a literature review and statistical analysis. Aust N Z J Obstet Gynaecol. 2001;41:45–55. doi: 10.1111/j.1479-828x.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 19.Tiller H, Kamphuis MM, Flodmark O, et al. Fetal intracranial haemorrhages caused by fetal and neonatal alloimmune thrombocytopenia: an observational cohort study of 43 cases from an international multicentre registry. BMJ Open. 2013;3:e002490. doi: 10.1136/bmjopen-2012-002490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkowitz RL, Lesser ML, McFarland JG, et al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial. Obstet Gynecol. 2007;110:249–55. doi: 10.1097/01.AOG.0000270302.80336.dd. [DOI] [PubMed] [Google Scholar]
- 21.Bussel JB, Berkowitz RL, Hung C, et al. Intracranial hemorrhage in alloimmune thrombocytopenia: stratified management to prevent recurrence in the subsequent affected fetus. Am J Obstet Gynecol. 2010;203:135.e1–14. doi: 10.1016/j.ajog.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Rayment R, Brunskill SJ, Soothill PW, et al. Antenatal interventions for fetomaternal alloimmune thrombocytopenia. Cochrane Database Syst Rev. 2011:CD004226. doi: 10.1002/14651858.CD004226.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birchall JE, Murphy MF, Kaplan C, et al. European collaborative study of the antenatal management of feto-maternal alloimmune thrombocytopenia. Br J Haematol. 2003;122:275–88. doi: 10.1046/j.1365-2141.2003.04408.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamphuis MM, Oepkes D. Fetal and neonatal alloimmune thrombocytopenia: prenatal interventions. Prenat Diagn. 2011;31:712–9. doi: 10.1002/pd.2779. [DOI] [PubMed] [Google Scholar]
- 25.te Pas AB, Lopriore E, van den Akker ES, et al. Postnatal management of fetal and neonatal alloimmune thrombocytopenia: the role of matched platelet transfusion and IVIG. Eur J Pediatr. 2007;166:1057–63. doi: 10.1007/s00431-006-0389-4. [DOI] [PubMed] [Google Scholar]
- 26.Kiefel V, Bassler D, Kroll H, et al. Antigen-positive platelet transfusion in neonatal alloimmune thrombocytopenia (NAIT) Blood. 2006;107:3761–3. doi: 10.1182/blood-2005-06-2235. [DOI] [PubMed] [Google Scholar]
- 27.Allen D, Verjee S, Rees S, et al. Platelet transfusion in neonatal alloimmune thrombocytopenia. Blood. 2007;109:388–389. doi: 10.1182/blood-2006-05-026419. [DOI] [PubMed] [Google Scholar]
- 28.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio: Pearson; 2009. [Google Scholar]
- 29.Mechoulan A, Kaplan C, Muller JY, et al. Fetal alloimmune thrombocytopenia: is less invasive antenatal management safe? J Matern Fetal Neonatal Med. 2011;24:564–7. doi: 10.3109/14767058.2010.511333. [DOI] [PubMed] [Google Scholar]
- 30.Bussel JB, Berkowitz RL, Lynch L, et al. Antenatal management of alloimmune thrombocytopenia with intravenous gamma-globulin: a randomized trial of the addition of low-dose steroid to intravenous gamma-globulin. Am J Obstet Gynecol. 1996;174:1414–23. doi: 10.1016/s0002-9378(96)70582-3. [DOI] [PubMed] [Google Scholar]
- 31.Radder CM, Brand A, Kanhai HH. Will it ever be possible to balance the risk of intracranial haemorrhage in fetal or neonatal alloimmune thrombocytopenia against the risk of treatment strategies to prevent it? Vox Sang. 2003;84:318–25. doi: 10.1046/j.1423-0410.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan C, Morel-Kopp MC, Kroll H, et al. HPA-5b (Br(a)) neonatal alloimmune thrombocytopenia: clinical and immunological analysis of 39 cases. Br J Haematol. 1991;78:425–9. doi: 10.1111/j.1365-2141.1991.tb04459.x. [DOI] [PubMed] [Google Scholar]
- 33.Yinon Y, Spira M, Solomon O, et al. Antenatal noninvasive treatment of patients at risk for alloimmune thrombocytopenia without a history of intracranial hemorrhage. Am J Obstet Gynecol. 2006;195:1153–7. doi: 10.1016/j.ajog.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 34.Radder CM, Beekhuizen H, Kanhai HH, et al. Effect of maternal anti-HPA-1a antibodies and polyclonal IVIG on the activation status of vascular endothelial cells. Clin Exp Immunol. 2004;137:216–22. doi: 10.1111/j.1365-2249.2004.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Akker ES, Oepkes D. Fetal and neonatal alloimmune thrombocytopenia. Best Pract Res Clin Obstet Gynaecol. 2008;22:3–14. doi: 10.1016/j.bpobgyn.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Ni H, Chen P, Spring CM, et al. A novel murine model of fetal and neonatal alloimmune thrombocytopenia: response to intravenous IgG therapy. Blood. 2006;107:2976–83. doi: 10.1182/blood-2005-06-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjeldsen-Kragh J, Killie MK, Tomter G, et al. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110:833–9. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]