Abstract
Background
The Indian blood group antigens, Ina and Inb, are clinically significant in transfusion medicine. However, antisera to type these antigens are difficult to obtain. The Inb antigen is a high frequency antigen present in all populations, while the frequency of the antithetical Ina ranges from 0.1% in Caucasians up to 11% in Middle Eastern groups. This antigen polymorphism is encoded by the single nucleotide polymorphism (SNP) 252G>C in CD44. The aim of this study was to establish and compare two genotyping methods to measure the frequency of the IN*A and IN*B alleles in a blood donor cohort.
Materials and methods
Donor blood samples (n=151) were genotyped by a novel real-time polymerase chain reaction (PCR) high-resolution meltcurve (HRM) analysis and a custom matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) assay. Samples with the rare IN*A allele were further investigated by nucleotide sequencing, red cell agglutination, and flow cytometry techniques.
Results
In this study group, 149 IN*B homozygous and 2 IN*A/B heterozygous samples were detected with 100% concordance between HRM and MALDI-TOF MS methods. For PCR HRM, amplicon melting alone did not differentiate IN*A and IN*B alleles (class 3 SNP), however, the introduction of an unlabelled probe (UP) increased the resolution of the assay. Sequencing confirmed that the two non-homozygous samples were IN*A/B heterozygous and phenotyping by red cell agglutination, and flow cytometry confirmed both Ina and Inb antigens were present as predicted.
Discussion
Genotyping permits conservation of rare antisera to predict blood group antigen phenotype. In PCR UP-HRM the IN*A and IN*B alleles were discriminated on the basis of their melting properties. The Ina frequency in this selected donor population was 1.3%. Application of genotyping methods such as these assists in identifying donors with rare blood group phenotypes of potential clinical significance.
Keywords: Indian blood group genotyping, high-resolution melting analysis, MALDI-TOF MS
Introduction
The CD44 gene, located on chromosome 11p13 consists of 20 exons, encoding numerous isoforms of the CD44 molecule1,2. The CD44 isoform present on hematopoietic cells is CD44s1,3 and approximately 2,000–5,000 copies of this molecule are expressed on mature red blood cells4. The CD44 glycoprotein carries antigens of the Indian (IN) blood group system5, namely: Ina, Inb, INFI, and INJA6–9. The Ina and Inb are antithetical antigens and have been associated with a single nucleotide polymorphism (SNP) from guanine (encoding Inb) to cytosine (encoding Ina) at nucleotide 252 in exon 2 of CD44. This SNP results in an amino acid substitution Arg46Pro of the CD44 molecule10.
The Ina and Inb antigens are efficient immunogens and antibodies to these polymorphisms are clinically significant11–14. Anti-Ina and anti-Inb antibodies have been associated with immediate and delayed haemolytic transfusion reactions7,13,15. The Inb antigen is a high frequency antigen expressed in all populations. While Ina is expressed in 0.1% of Caucasians4, it is present in 2.9% of Indians, 10.6% of Iranians, and 11.8% of Arab populations living in Mumbai (Bombay, India)7,16. These studies on the population frequency of Ina and Inb were performed over 30 years ago7,11,16,17.
The antisera required for typing the Ina and Inb antigens are rare, and difficult to obtain. The SNP associated with the Ina and Inb antigens has been defined; however, commercially-available genotyping systems such as BLOODchip v4.1 (Progenika, Vizcaya, Spain) and Human Erythrocyte Antigen (HEA) BeadChip Kit (BioArray Solutions Ltd, Warren, NJ, USA) do not incorporate this polymorphism.
The aim of this study was to apply two DNA methods to genotype the IN*A and IN*B alleles and to investigate the frequency of Ina and Inb in a selected population of Australian blood donors. We describe a novel high-resolution meltcurve (HRM) analysis method and a custom matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) assay to genotype the IN*A and IN*B alleles. Molecular typing results were validated by comparing data obtained between genotyping methods, with phenotype determined by standard serological techniques, and Sanger DNA sequencing.
Materials and methods
Donor selection and DNA preparation
Blood samples collected into EDTA (n=151) were retrieved from volunteer blood donors who presented at collection sites where the population includes South Asian and West Asian (Middle Eastern) individuals. This study had ethics approval from the Human Research Ethics Committee, Australian Red Cross Blood Service.
Two additional samples, In(a+b−) and In(a−b+), were used as controls in genotyping experiments. The In(a+b−) and In(a−b+) control samples were phenotyped by the Blood Service Red Cell Reference Laboratories. The In(a+b−) control was kindly provided by the Sydney Red Cell Reference Laboratory.
Genomic DNA was extracted from whole blood using the EZ1 DNA Blood kit in an automated BioRobot EZ1 Workstation (QIAGEN, Doncaster, VIC, Australia) in accordance with the manufacturer’s instructions.
Polymerase chain reaction unlabelled probe high-resolution meltcurve analysis
HRM analysis is a post-polymerase chain reaction (PCR), DNA analysis method used for genotyping and scanning DNA sequence variants18. HRM uses intercalating dyes that fluoresce when bound to double-stranded (ds) DNA only. Following PCR, dsDNA is melted by gradually increasing the temperature. As dsDNA dissociates into single-stranded products, total fluorescence is reduced and monitored over time19. The standard HRM method is performed by amplicon melting only. A variation of this method is the addition of an unlabelled probe to improve specificity of the assay to detect the target polymorphism during denaturation20–23.
The primers and probe used in this method were designed in reference to GenBank accession number M59040. Oligonucleotide sequences are listed in Table I. The Indian HRM assay primers amplify a 104-bp product. The UP is a complete match to IN*B and is therefore a single-base mismatch to IN*A. It has an amino-C6 modification on the 3′-end to prevent polymerase extension during PCR24. The primers and probe were ordered from Sigma-Aldrich (Sigma-Aldrich, Castle Hill, NSW, Australia).
Table I.
Primer and probe sequences used in molecular typing for 252C>G associated with IN*A/B.
| Method | Oligonucleotide | Sequence |
|---|---|---|
| HRM assay | IN-Ex2 forward primer | 5′–CGCTTTGCAGGTGTATTCC–3′ |
| IN-Ex2 reverse primer | 5′–ATTGTGGGCAAGGTGCTATT–3′ | |
| IN-33F probe | 5′–ATGGTCGCTACAGCATCTCTCGGACGGAGGCCG–3′ | |
|
| ||
| Exon 2 sequencing | Forward primer9 | 5′–TGTTAACCAGGCTGGTCTTGAG–3′ |
| Reverse primer9 | 5′–AGTTCTAAGCCCAGCTGCCTG–3′ | |
The nucleotide base underlined in the IN33-F probe sequence is a complete match to IN*B and a single mismatch to IN*A.
The PCR reaction volume was 20 μL and contained 150 nM forward primer (limiting), 750 nM reverse primer (excess), 750 nM unlabelled probe, 20 ng of genomic DNA, and 12.5 μL of HRM PCR master mix (QIAGEN Type-it HRM PCR kit). This assay utilises an asymmetric PCR technique resulting in the generation of excess single-stranded template available for hybridisation of the probe.
The PCR was performed with an initial activation step of 95 °C for 5 minutes followed by 40 cycles of 95 °C for 10 seconds and an annealing/extension step of 60 °C for 30 seconds. Fluorescence data were acquired on the Green Channel during PCR. After the last cycle, samples were held at 95 °C for 1 minute prior to lowering the temperature to 65 °C. Melting was then performed by steadily raising the temperature at increments of 0.5 °C every 2 seconds from 65 °C to 90 °C. The rate of change in fluorescence was monitored on the HRM channel.
Both PCR and HRM steps were performed using real-time PCR equipment, Rotor-Gene 6000 and Rotor-Gene Q, 5plex HRM platform (QIAGEN). Rotor-Gene Q Series Software was used for data analysis. A confidence threshold was set at 90% for genotype call assignment.
Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry
MassARRAY (Sequenom Inc., San Diego, CA, USA) is a SNP genotyping platform which utilises MALDI-TOF MS technology. SNP genotyping using MassARRAY was performed following the manufacturer’s recommendations and involves target-specific PCR amplification followed by target-specific primer single base extension25. MassARRAY provides customisation via the design of amplification and extension primers specific for the SNP of interest26,27 using Assay Design 4 software. During the primer extension stage an oligonucleotide primer anneals immediately adjacent to the SNP being genotyped25 and is extended a single base, incorporating the nucleotide at the SNP position. During MALDI-TOF MS, samples are individually irradiated with a short laser pulse which causes the matrix and DNA fragments to desorb, ionise and be accelerated in an electric field25. The time-of-flight and mass of each extension primer is calculated from the time of arrival at the detector, allowing the nucleotide at the SNP to be determined25 in an automated fashion using Typer 4.0 software.
In this study, SNP genotyping was performed following the Sequenom MassARRAY iPLEX Pro genotyping protocol. Primer sequences are available upon request from Sequenom, Inc.
Preparation of the polymerase chain reaction product for DNA sequencing
Primers published by Poole et al.9 targeting exon 2 of CD44 were used to amplify a 430-bp product. The FastStart High Fidelity PCR System, dNTPack (Roche Diagnostics, Castle Hill, NSW, Australia) PCR kit was used to make up 50 μL of master mix according to the manufacturer’s recommendation.
Briefly, the PCR started at 95 °C for 2 minutes followed by 35 cycles at 95 °C for 30 seconds, 65 °C for 30 seconds, 72 °C for 1 minute, and a final extension step at 72 °C for 7 minutes on a Veriti Dx Thermal Cycler (Applied Biosystems, Life Technologies, VIC, Australia). PCR products were purified using a MinElute PCR Purification Kit (QIAGEN) according to the manufacturer’s protocol. Purified PCR products were sent to the Australian Genome Research Facility (AGRF), The University of Queensland, QLD, Australia, for DNA sequencing. Data were analysed using Chromas Lite 2.01 software.
Low ionic strength solution-indirect antiglobulin testing
Red cells of all 151 samples were washed in phosphate-buffered saline and resuspended in low ionic strength solution (LISS). Samples were tested in DiaMed Card LISS/Coombs (Bio-Rad, NSW, Australia) using a standard protocol for indirect antiglobulin testing (IAT). Samples were incubated with anti-Inb at 37 °C for 15 minutes and then centrifuged using the DiaMed-ID Micro Typing System (Bio-Rad) at 910 rpm for 10 minutes. Samples were examined for agglutination. The anti-Inb human polyclonal antiserum was from the Serum, Cells, And Rare Fluids (SCARF) exchange programme.
A limited supply of anti-Ina human polyclonal antiserum was used to perform standard LISS-IAT, as described above, on samples found to possess the IN*A allele. Anti-Ina antiserum was kindly provided by Joyce Poole and Nicole Thorton of the International Blood Group Reference Laboratory, Bristol, UK.
Flow cytometry
A flow cytometry experiment was performed on samples positive for the Ina antigen by LISS-IAT. Cell suspensions were incubated with anti-Ina and anti-Inb (the same antisera were used in the LISS-IAT) in separate tubes at 37 °C for 30 minutes. Cells were washed in phosphate-buffered saline and incubated with PE Mouse Anti-Human IgG (Cat No. 555787, BD Biosciences, Franklin Lakes, NJ, USA) at room temperature in the dark for 30 minutes. Following secondary antibody incubation, cells were washed and resuspended in phosphate-buffered saline. Samples were analysed using a FACS CANTO II flow cytometer and FACSDiva software (BD Biosciences).
Results
Development of the polymerase chain reaction unlabelled probe high-resolution meltcurve assay
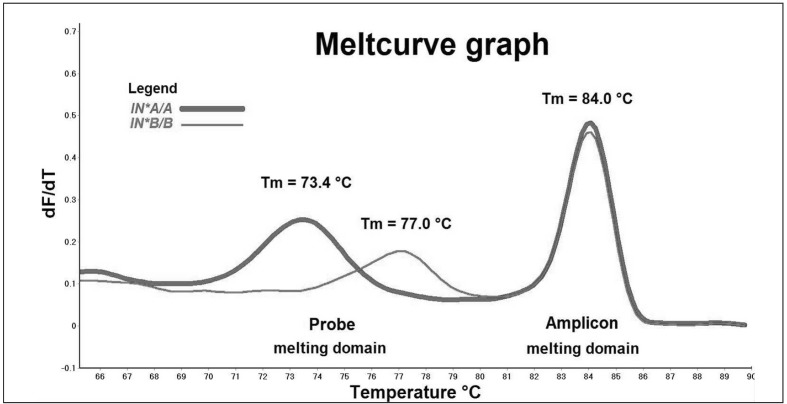
The PCR products derived from IN*A/A and IN*B/B control samples were denatured between 65.0 °C and 90.0 °C, and generated two distinct domains: an amplicon melting domain from 80.5 °C to 87.0 °C and a probe melting domain from 69.0 °C to 80.5 °C (Figure 1a).
Figure 1a.
Representative melt profiles of samples homozygous for IN*A/A (thick line) and IN*B/B (thin line). Meltcurve graph plots the rate of change in fluorescence (y-axis) against temperature (x-axis).
In the amplicon melting domain, the two control samples produced identical melting temperatures (Tm) at 84.0 °C. Melt profiles in this domain showed indistinguishable melt profiles masking the single nucleotide difference between IN*A and IN*B. Data in this domain were unsuitable for genotyping analysis.
In the probe melting domain, control samples displayed distinct melting behaviour. The Tm was 73.4 °C for the IN*A/A control and 77.0 °C for the IN*B/B control, a temperature shift of 3.6 °C. These unique melt patterns supported the assignment of homozygous IN*A/A and IN*B/B genotypes to the two control samples In(a+b−) and In(a−b+) respectively. These melt profiles were then used to assign genotype to our sample cohort.
Polymerase chain reaction unlabelled probe high-resolution meltcurve genotyping analysis
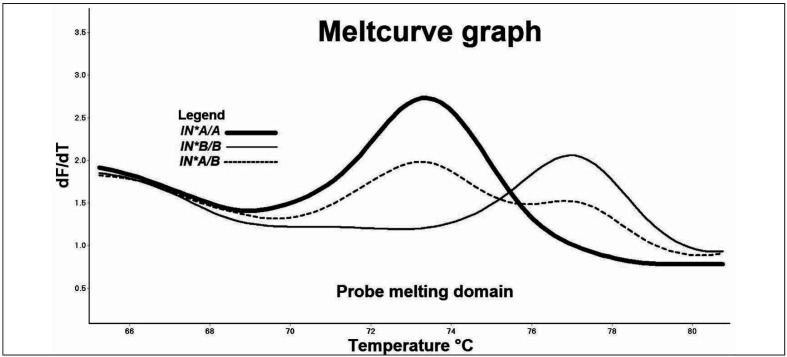
The melt profiles of 149 (149/151) unknown samples matched the IN*B/B genotype profile with a percentage confidence of at least 98% (Figure 1b). Two samples (2/151) produced peaks at 73.3 °C and 76.6 °C, in the probe Tm melting domain of melting curves for IN*A and IN*B, respectively (Table II).
Figure 1b.
Representative melt profile of IN*A/B heterozygous sample (dash) produced double peaks in the probe melting domain at 73.3±0.3 °C and 76.6±0.2 °C compared to the single peaks produced by IN*A (Tm=73.4 °C) and IN*B (Tm=77.0 °C) homozygous samples.
Table II.
Average Tm of homozygous IN*A/A and IN*B/B and donor cells: mean Tm and two standard deviations (SD) were calculated from 30 replicate assays.
| Sample | Melting domain Tm Probe±2SD | Melting domain Tm Amplicon±2SD |
|---|---|---|
| Control IN*A/A | 73.4±0.3 °C | 84.0±0.3 °C |
| Control IN*B/B | 77.0±0.3 °C | 84.0±0.3 °C |
| Donor 1 IN*A/B | 73.3±0.3 °C and 76.6±0.2 °C | 83.8±0.2 °C |
| Donor 2 IN*A/B | 73.3±0.3 °C and 76.6±0.3 °C | 83.8±0.2 °C |
Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry genotyping analysis
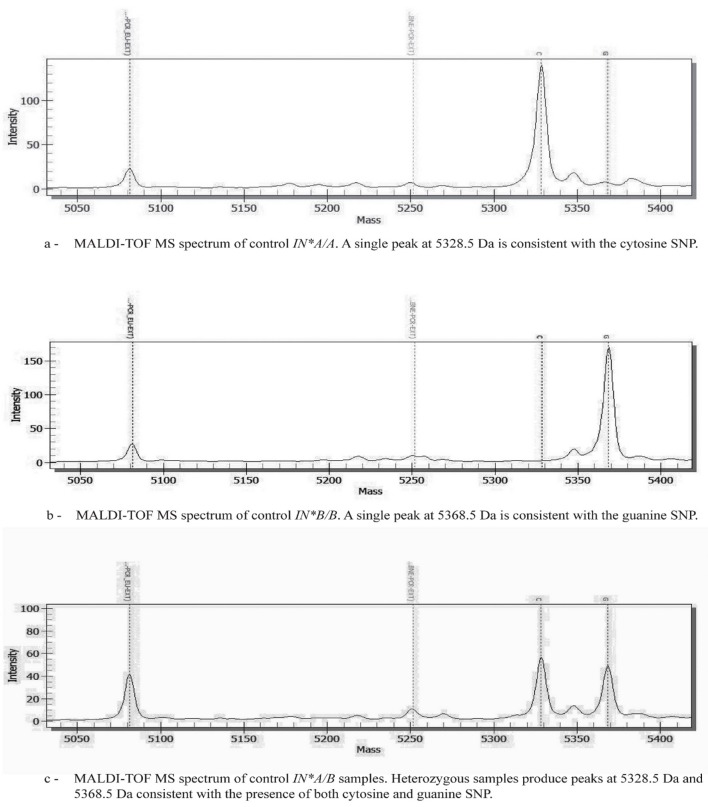
On analysis the In(a+b−) control produced a single peak with a mass of 5328.5 Dalton (Da) consistent with the cytosine SNP (Figure 2a). The In(a−b+) control produced a single peak of 5368.5 Da consistent with the guanine SNP (Figure 2b). One hundred and forty-nine samples produced single peaks with a mass of 5368.5 Da and were therefore genotyped as homozygous for guanine (IN*B/B). Two samples produced peaks at both 5328.5 Da and 5368.5 Da indicating they were heterozygous for cytosine and guanine (IN*A/B) (Figure 2c). MALDI-TOF MS genotyping results of the control samples and all 151 unknown samples were fully concordant with genotypes determined by unlabelled probe (UP)-HRM.
Figure 2.
Representative MALDI-TOF MS spectra. Genotypes are determined by plotting peak intensity (y-axis) against mass (Da) (x-axis).
DNA sequencing
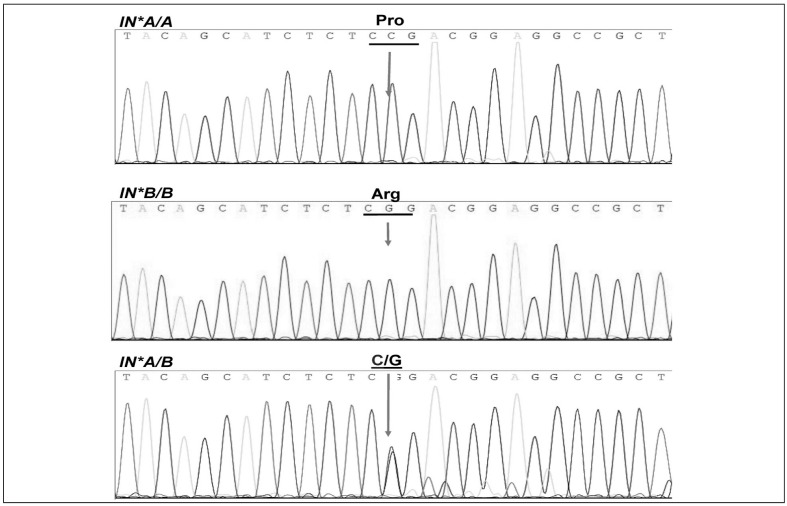
Sequencing data of the two homozygous controls and the two identified IN*A/B heterozygous samples were aligned with the reference allele M59040 using the Basic Local Alignment Tool (BLAST) on the NCBI database website (http://www.ncbi.nlm.nih.gov). Chromatograms (Figure 3) show that a cytosine base for IN*A/A was substituted by a guanine base in IN*B/B, whereas for the two heterozygous IN*A/B samples, both cytosine and guanine were detected in the same position.
Figure 3.
Nucleotide sequencing chromatograms showing the 252C>G nucleotide polymorphism.
The arrow indicates the location of cytosine (C) in IN*A/A to form proline and guanine (G) in IN*B/B to form arginine. Both C and G are co-incident for the heterozygous samples IN*A/B.
Serology and Ina frequency in the selected population
All 151 samples were agglutinated by anti-Inb antiserum, indicating the presence of the Inb antigen.
Red blood cells of the two samples genotyped as IN*A/B were further tested using anti-Ina antiserum. Both cells were positive for agglutination, confirming the phenotype In(a+b+). The frequency of Ina was 1.3% in this selected group.
Flow cytometry
Flow cytometry was performed with anti-Ina and anti-Inb antisera (Table III). To calculate mean fluorescence intensity (MFI) ratios, we divided the MFI value of the sample by the MFI value of the negative control. In(a+b−) and In(a−b+) cells were used as negative controls for anti-Inb and anti-Ina flow cytometry assays, respectively. Negative controls were given an MFI ratio of 1.0. The two donor samples identified as IN*A/B gave MFI values between those of the control cells, further confirming that these donor cells express Ina and Inb antigens. The MFI ratios of the two heterozygous samples differed in their reactivity to each particular antisera, possibly because of variable expression of antigens on the cell surface. Due to the limited supply of antisera, it was not possible to repeat this assay.
Table III.
Mean fluorescence intensity (MFI) ratio of control In(a+b−), control In(a−b+), and two identified In(a+b+) red cells measured with anti-Ina and anti-Inb. Due to the limited supply of antisera, the MFI ratio values supplied in this table were taken from a single experiment only.
| Sample | MFI ratio using anti-Ina | MFI ratio using anti-Inb |
|---|---|---|
| Control In(a+b−) | 4.9 | 1.0 |
| Control In(a−b+) | 1.0 | 4.7 |
| Donor 1 In(a+b+) | 2.7 | 1.8 |
| Donor 2 In(a+b+) | 3.2 | 4.2 |
Discussion
In this study on the detection of Ina blood group antigen in the Australian population, this antigen was detected in 1.3% of a cohort of donors. This rate is intermediate between the 0.1% occurrence in Caucasians4 and the high rate observed for Middle East groups16. Two genotyping methods, one novel PCR-based HRM assay and a commercial MassARRAY system, detected two (2/151) blood donors exhibiting the IN*A SNP along with the IN*B SNP. Serology testing using the rare anti-Ina confirmed heterozygosity for the Ina antigen in these samples. The concordance rate between genotyping systems and serotyping for the high frequency Inb was 100%.
The design of both the genotyping assays here was based on the SNP from guanine (encoding Inb) to cytosine (encoding Ina) at nucleotide 252 in exon 2 of CD44. This is the first application of HRM genotyping for Indian antigens although standard HRM analysis by amplicon melting has been applied to other blood groups28. However, our experience with the HRM technique showed that standard amplicon melting alone is insufficient to detect a nucleotide change between “C” and “G”. The amplicon Tm difference in homozygotes with class 3 (C::G) polymorphisms is very small, usually <0.4 °C as class 3 SNP are a simple inversion of a complementary base pair19. To overcome this complexity, we introduced an unlabelled probe into the reaction mix. This addition improved the specificity of our HRM genotyping assay by generating distinct melt profiles for samples homozygous for IN*A or IN*B The novel, closed-tube, real-time PCR method does not require post-amplification sample processing compared to IN*A/B genotyping methods by DNA sequence typing9 and gel electrophoresis29. The PCR UP-HRM described here is a medium-throughput assay capable of genotyping 32 samples in duplicate in 96 minutes. This assay is suitable for referral testing in specialised laboratories.
In contrast, the MassARRAY system has the advantage of being a high-throughput system capable of performing large-scale screening of up to 4,000 samples per day. The MassARRAY system which utilises MALDI-TOF MS technology was adapted through the design of primers for the IN*A and IN*B SNP. Sequenom Inc. in collaboration with the Blood Transfusion Service Zurich of the Swiss Red Cross are currently developing and evaluating a MassARRAY module for genotyping rare red blood cell polymorphisms, including IN*A and IN*B30. Gassner et al.30 reported a zero allele frequency for IN*A (IN*01) among 3,040 donors in this Swiss study. This is in contrast to the 1.3% found here in our population. The present study is limited by the sample size and the fact that it is confined to one Metropolitan area. Interestingly, 2% of the Australian population identify as having Indian ancestry31. Joshi and Vasantha described serological screening of 2,000 blood units to find two compatible In(a+b−) cells for transfusion to a patient with anti-Inb antibody32, demonstrating that serological typing to find donors with rare phenotypes is challenging when antisera are rare and in limited supply33. As the antisera supply was limited in this study, serological typing for Ina was restricted to the two samples genotyped as IN*A/B (by both PCR UP-HRM and MassARRAY). Serology by agglutination and flow cytometry studies confirmed that the Ina antigen was present and the flow cytometry findings were consistent with single-dose gene expression. In future studies, flow cytometry may be preferred as less antisera is required compared to that necessary for red cell agglutination. Additionally, future studies could apply either genotyping methods described here to a larger number of samples to reliably establish the IN*A/B allele frequency within the Australian population.
In conclusion, the frequency of ethnically-associated blood group antigens is expected to vary as the population demographic changes. The Australian Red Cross Blood Service has an active policy to recruit donors from different backgrounds to prepare to meet patients’ needs in the future. Serological typing to find donors with uncommon phenotypes is challenging especially when antisera are rare and in limited supply33. Genotyping methods will play a growing role in defining rare alleles such as IN*A in the Australian blood donor panel and direct serology to confirm blood donors with the rare In(a+b−) phenotype. As evidenced by the finding of IN*A/B in this sample group, PCR UP-HRM and MassARRAY are viable methods for the detection of the IN*A and IN*B alleles. Molecular typing is a useful adjunct for Indian typing when antisera are limited in supply.
Acknowledgements
The Australian Government fully funds the Australian Red Cross Blood Service for the provision of blood products and services to the Australian community. We thank Dr Melinda Dean and Dr Stacy Scott for their contribution to the flow cytometry data.
We are very grateful to the Serum, Cells, And Rare Fluids exchange programme for the gift of anti-Inb antiserum and to Joyce Poole and Nicole Thorton of the International Blood Group Reference Laboratory, Bristol, UK, for the gift of anti-Ina antiserum. We thank Andrew Davis from the Red Cell Reference Laboratory, Australian Red Cross Blood Service, Sydney, NSW, for kindly supplying the Ina cell.
This study was approved by the Australian Red Cross Blood Service Ethics Committee (reference number 2010#07).
Footnotes
Authorship contribution
All Authors contributed in writing and reviewing the manuscript. Genghis H. Lopez and Rhiannon S. McBean contributed equally in designing the experiments, performing molecular testing and data analysis. Darryl L. Irwin provided expert advice on the MALDI-TOF MS technology and assay design. Brett Wilson and Yew-Wah Liew performed phenotyping tests and analysed serological data. Catherine A. Hyland and Robert L. Flower supervised the study design and reviewed molecular and serological data.
Disclosure and competing interests statement
The Authors from the Australian Red Cross Blood Service have no competing interests. Darryl L. Irwin is the Applications and Technology Director of Sequenom Inc. Asia Pacific.
References
- 1.Screaton GR, Bell MV, Jackson DG, et al. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160–4. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels G. Human Blood Groups. 3rd ed. Oxford, UK: Wiley-Blackwell Publishing; 2013. [Google Scholar]
- 3.Tolg C, Hofmann M, Herrlich P, Ponta H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993;21:1225–9. doi: 10.1093/nar/21.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid ME, Lomas-Francis C, Olsson ML. The Blood Group Antigen Facts Book. 3rd ed. Elsevier; Academic Press; 2012. [Google Scholar]
- 5.Spring FA, Dalchau R, Daniels GL, et al. The Ina and Inb blood group antigens are located on a glycoprotein of 80,000 MW (the CDw44 glycoprotein) whose expression is influenced by the In(Lu) gene. Immunology. 1988;64:37–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Badakere SS, Joshi SR, Bhatia HM, et al. Evidence for a new blood group antigen in the Indian population (a preliminary report) Indian J Med Res. 1973;61:563. [PubMed] [Google Scholar]
- 7.Badakere SS, Parab BB, Bhatia HM. Further observations on the Ina (Indian) antigen in Indian populations. Vox Sang. 1974;26:400–3. doi: 10.1111/j.1423-0410.1974.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 8.Giles CM. Antithetical relationship of anti-In-a with the Salis antibody. Vox Sang. 1975;29:73–6. doi: 10.1111/j.1423-0410.1975.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 9.Poole J, Tilley L, Warke N, et al. Two missense mutations in the CD44 gene encode two new antigens of the Indian blood group system. Transfusion. 2007;47:1306–11. doi: 10.1111/j.1537-2995.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 10.Telen MJ, Udani M, Washington MK, et al. A blood group-related polymorphism of CD44 abolishes a hyaluronan-binding consensus sequence without preventing hyaluronan Bbnding. J Biol Chem. 1996;271:7147–53. doi: 10.1074/jbc.271.12.7147. [DOI] [PubMed] [Google Scholar]
- 11.Longster GH, North DI, Robinson EA. Four further examples of anti-Inb detected during pregnancy. Clin Lab Haematol. 1981;3:351–6. [PubMed] [Google Scholar]
- 12.Bhatia H. Studies on the blood group antigen Ina. Immunol Commun. 1980;9:203. doi: 10.3109/08820138009065994. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson D, Gaal H. Some observations on the Inb antigen and evidence that anti-Inb causes accelerated destruction of radiolabeled red cells. Transfusion. 1988;28:479–82. doi: 10.1046/j.1537-2995.1988.28588337342.x. [DOI] [PubMed] [Google Scholar]
- 14.Anstee DJ. Red cell genotyping and the future of pretransfusion testing. Blood. 2009;114:248–56. doi: 10.1182/blood-2008-11-146860. [DOI] [PubMed] [Google Scholar]
- 15.Joshi SR. Immediate haemolytic transfusion reaction due to anti-Inb. Vox Sang. 1992;63:232–3. doi: 10.1111/j.1423-0410.1992.tb05107.x. [DOI] [PubMed] [Google Scholar]
- 16.Badakere SS, Vasantha K, Bhatia HM, et al. High frequency of Ina antigen among Iranians and Arabs. Hum Hered. 1980;30:262–3. doi: 10.1159/000153140. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia HM, Badakere SS, Mokashi SA, Parab BB. Studies on the blood group antigen Ina. Immunol Commun. 1980;9:203–15. doi: 10.3109/08820138009065994. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery J, Wittwer CT, Palais R, Zhou L. Simultaneous mutation scanning and genotyping by high-resolution DNA melting analysis. Nat Protoc. 2007;2:59–66. doi: 10.1038/nprot.2007.10. [DOI] [PubMed] [Google Scholar]
- 19.Liew M, Pryor R, Palais R, et al. Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clin Chem. 2004;50:1156–64. doi: 10.1373/clinchem.2004.032136. [DOI] [PubMed] [Google Scholar]
- 20.Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8:597–608. doi: 10.2217/14622416.8.6.597. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Myers AN, Vandersteen JG, et al. Closed-tube genotyping with unlabeled oligonucleotide probes and a saturating DNA dye. Clin Chem. 2004;50:1328–35. doi: 10.1373/clinchem.2004.034322. [DOI] [PubMed] [Google Scholar]
- 22.Erali M, Voelkerding KV, Wittwer CT. High resolution melting applications for clinical laboratory medicine. Exp Mol Pathol. 2008;85:50–8. doi: 10.1016/j.yexmp.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew M, Seipp M, Durtschi J, et al. Closed-tube SNP genotyping without labeled probes/a comparison between unlabeled probe and amplicon melting. Am J Clin Pathol. 2007;127:341–8. doi: 10.1309/N7RARXH3623AVKDV. [DOI] [PubMed] [Google Scholar]
- 24.Dames S, Margraf RL, Pattison DC, et al. Characterization of aberrant melting peaks in unlabeled probe assays. J Mol Diagn. 2007;9:290–6. doi: 10.2353/jmoldx.2007.060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer S, Gut IG. Genotyping single-nucleotide polymorphisms by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry. J Chromatogr B. 2002;782:73–87. doi: 10.1016/s1570-0232(02)00692-x. [DOI] [PubMed] [Google Scholar]
- 26.Tost J, Gut IG. Genotyping single nucleotide polymorphisms by MALDI mass spectrometry in clinical applications. Clin Biochem. 2005;38:335–50. doi: 10.1016/j.clinbiochem.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Garritsen HS, Fan AX, Bosse N, et al. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for genotyping of human platelet-specific antigens. Transfusion. 2009;49:252–8. doi: 10.1111/j.1537-2995.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Takahahi J, Hirayama F, Tani Y. High-resolution melting analysis for genotyping Duffy, Kidd and Diego blood group antigens. Leg Med (Tokyo) 2011;13:1–6. doi: 10.1016/j.legalmed.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Jungbauer C, Hobel CM, Schwartz DW, Mayr WR. High-throughput multiplex PCR genotyping for 35 red blood cell antigens in blood donors. Vox Sang. 2012;102:234–42. doi: 10.1111/j.1423-0410.2011.01542.x. [DOI] [PubMed] [Google Scholar]
- 30.Gassner C, Meyer S, Frey BM, Vollmert C. Matrix-assisted laser desorption/ionisation, time-of-flight mass spectrometry-based blood group genotyping--the alternative approach. Transfus Med Rev. 2013;27:2–9. doi: 10.1016/j.tmrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Cultural Diversity in Australia, Reflecting a Nation: Stories from the 2011 Census. Australian Bureau of Statistics; [Accessed on 16/12/2013]. Available at: http://www.abs.gov.au. [Google Scholar]
- 32.Joshi SR, Vasantha K. A profile of rare bloods in India and its impact in blood transfusion service. Asian J Transfus Sci. 2012;6:42–3. doi: 10.4103/0973-6247.95050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid ME. DNA analysis to find rare blood donors when antisera is not available. Vox Sang. 2002;83(Suppl 1):91–3. doi: 10.1111/j.1423-0410.2002.tb05275.x. [DOI] [PubMed] [Google Scholar]