Abstract
Background
Recombinant activated factor VII (rFVIIa) has been widely used as an off-licence pan-haemostatic agent in patients with critical bleeding. However, outside the trauma setting, there is relatively little high quality evidence on the risks and benefits of this agent. The Haemostasis Registry was established to investigate the extent of use, dosing, safety and outcomes of patients after off-licence rFVIIa treatment of critical bleeding.
Materials and methods
The Registry recruited non-haemophiliac patients treated with rFVIIa from 2000–2009 (inclusive) in Australia and New Zealand. Detailed information was gathered on patients’ demographics, context of bleeding, rFVIIa administration, laboratory results, blood component and other therapies, and outcomes. Outcome measures included subjectively assessed effect of rFVIIa on bleeding (response), adverse events (thromboembolic and other) and 28-day mortality.
Results
The registry included 3,446 cases in 3,322 patients (median [IQR] age 56 [33–70] years, 65% (n=2,147) male). Clinical indications included cardiac surgery (45%), other surgery (18%), trauma (13%), medical bleeding (6%), liver disease (6%), and obstetric haemorrhage (5%). The median [IQR] dose was 91 [72–103] μg/kg and 77% received a single dose. Reduction or cessation of bleeding was reported in 74% and 28-day survival was 71% but outcomes varied depending on clinical context. pH strongly correlated with outcome measures; 81% of patients with pH <7.1 died. Approximately 11% of patients had thromboembolic adverse events. In multivariate analysis, pH prior to administration and bleeding context were independently associated with reported response to rFVIIa and 28-day mortality.
Discussion
The Haemostasis Registry is the largest dataset of its kind and provides observational data on the off-licence use of rFVIIa over a 10-year period. It has been an invaluable resource for rigorously tracking adverse events and helping to inform clinical practice.
Keywords: Haemostasis Registry, rFVIIa, NovoSeven®, critical bleeding, haemostasis
Introduction
Uncontrolled bleeding is associated with an increased risk of morbidity and mortality and is a potential threat in a variety of clinical settings, including complex surgery (e.g. cardiac surgery and liver transplantation), medical bleeding, obstetric complications and serious trauma. Current clinical strategies employed to minimise or control excessive blood loss and its effects include transfusion of blood components, volume replacement, management of metabolic and biochemical derangements and surgical interventions. Despite this, standard therapeutic options may be quickly exhausted and imminent exsanguination approached. The clinical appetite for new strategies is high, in particular pharmacological approaches that have the potential to rapidly activate or rescue failing coagulation by improving haemostatic function.
The successful use of the procoagulant, recombinant activated factor VII (rFVIIa, eptacog alfa, NovoSeven®, Novo Nordisk, Bagsvaerd, Denmark) for the treatment of bleeding in haemophilia patients1,2 provided a promising paradigm. Licensed in many countries for use in the treatment of spontaneous and surgical bleeding in patients with inhibitors to factors VIII and IX, congenital factor VII deficiency and Glanzmann’s thrombasthenia, rFVIIa has been demonstrated to bind to the surface of activated platelets and directly activate factor X, thus bypassing the early steps of the coagulation cascade. Encouraged by the observation that supraphysiological levels of rFVIIa did not appear to lead to systemic activation of the coagulation cascade and inappropriate thrombosis3–5, the potential for rFVIIa to be used in a variety of other clinical situations was entertained6.
The first report of rFVIIa use for treatment of critical bleeding in non-haemophilia patients was in the context of trauma7 and despite the lack of higher level evidence, a steady stream of case reports and case series on the off-label use of rFVIIa in cardiac surgery, liver transplantation and post-partum haemorrhage entered the literature8. To date, 16 randomised controlled trials (RCT) have examined prophylactic use of rFVIIa in patients at high risk of bleeding and a further 13 RCT have attempted to evaluate the safety and efficacy of therapeutic use of rFVIIa to treat established bleeding9. The latter trials include two simultaneous, double-blind, RCT that reported a statistically significant reduction in red blood cell transfusions in blunt trauma10, although patients who died early were excluded from the analysis. While these studies were not powered for analysis of mortality, there was a trend toward a reduction in deaths. The results of these studies were used to inform the only study designed to evaluate the effect of rFVIIa on mortality, the CONTROL Trial, which was stopped early due to lower than expected mortality11. Despite a disappointing outcome, the authors published a follow-up article reporting safety data which indicated that the use of rFVIIa in trauma patients was not associated with an increased risk of adverse events (AE), including thromboembolic adverse events (TAE). However, a recent Cochrane review and meta-analysis12 examined results from 13 RCT (involving 2,929 patients) on the therapeutic use of rFVIIa, including treatment of intra-cerebral haemorrhage and gastrointestinal bleeding, and concluded that, while there was a trend toward decreased mortality and increased TAE, there was little evidence of benefit for clinical use of rFVIIa outside the approved indications. In addition, a number of publications have highlighted concerns regarding the safety of rFVIIa in the light of reported TAE following its use13,14.
A consensus conference on massive transfusion in trauma, organized by the Canadian National Advisory committee on Blood and Blood Products in 2011, unanimously agreed that rFVIIa was “not recommended for the prevention or management of haemorrhage in trauma patients”15 and the Australian National Health and Medical Research Council Patient Blood Management Guidelines concluded that the “routine use of rFVIIa in trauma patients with critical bleeding requiring massive transfusion is not recommended because of its lack of effect on mortality and variable effect on morbidity”16. Thus, more than 10 years of research and efforts to improve the evidence-base for off-licence use of rFVIIa has not proven the clinical worth of the product in non-haemophiliac patients.
Herein we summarise 10 years of data captured by the Haemostasis Registry from non-haemophiliac patients treated off-licence with rFVIIa for episodes of critical bleeding in Australia and New Zealand. The Haemostasis Registry is the only bi-national registry of rFVIIa use worldwide and these results provide the largest published case series of rFVIIa use and include the largest case series in many individual clinical specialties. Data from the registry have proven to be an important source of information, especially on the use of FVIIa in rare conditions and, despite not having the internal rigour of prospective, randomised studies, has allowed extensive exploration and sub-analyses of rFVIIa use in a variety of clinical settings, contributions that remain unattainable through RCT alone.
Materials and methods
The Haemostasis Registry was established by the Department of Epidemiology and Preventive Medicine, Monash University and funded through an unrestricted educational grant from Novo Nordisk Pharmaceuticals Pty Ltd., Baulkham Hills, NSW, Australia. The registry was established in 2005 and included retrospective data collection resulting in cases spanning a 10-year period (2000–2009). Ninety-six hospitals across Australia and New Zealand were granted approval by local Human Research Ethics Committees to collect non-identifiable data with waived consent. These included all major metropolitan hospitals in the two countries and are estimated to account for more than 95% of off-licence use of rFVIIa during the period.
Patients
Patients receiving rFVIIa to pre-empt or treat critical bleeding episodes outside the approved indications were eligible for inclusion in the Registry. Data from patients with acquired haemophilia were also recorded but are not reported in this paper. Seventy-five hospitals from all Australian states and from New Zealand contributed data to the registry, the remaining 21 hospitals that had obtained a priori ethical approval did not ultimately use the drug for off-label conditions. Patients were identified by local investigators at each hospital using blood bank and/or pharmacy records. It was mandated by the registry that participating hospitals register every case of off-licence use of rFVIIa at their institution. Completeness of case accrual was audited on an annual basis against blood bank and/or pharmacy records and hospital purchase records. Data were collected from the medical record by trained data collectors at each site, typically research or transfusion nurses or interested clinicians, using a standardised electronic case report form for direct web-based entry. All data entries were verified and validated by central registry staff prior to inclusion in the registry. Any additional information required was ascertained from local investigators. The report of a detailed data audit has previously been published17.
Data items
Data were collected in the following categories; patients’ demographics (age, gender), context of bleeding, volume of rFVIIa dose administered, patient-related factors (temperature, pH), results of blood tests performed before and after rFVIIa use (prothrombin time [PT], activated partial thromboplastin time [APTT], International Normalised Ratio [INR], haemoglobin, haematocrit, fibrinogen, platelet count), blood components transfused within 24 hours before and after rFVIIa use (red blood cells, fresh frozen plasma, cryoprecipitate, platelets), co-interventions (surgical, topical and pharmaceutical) and outcomes.
Outcome measures were recorded up to 28 days following rFVIIa administration and included response to rFVIIa (stopped, decreased or unchanged after each dose as judged by attending clinician), adverse events (thromboembolic and other) and 28-day mortality. All adverse events were recorded, regardless of whether or not they were considered to be linked to rFVIIa treatment and were collected retrospectively after 28 days had elapsed. A second or subsequent linked case was recorded in patients who were treated with rFVIIa more than 48 hours after a previous dose. Analysis of outcomes was adjusted to allow for linked cases (i.e. mortality and adverse event data were scored per patient, but effect on bleeding was scored per dose and per case). Missing data are shown in Appendix Table A.
Information was also collected from each hospital regarding the use of protocols or guidelines for the administration of rFVIIa. That information was the subject of a previous publication18 and is not presented here.
Structured feedback regarding off-licence use of rFVIIa was provided to participating hospitals through web-based data reports which could be generated ad hoc. Individual site data were presented in customised reports with aggregate data from other contributing hospitals, to facilitate meaningful comparisons.
Statistical analysis
Summary statistics are presented as percentage values, mean and standard deviation (SD) for normally distributed data, and median and inter-quartile range (IQR) for non-normally distributed data. Regression analyses included data only from adult patients (aged ≥ ≥16 years). Logistic regression was used to assess the association between covariates and the outcome measures of mortality and response to first dose of rFVIIa. A matrix of correlation coefficients with a cut-off of 0.5 was used to exclude highly correlated variables from the multiple regression models. Multiple regression analysis modelled the association between covariates (bleeding context, temperature, pH, laboratory values prior to first rFVIIa dose, blood products transfused in 24 hours prior to first rFVIIa dose and use of adjunctive therapies prior to rFVIIa) and the outcome measures. A P-value less than 0.05 was considered statistically significant. All analyses were performed using Stata v. 11.0 (Stata Corp, College Station, Texas, USA). Analyses were adjusted to take account of missing data. The effect of missing data and potential remedial methods, such as multiple imputation, will be explored in a subsequent publication.
Results
The Haemostasis Registry includes 3,446 cases of off-label rFVIIa use from 3,322 patients (Table I) treated at 75 hospitals across Australia and New Zealand. The earliest case reported to the registry, which was also the first off-licence use of rFVIIa in Australia, was in 2000. Thereafter there was a steady increase in the number of cases between 2002 (n=48) and 2004 (n=278), before a steep rise in case numbers in 2005 (n=510). The number of cases plateaued in 2006–2008 (range; n=613–633), followed by a slight decrease in 2009 (n=559).
Table I.
Bleeding contexts included in the Haemostasis Registry.
| Context of bleeding | Cases (%) |
|---|---|
| Total Cases | 3,446 (100) |
|
| |
| Cardiac Surgery | 1,535 (45) |
| Isolated coronary artery bypass grafting (CABG) | 203 |
| Isolated valve surgery | 249 |
| CABG and valve surgery | 157 |
| Other cardiac surgery | 926 |
|
| |
| Other surgery | 604 (18) |
| Abdominal (non-vascular) | 257 |
| Vascular - abdominal aortic aneurysm | 103 |
| Thoracic (non-cardiac) | 83 |
| Vascular - other | 42 |
| Neurosurgery | 26 |
| Orthopaedic | 24 |
| Plastics | 21 |
| Urology | 18 |
| Other | 30 |
|
| |
| Trauma | 461 (13) |
| Blunt | 374 |
| Penetrating | 52 |
| Burns | 20 |
| Unknown | 15 |
|
| |
| Medical/other non-surgical | 212 (6) |
| Intra-abdominal haemorrhage | 52 |
| Gastrointestinal bleeding | 47 |
| Pulmonary haemorrhage | 30 |
| Per rectum bleeding | 18 |
| Sepsis | 18 |
| Haemothorax | 17 |
| Bleeding from puncture sites | 6 |
| Other | 20 |
| Unknown | 4 |
|
| |
| Liver | 189 (5) |
| Orthotopic liver transplant | 59 |
| Upper gastrointestinal bleeding | 59 |
| Other liver surgery | 65 |
| Prophylaxis | 6 |
|
| |
| Obstetric haemorrhageab | 177 (5) |
| Placenta praevia | 46 |
| Uterine atony | 39 |
| Placenta accreta/percreta | 30 |
| Intra-uterine foetal death | 23 |
| Pre-eclampsia/eclampsia | 20 |
| Placental abruption | 17 |
| Other | 2 |
|
| |
| Haematology/oncology | 166 (5) |
| Acute myeloid leukaemia | 53 |
| Non-Hodgkin’s lymphoma | 43 |
| Acute lymphoblastic leukaemia | 21 |
| Multiple myeloma | 18 |
| Other | 31 |
|
| |
| Intra-cranial haemorrhage | 83 (2) |
| Intra-cerebral haemorrhage | 52 |
| Sub-dural haemorrhage | 26 |
| Sub-arachnoid haemorrhage | 11 |
| Extra-dural haemorrhage | 2 |
|
| |
| Known clotting disorders | 19 (1) |
| Idiopathic thrombocytopenia purpura | 6 |
| Other | 13 |
Patients commonly reported multiple factors possibly contributing to obstetric haemorrhage;
including 5 patients diagnosed with amniotic fluid embolism.
Demographics
The majority of patients reported to the registry were male (n=2,147, 65%). The patients’ age ranged from a few days to more than 85 years; the largest proportion (n=1,187, 36%) was aged between 55 and 74 years. Approximately 11% of patients were ≤16 years of age (n=361) and of these, 32% (n=114, 3% all patients) were neonates (<1 month). The largest proportion of cases originated in the Australian state of New South Wales (n=1,457, 44%) reflecting the largest population but on a per-capita basis, use was highest in the Northern Territory and lowest in Tasmania (28 vs 2 per 100,000 over 10 years).
Context of bleeding
An overview of off-label rFVIIa use by clinical context is shown in Table I. Over the 10-year period of data collection, cardiac surgery was consistently the largest category with 45% (n=1,535) of cases including 60% (n=926) related to complex procedures such as those involving the aorta or heart transplantation (Table I).
Dosing
Overall, 4,830 doses were administered in the 3,446 cases in 3,322 patients; 2,650 (77%) cases received a single dose, 611 (18%), 114 (3%), 32 (1%) and 14 (0.4%) received two, three, four and five doses, respectively and the remaining 25 (0.7%) cases received between 6 and 84 consecutive doses. Of the latter group, 11 (44%) were cases of haematological or other cancers and six (24%) were treated for known bleeding disorders (1 with idiopathic pulmonary haemosiderosis, 5 with idiopathic thrombocytopenia purpura) refractory to other therapies. The number of doses per patient did not differ over the 10-year period; a similar percentage of cases received single or subsequent doses when normalised against annual case numbers. Fifty-two percent of patients received the first dose of rFVIIa in the operating theatre; 41% in the Intensive Care Unit; 2% in the Emergency Department and the remaining 5% elsewhere. As shown in Table II, the median (IQR) first dose of rFVIIa administered was 91 μg/kg (72–103) with a range from 7 to 2,250 μg/kg, the latter the result of a dosing error. These doses correspond to a median (IQR) dose volume of 6.0 (4.8–7.2) mg reflecting the available packaged vials and the typical use of whole vials. The size of dose administered did not differ according to clinical context of bleeding but was significantly higher in paediatric cases (see also McQuilten et al.19 for further details regarding the paediatric cohort).
Table II.
Dose and outcome measures according to bleeding context.
| All registry (%) | Cardiac (%) | Trauma (%) | Liver (%) | Other surgery (%) | Obstetric (%) | Haem/onc (%) | ICH (%) | Medical other (%) | Known coagulopathy (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Total cases | 3,446 (100) | 1,535 (45) | 461 (13) | 189 (5) | 604 (18) | 177 (5) | 166 (5) | 83 (2) | 212 (6) | 19 (1) |
|
| ||||||||||
| Total Patients | 3,322 (100) | 1,513 (46) | 453 (14) | 168 (5) | 577 (17) | 175 (5) | 146 (4) | 81 (2) | 191 (6) | 18 (1) |
| Dose | ||||||||||
| Single dose rFVIIa | 2,650 (77) | 1,217 (79) | 352 (76) | 140 (74) | 455 (75) | 134 (76) | 111 (67) | 72 (87) | 156 (74) | 13 (68) |
| Median first dose μg/kg [IQR] | 91 [72–103] | 91 [73–103] | 95 [80–108] | 89 [67–104] | 90 [70–102] | 90 [69–100] | 90 [77–100] | 90 [77–102] | 88 [73–100] | 99 [73–113] |
|
| ||||||||||
| Effect on bleeding after initial dose | ||||||||||
| Stopped | 408 (12) | 207 (13) | 39 (8) | 23 (12) | 61 (10) | 26 (15) | 13 (8) | 11 (13) | 26 (12) | 2 (11) |
| Decreased | 1,600 (46) | 863 (56) | 156 (34) | 61 (32) | 268 (44) | 73 (41) | 62 (37) | 23 (28) | 87 (41) | 7 (37) |
| Unchanged | 949 (28) | 290 (19) | 180 (39) | 77 (41) | 185 (31) | 56 (32) | 61 (37) | 21 (25) | 74 (35) | 5 (26) |
| Effect not known | 489 (14) | 175 (11) | 89 (19) | 28 (15) | 90 (15) | 22 (12) | 30 (18) | 28 (34) | 25 (12) | 5 (26) |
|
| ||||||||||
| Effect on bleeding after final dose | ||||||||||
| Stopped | 485 (14) | 250 (16) | 44 (10) | 28 (17) | 72 (12) | 29 (16) | 17 (10) | 11 (13) | 33 (16) | 1 (5) |
| Decreased | 1,700 (49) | 914 (60) | 168 (36) | 58 (35) | 280 (46) | 85 (48) | 66 (40) | 23 (28) | 89 (42) | 6 (32) |
| Unchanged | 770 (22) | 194 (13) | 162 (35) | 56 (33) | 165 (27) | 41 (23) | 56 (34) | 21 (25) | 61 (29) | 5 (26) |
| Effect not known | 491 (14) | 177 (12) | 87 (19) | 26 (15) | 87 (14) | 22 (12) | 27 (16) | 28 (34) | 29 (14) | 7 (37) |
|
| ||||||||||
| 28-day mortality | ||||||||||
| Deceased | 945 (28) | 249 (16) | 207 (46) | 73 (43) | 205 (36) | 11 (6) | 79 (54) | 39 (48) | 78 (41) | 4 (22) |
Final dose equals first dose in patients who received only a single dose.
Haem/Onc: haematology/oncology; ICH: intracranial haemorrhage.
Coagulation parameters
Prior to rFVIIa administration, the median values of all laboratory parameters were deranged. After rFVIIa administration, all coagulation parameters had significantly changed when compared with the same parameters before rFVIIa administration (Wilcoxon’s matched-pairs, signed-rank tests, all p<0.001). After rFVIIa administration the following median (IQR) values were reduced: PT, 18 seconds (15–22) to 13 seconds (11–15); APTT, 45 seconds (36–66) to 40 seconds (34–55); INR, 1.5 (1.3–1.9) to 1.0 (0.9–1.2); and platelet count, 106×109/L (70–162) to 103×109/L (71–144), while there were increases in: haemoglobin, 84 g/L (71–101) to 90 g/L (78–103); haematocrit, 0.25% (0.21–0.30) to 0.26% (0.23–0.30); and fibrinogen 1.9 g/L (1.4–2.6) to 2.1 g/L (1.6–2.8). It should be noted, however, that the presence of circulating rFVIIa has been shown to react in vitro, artificially shortening clot time and consequently PT, APTT and INR results may only indicate that rFVIIa has been given, rather than that there has been any real improvement in clotting profile.
Blood components
Similarly, the number of units of blood components transfused was also significantly lower following rFVIIa administration (Wilcoxon’s matched-pairs, signed-rank tests, all p<0.001). The median (IQR) number of units decreased as follows: red blood cells from 6 (2–13) to 2 (0–4), fresh frozen plasma from 6 (2–10) to 0 (0–4), cryoprecipitate from 4 (0–10) to 0 (0–3) and platelets from 2 (1–4) to 0 (0–2), equating to an overall decrease of total blood component transfusion from 22 (10–35) to 4 (1–14) units.
Notably, 169 patients in the Registry did not receive any blood components before rFVIIa administration, of whom 100 also did not receive any blood components after rFVIIa administration. Of the 100 patients receiving no transfusion, 32 (32%) refused blood transfusion for religious reasons, 14 (14%) were treated prophylactically in situations of high bleeding risk, and a further 15 (15%) were treated for intracranial haemorrhage. In the remaining 39 (40%), the reasons for not using any blood component therapy are not obvious.
Adverse events
In total, the Haemostasis Registry has records of 370 TAE in 361 patients, representing 10.9% of patients (Table III). Arterial thromboses were recorded in 6.3% of patients. The most common event was stroke, which was recorded in 129 patients and included 122 cerebrovascular accidents and eight transient ischaemic attacks. Seventy-three patients were reported to have disseminated intravascular coagulation. Additionally, many non-thromboembolic events were reported such as multi-organ failure and acute respiratory distress syndrome, although it was suspected that these were under-reported due to difficulties with definitions, establishing whether the AE commenced before rFVIIa use or after, and because many patients were extremely ill and the AE were not considered to be related to the treatment and hence not reported. Ten patients reported (minor) allergic reactions following rFVIIa administration.
Table III.
Summary of reported thromboembolic adverse events.
| Adverse events | All registry (%) | Cardiac (%) | Trauma (%) | Liver (%) | Other surgery (%) | Obstetric (%) | Haem/onc (%) | ICH (%) | Medical other (%) |
|---|---|---|---|---|---|---|---|---|---|
| Total cases | 3,446 | 1,535 | 461 | 189 | 604 | 177 | 166 | 83 | 212 |
|
| |||||||||
| Total patients | 3,322 (100) | 1,513 (100) | 453 (100) | 168 (100) | 577 (100) | 175 (100) | 146 (100) | 81 (100) | 191 (100) |
|
| |||||||||
| Thromboembolic adverse events | |||||||||
| Patients with arterial thrombosisa | 208 (6.3) | 129 (8.5) | 10 (2.2) | 2 (1.2) | 44 (7.6) | 2 (1.1) | 6 (4.1) | 5 (6.2) | 10 (5.2) |
| Total arterial thrombosis events | 220 | 136 | 11 | 4 | 46 | 2 | 6 | 5 | 10 |
| Cerebrovascular accident | 122 (3.7) | 93 (6.1) | 7 (1.5) | 1 (0.6) | 10 (1.7) | 1 (0.6) | 3 (1.8) | 3 (3.7) | 4 (2.1) |
| Transient ischaemic attack | 8 (0.2) | 6 (0.4) | 0 (0) | 0 (0) | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Acute myocardial infarction | 48 (1.4) | 16 (1.1) | 1 (0.2) | 2 (1.2) | 23 (4.0) | 1 (0.6) | 0 (0) | 1 (1.2) | 4 (2.1) |
| Other arterial thrombosis | 42 (1.3) | 21 (1.4) | 3 (0.7) | 1 (0.6) | 11 (1.9) | 0 (0) | 3 (1.8) | 1 (1.2) | 2 (1.0) |
| Patients with venous thrombosisa | 99 (3.0) | 41 (2.7) | 16 (3.5) | 1 (0.6) | 24 (4.2) | 5 (2.9) | 2 (1.4) | 3 (3.7) | 7 (3.7) |
| Venous thrombosis events | 102 | 43 | 17 | 1 | 24 | 5 | 2 | 3 | 7 |
| Deep vein thrombosis (DVT) | 41 (1.2) | 14 (0.9) | 6 (1.3) | 0 (0) | 14 (2.4) | 1 (0.6) | 1 (0.7) | 2 (2.5) | 3 (1.6) |
| Pulmonary embolism (PE) | 25 (0.8) | 7 (0.5) | 9 (2.0) | 1 (0.6) | 4 (0.7) | 1 (0.6) | 1 (0.7) | 0 (0) | 2 (1.0) |
| Other thrombosis | 36 (1.1) | 22 (1.5) | 2 (0.4) | 0 (0) | 6 (1.0) | 3 (1.7) | 0 (0) | 1 (1.2) | 2 (1.0) |
| Patients with DICa | 73 (2.2) | 13 (0.9) | 15 (3.3) | 3 (1.8) | 14 (2.4) | 9 (5.1) | 6 (4.1) | 4 (4.9) | 9 (4.7) |
| Total events | 370 | 176 | 41 | 7 | 81 | 15 | 14 | 13 | 23 |
| Total patients with TAEa | 361 (10.9) | 174 (11.5) | 40 (8.8) | 6 (3.6) | 78 (13.5) | 15 (8.6) | 13 (8.9) | 12 (14.8) | 23 (12.0) |
Multiple thrombotic events were reported in some patients.
Haem/Onc: haematology/oncology; ICH: intracranial haemorrhage; TAE: thromboembolic adverse event, DIC: disseminated intravascular coagulopathy.
Summarised by clinical context of bleeding, patients categorised as undergoing cardiac or other surgery had the highest incidence of all TAE (n=176 and n=81, respectively) (Table III) and arterial thromboses (8.5% and 7.6%, respectively) and patients with obstetric or liver disease had the lowest incidence (arterial thromboses 1.2% and 1.8%, respectively). The likelihood of TAE did not increase with higher doses rFVIIa (p=0.769), nor did logistic regression reveal any of the independent variables recorded as predictors of TAE.
Outcome measures
Bleeding response following rFVIIa administration and mortality at 28 days after rFVIIa administration were the main outcomes examined in the Haemostasis Registry. Reported bleeding outcome was available for 86% of all cases (n=2,957). A reduction or cessation of bleeding was reported in 68% of these patients (58% of all cases) after the first dose, rising to 74% (63% of all cases) with subsequent doses of rFVIIa. In a total of 485 cases, bleeding was reported to have stopped (17%, 14% of all cases) and 1,700 cases there were reports of decreased bleeding (57% or 49% of all cases).
Mortality data were available for all patients. At 28 days after rFVIIa administration, 72% (n=2,377) of patients had survived. Of those alive (and for whom response was reported, n=2,054), 20% (n=420) reported cessation of bleeding and 66% (n=1,350) had reduced bleeding after their final dose of rFVIIa. In those patients who died (and for whom response was reported, n=793), the majority (n=448, 56%) had not experienced any change in bleeding following the use of rFVIIa (c22, p<0.001).
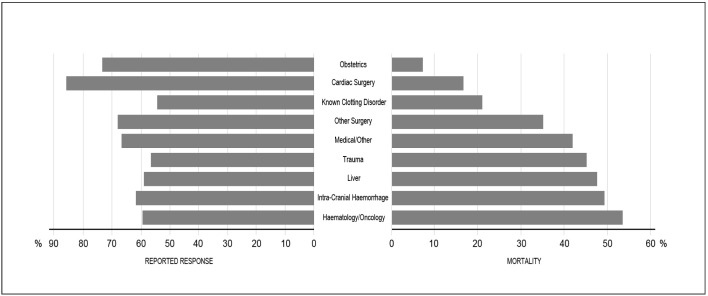
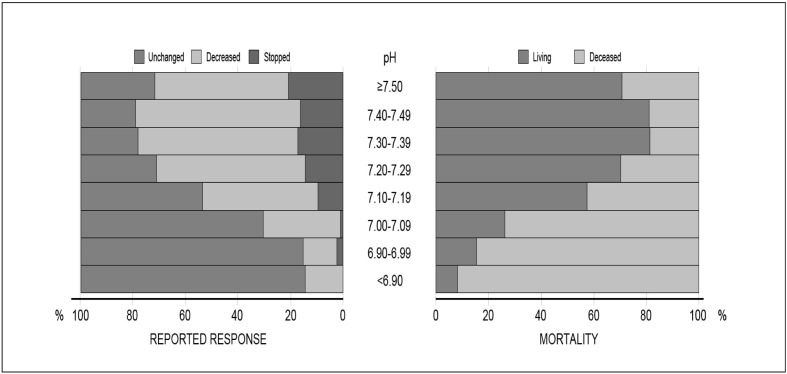
As illustrated in Figures 1 and 2 and Table II, bleeding context and pH correlated with the outcome measures. The proportion of reduced bleeding reported varied according to the context of bleeding (Figure 1, Table I), although in all contexts the majority of patients had a reduction in bleeding. The largest proportion of patients noted to have had a reduction in bleeding was seen in cardiac patients (86%) followed by those with obstetric haemorrhage (74%), and the lowest proportions in patients with known bleeding disorders (55%) and trauma (57%). Similarly, mortality at 28 days after rFVIIa use was examined according to bleeding context (Figure 1, Table II). The highest mortality rate was seen in haematology/oncology patients (54%) and patients treated for intracranial haemorrhage (49%), whereas women treated for obstetric haemorrhage had the lowest mortality rate (7%). Likewise, as shown in Figure 2, pH was strongly correlated with the outcome measures. As patients became more acidotic (pH decreased), they were less likely to have stopped bleeding or reported a decrease in bleeding (c214, p<0.001) (Figure 2) and were more likely to die (c27, p<0.001) (Figure 2).
Figure 1.
Outcome measures according to bleeding context.
Figure 2.
Effect of pH on outcome measures.
PT and INR were found to be closely correlated with APTT and haematocrit with haemoglobin and consequently only APTT and haemoglobin were included in the multivariate analysis (Appendix Table B). In multivariate analysis, clinical context and pH remained associated with reported reduction in bleeding following the first dose of rFVIIa dose (Table IV), with patients with lower pH or being treated for intracranial haemorrhage, medical/other, haematology/oncology and liver conditions less likely to have a reported reduction in bleeding than other patients (p<0.001). In addition to clinical context and pH, prolonged APTT and reduced platelet level before rFVIIa administration were also associated with increased 28-day mortality in multivariate analysis (Table IV). Compared with cardiac surgery, patients treated for bleeding in the contexts of intracranial haemorrhage, medical/other, haematology/oncology and liver conditions were more likely to die, whereas obstetric patients were less likely to die (p<0.001). Patients with a less deranged (higher) pH or (shorter) APTT and higher platelet level prior to rFVIIa administration were less likely to die (p<0.001).
Table IV.
Variables associated with response to first dose of rFVIIa and 28-day mortality in univariate and multivariate analyses.
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Variables | Reported response | 28-day mortality | Reported response | 28-day mortality | ||||||||
|
|
|
|
|
|||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Bleeding context | ||||||||||||
| Cardiac surgery (ref) | 1.00 | - | - | 1.00 | - | - | 1.00 | - | - | 1.00 | - | - |
| Trauma | 0.31 | 0.24, 0.40 | <0.001 | 3.87 | 3.06, 4.89 | <0.001 | 0.61 | 0.36, 1.03 | 0.062 | 1.48 | 0.87, 2.52 | 0.148 |
| Obstetric | 0.51 | 0.36, 0.73 | <0.001 | 0.38 | 0.21, 0.67 | 0.001 | 0.75 | 0.40, 1.42 | 0.381 | 0.09 | 0.03, 0.29 | <0.001 |
| Intracranial haemorrhage | 0.56 | 0.30, 1.04 | 0.069 | 4.36 | 2.69, 7.05 | <0.001 | 0.23 | 0.067, 0.79 | 0.020 | 16.4 | 5.98, 45.1 | <0.001 |
| Medical/other | 0.44 | 0.31, 0.62 | <0.001 | 3.35 | 2.41, 4.67 | <0.001 | 0.35 | 0.19, 0.62 | 0.001 | 3.52 | 1.89, 6.55 | <0.001 |
| Other surgery | 0.49 | 0.39, 0.62 | <0.001 | 2.58 | 2.06, 3.22 | <0.001 | 0.72 | 0.45, 1.13 | 0.148 | 1.63 | 1.03, 2.58 | 0.037 |
| Haematology/oncology | 0.33 | 0.22, 0.49 | <0.001 | 6.07 | 4.17, 8.82 | <0.001 | 0.32 | 0.13, 0.78 | 0.014 | 15.9 | 6.22, 40.8 | <0.001 |
| Liver | 0.33 | 0.23, 0.46 | <0.001 | 4.18 | 3.02, 5.80 | <0.001 | 0.35 | 0.18, 0.68 | 0.002 | 2.81 | 1.46, 5.39 | 0.002 |
| pH at time of dose (0.1 increase) | 1.64 | 1.53, 1.76 | <0.001 | 0.58 | 0.54, 0.62 | <0.001 | 1.46 | 1.29, 1.64 | <0.001 | 0.59 | 0.53, 0.67 | <0.001 |
| APTT prior to dose (30 sec increase) | 0.74 | 0.70, 0.79 | <0.001 | 1.41 | 1.33, 1.48 | <0.001 | 0.91 | 0.82, 1.01 | 0.068 | 1.19 | 1.08, 1.33 | 0.001 |
| Platelet level prior to dose (100×109 increase) | 1.28 | 1.14, 1.43 | <0.001 | 0.59 | 0.52, 0.67 | <0.001 | 1.04 | 0.82, 1.31 | 0.765 | 0.55 | 0.43, 0.72 | <0.001 |
Discussion
The observations reported here are based on 10 years’ of data on the off-licence use of rFVIIa to treat critical bleeding in non-haemophiliac patients in Australia and New Zealand and represent the collective effort of epidemiologists and clinicians from throughout the two countries. The Haemostasis Registry is the largest database of its kind and currently stands as the only bi-national registry of rFVIIa use worldwide. The data presented show that rFVIIa use has expanded to include many unforeseen clinical contexts including rare medical conditions (e.g. amniotic fluid embolus) and surgical settings (Table I). The use of rFVIIa was reported to be temporally associated with a reduction or cessation in bleeding in some, but not all, patients (Table II); clinical context of bleeding (Figure 1) and pH (Figure 2) were the most important factors statistically associated with likelihood of reported reduction in bleeding and death (Table IV).
Diversity in clinical use
A major strength of the registry proved to lie in the capacity to monitor trends in the use of rFVIIa across all clinical contexts and detect new and unexpected clinical areas of use. From the beginning, trauma was proposed as a clinically promising area of off-licence use7. Despite this, the earliest cases of off-licence rFVIIa use reported to the Haemostasis Registry were in patients with liver conditions. Possibly illustrating how practice is affected by publications, reports of rFVIIa use in trauma patients were halved in 2008–2009 following the termination of the CONTROL trial, even though its termination was not related to efficacy or safety. While Dutton et al.20 presented important safety data from the CONTROL trial; continued extensive use of rFVIIa in trauma appears to be unlikely, especially with the availability of alternative haemostatic agents, such as tranexamic acid, which have been reported to be safe and efficacious in the trauma setting21.
Cardiac surgery, on the other hand, was consistently the largest use (almost 50% of cases) reported to the registry over the 10-year period and unlike trauma, case numbers for this use remained relatively stable. Of interest, 10% of all cardiac cases in the registry were paediatric patients, mostly treated during surgical correction of congenital heart defects (see also19). A small dose-escalation RCT suggested that rFVIIa may be effective in reducing allogeneic transfusion and the need for reoperation in cardiac surgery22 yet routine use has not been adopted due to sustained concerns of increased risk of TAE, in particular stroke22,23.
The use of rFVIIa in the management of obstetric bleeding was one of the few clinical areas for which the majority of publications reported a positive effect with high survival rates and few adverse effects (see also24). Indeed, the majority of case series in all areas of use have included mostly positive cases of rFVIIa use, although this is almost certainly due, at least in part, to publication bias. Unfortunately no RCT have been undertaken in obstetric haemorrhage. The usefulness of rFVIIa in this setting may increase with the introduction of a temperature-stable product (NovoSeven® RT), particularly in remote locations where availability of other treatments, including labile blood components, are limited and the occurrence of obstetric haemorrhage may be unpredictable and develop rapidly.
Intracranial haemorrhage seemed to be an important potential use for rFVIIa, especially following results of the first studies in this area25,26. However, cases in this context halved after 2008 following publication of the results from the FAST trial which demonstrated a statistically significant reduction in the growth of haemorrhage volume following administration of rFVIIa, but no effect on primary end-points including severe disability and death, and a small increased risk in adverse outcomes27,28.
Safety
Given the inherent risk of TAE associated with rFVIIa use, one of the primary objectives of the Haemostasis Registry was to monitor and report on all adverse events following treatment with rFVIIa. Findings from a systematic review of seven RCT in patients undergoing major surgery found no significant difference in the proportion of TAE between the rFVIIa and placebo-control groups (7.1% vs 5.3%; OR 1.32; 95% CI 0.69–2.52; p=0.40)29 and the most recent Cochrane Review found a non-statistically significant increase in relative risk in 13 therapeutic RCT (RR 1.14; 95% CI 0.89–1.47) although combining these with 16 trials involving prophylactic rFVIIa use resulted in a significant increase in relative risk (RR 1.45; 95% CI 1.02–2.05)9. Levi and Colleagues30 combined data from 35 placebo-controlled RCT including prophylactic and therapeutic rFVIIa use and “healthy volunteer” studies and found significantly higher rates of arterial TAE (5.5% vs 3.2%; OR 1.68; 95% CI 1.2–2.36; p=0.003), though no statistically significant increase in either venous TAE or overall TAE.
The overall unadjusted TAE rate in the Haemostasis Registry was 10.9%, with 48% of all TAE occurring in cardiac surgery patients. The majority of TAE were arterial (6.3%) and occurred at a higher rate than that observed in the study by Levi and colleagues30. The proportion of cases with reported TAE varied depending on the clinical context (Table III) but number and size of doses were not associated with increased adverse events.
In the absence of a suitable control group, it is difficult to draw any meaningful conclusions on the overall rate of TAE reported to the registry. One possible reason for the higher rate of TAE reported to the registry is that participation in the Haemostasis Registry required that all cases were reported regardless of outcome. Cases in the Haemostasis Registry are also likely to include patients at high risk of TAE who would be excluded from RCT. On the other hand, the TAE recorded in the registry may be an underestimate because no active surveillance for adverse events is undertaken beyond normal patient care.
In a case-control study using Haemostasis Registry patients and a comparable group of patients from the Australasian Society for Cardiac and Thoracic Surgeons cardiac surgery database, Mitra and colleagues31 found no increase in stroke or myocardial infarction associated with the use of rFVIIa. Furthermore, the rate of TAE in cardiac patients reported to the registry was not found to be associated with the size of dose32, despite these patients forming a high-risk cohort.
Likewise, the incidence of TAE reported in trauma patients in the registry was markedly lower than the TAE rate in trauma patients not treated with rFVIIa reported elsewhere33. Apart from intracranial haemorrhage27, trauma remains the only area of use in which a RCT has specifically addressed the issue of safety20. While the overall rate of TAE in the CONTROL trial was higher than that in a previous large case series (9%)33 and the Haemostasis Registry (5.7%), there was no statistically significant increased risk of TAE in patients treated with rFVIIa versus placebo20.
Obstetric patients were also found to experience very few adverse events despite being highly susceptible to TAE. One exception was women diagnosed with amniotic fluid embolism, which is known to result in high levels of circulating tissue factor, rendering patients susceptible to thrombotic complications. In a recent systematic case review by Leighton et al.34, patients with amniotic fluid embolism treated with rFVIIa were found to have significantly worse outcomes including death and permanent disability, compared to the cohort that did not receive rFVIIa, although the review was likely significantly influenced by publication bias against untreated cases with poor outcome. Notably, the Haemostasis Registry contributed five of the 16 cases of rFVIIa use in patients with amniotic fluid embolism reported world-wide, marking a significant contribution to the knowledge base on the use of rFVIIa in this rare medical condition.
Efficacy
Registry data, by their very nature, are unable to address the question of efficacy of any treatment: a question best answered by a RCT. Registry data can, however, contribute to the body of evidence in favour (or against) causation of effect.
Potential surrogate markers for reduction in bleeding, administration of red cells and other blood components (fresh frozen plasma, platelets and cryoprecipitate), were significantly lower following treatment with rFVIIa in the vast majority of Haemostasis Registry patients. Reductions in transfusion requirements following rFVIIa administration have been extensively reported elsewhere; however, due to the potential for mortality bias, it is an unreliable outcome measure. In addition, concomitant or temporally close use of other pharmacological agents or physical interventions further compromise transfusion reduction as a valid measure.
Similarly, significant improvements in coagulation parameters, including PT, INR and APTT were observed, suggesting that rFVIIa may correct patients’ coagulation profiles. However, the usefulness of routine laboratory tests in reflecting real changes in coagulopathy, especially when performed after administration of rFVIIa, is also questionable. The presence of circulating rFVIIa has been shown to shorten in vitro clot time artificially, resulting in false positive readings. Nonetheless, haemoglobin, haematocrit and fibrinogen levels all increased significantly following administration of rFVIIa, an effect unlikely to be attributed to circulating rFVIIa levels but rather improvements associated with successful blood component therapy and the stemming of critical bleeding. Platelet levels were not shown to increase following rFVIIa which may either reflect a failure in replacement therapy and/or an increased consumption, as activated platelets provide the substrate upon which rFVIIa acts.
pH
In vitro studies have shown that reducing the pH from 7.4 to 7.0 reduces rFVIIa enzymatic activity by 60–90%35. Although rFVIIa administration increases circulating FVIIa levels approximately 100-fold, the impact of pH on rFVIIa activity in vivo has not been quantified. The Haemostasis Registry data consistently showed pH to be the single most important predictor of reported reduction in bleeding and likelihood of death following use of rFVIIa (Table IV), irrespectively of the clinical context (see also36–38). As pH decreased, patients were less likely to have a reported reduction in bleeding and patients were more likely to die (Figure 2). Nonetheless, pH prior to rFVIIa administration is a statistically significant and probably clinically important predictor of both response of bleeding to rFVIIa use and mortality. On the other hand, while temperature is important clinically, and was also reported by Meng and Colleagues to influence rFVIIa activity in vitro35, it was not observed to have a significant association with outcome measures in patients in the Haemostasis Registry.
Limitations
There are several important limitations that should be considered in any retrospective observational study, no matter how robustly designed and conducted. Most obviously, there is no control group with which to compare outcome measures, meaning conclusions regarding efficacy and safety are limited. Unfortunately, RCT are expensive, time-consuming and, in the setting of resuscitation and approaching exsanguination, often ethically and logistically difficult. Clinical trials in all areas of rFVIIa use, though desirable, are not a realistic prospect and monitoring of use over an extended period by the Haemostasis Registry is likely to provide the most rigorous data for many years in some of the clinical areas of use.
As the registry ceased collecting data at the end of 2009, the data presented may not reflect current patterns of use. Nonetheless, in Australia and New Zealand, apart from trauma patients for whom off-licence use of rFVIIa is not recommended for critical bleeding16, rFVIIa continues to be used for the treatment of uncontrolled haemorrhage in salvageable patients when all other treatments (i.e. surgical, radiological, blood component therapy) have failed39, despite no supporting evidence from RCT.
Between-centre variation in the use of rFVIIa was inevitable when no study protocol or even national guidelines were available. Variation in practice between hospitals, particularly in the vastly different clinical environments of major tertiary centres in metropolitan centres and regional, rural and remote hospitals in Australia and New Zealand, has been previously assessed in a study32 that demonstrated differences in case complexity and use of labile blood component therapy between hospitals, but found no difference in reported outcomes.
Without the ability to mandate treatment protocols and laboratory testing regimens, as is the case in clinical trials, it is inevitable that there are some missing data in this type of study. To limit bias, patients were not excluded from the dataset due to a missing datum but were included in any analytical test where the requisite data items were present.
In such a broad study as the Haemostasis Registry, clinical diversity and co-morbidities of patients are likely to affect the outcome measures in an unmeasurable manner. For example, expectations of the response and outcomes of a young woman suffering an obstetric haemorrhage are quite different to those of an elderly patient undergoing complex cardiac surgery, or a patient with an end-stage haematological malignancy. To partially control for this, the clinical context of bleeding was retained in the regression model to prevent any single clinical context from skewing the data. However, the extent of the analyses was limited by these differences and more detailed analyses of Haemostasis Registry data have been reserved for publications reporting on specific groups of patients19,24,31,32,36–38,40.
Observational studies are often subject to bias, particularly those in which registration of patients is voluntary. To avoid this, the Haemostasis Registry operated with strict rules requiring the inclusion of all eligible patients and undertook audits to ensure compliance and to assess accuracy of reporting17. This increased the level of confidence in the observed proportions of reported responses and adverse events. Indeed, previously published compilations of adverse events following use of rFVIIa13, while helpful in raising awareness of the potential for adverse outcomes, are unable to give reliable estimates of proportional occurrence of adverse events or meaningful comparisons with other populations of patients due to the lack of a denominator.
Outcome measures in the Haemostasis Registry were undoubtedly a potential source of bias as clinicians were not blinded to the patients’ treatment. In addition, clinicians were asked to make a subjective determination of whether bleeding had altered or not following the use of rFVIIa. In many of the situations reported, when critical bleeding was occurring, multiple treatments may have been given in close temporal proximity, meaning that it would be difficult to separate out effects of any individual treatment.
Despite these limitations, the greatest strength of the Haemostasis Registry is that it is a well-designed and rigorously audited (i.e. accuracy of data and completeness of case ascertainment) database. Participation in the registry required that all cases were entered regardless of outcome and data were independently collected by hospital employed staff. In addition, although financial support for the registry was provided by the manufacturer of rFVIIa (NovoNordisk Pharmaceuticals), extreme care was taken to maintain independence of data collection, analysis and publication. This was important, not only to maintain real and perceived independence of reporting, but also for clinician and local investigator confidence in the registry and the handling of data they submitted.
Conclusions
This is the largest published case series of off-licence use of rFVIIa in the management of critical bleeding. Our data are not able to provide any causal link between rFVIIa use and survival but show that patients with a reported decrease in bleeding following rFVIIa use are more likely to survive. The clinical context of bleeding and pH are the most important predictors of outcome, with the utility of therapy substantially reduced in certain clinical situations and at very low pH (<7.1). Overall, 10.9% of patients experienced a TAE. While it is clearly desirable that pharmaceutical agents such as rFVIIa are subject to rigorous RCT in all clinical areas, it is equally apparent that these trials are highly unlikely to occur. Meticulous and methodical collection of data on off-label use, such as that undertaken by the Haemostasis Registry, should be encouraged or mandated, particularly where the potential for significant harm is inherent in the use of the agent.
Acknowledgements
Haemostasis Registry Steering Committee: Professor James Isbister (chair), Professor Peter Cameron, Dr Scott Dunkley, Dr Roger Houben, Professor John McNeil, Dr Louise Phillips, Ms Wendy Thomas, Dr Amanda Zatta.
Footnotes
Participating hospitals
Australian Capital Territory: The Canberra Hospital.
New South Wales: Blacktown Hospital; Children’s Hospital at Westmead; Concord Hospital; Dalcross Private Hospital; John Hunter Hospital; Lismore Hospital; Liverpool Hospital; Nepean Hospital; North Shore Private Hospital; Port Macquarie Base Hospital; Prince of Wales Hospital; Royal Hospital for Women; Royal North Shore Hospital; Royal Prince Alfred Hospital; St George Private Hospital; St George Public Hospital; St Vincent’s Private Hospital; St Vincent’s Public Hospital; Sydney Adventist Hospital; Sydney Children’s Hospital; Tweed Heads Hospital; Westmead Hospital.
Northern Territory: Alice Springs Hospital; Royal Darwin Hospital.
Queensland: Allamanda Hospital; Gold Coast Hospital; Mackay Base Hospital; Mater Children’s Hospital; Mater Adult Hospital; Nambour Hospital; Princess Alexandra Hospital; Redcliffe Hospital; The Prince Charles Hospital; Royal Brisbane and Women’s Hospital; Royal Children’s Hospital; Townsville Hospital.
South Australia: Flinders Medical Centre; Flinders Private Hospital; Lyell McEwin Hospital; Modbury Public Hospital; Queen Elizabeth Hospital; Repatriation General Hospital; Royal Adelaide Hospital; Women’s and Children’s Hospital.
Tasmania: Launceston Hospital; North West Regional Hospital; Royal Hobart Hospital.
Victoria: Alfred Health; Austin Health; Bendigo Hospital; Box Hill Hospital; Dandenong Hospital; Epworth Hospital; Geelong Hospital; Knox Private Hospital; Mercy Hospital for Women; Monash Medical Centre; Northern Hospital; Peter MacCallum Cancer Centre; Royal Children’s Hospital; Royal Melbourne Hospital; Royal Women’s Hospital; St Francis Xavier Cabrini Hospital; St Vincent’s Hospital; Warringal Private Hospital; Warrnambool Hospital.
Western Australia: Fremantle Hospital; King Edward Memorial Hospital for Women; Mount Hospital; Princess Margaret Hospital for Children; Royal Perth Hospital; Sir Charles Gairdner Hospital; St John of God Hospital Murdoch; St John of God Hospital Subiaco.
New Zealand: Auckland City Hospital; Christchurch Hospital; Dunedin Public Hospital; Hawkes Bay Hospital; Middlemore Hospital; Nelson Hospital; North Shore Hospital; Rotorua Hospital; Tauranga Hospital; Timaru Hospital; Waikato Hospital; Wellington Hospital; Whangarei Hospital.
The Authors declare no conflicts of interest.
References
- 1.Hedner U, Glazer S, Falch J. Recombinant activated factor VII in the treatment of bleeding episodes in patients with inherited and acquired bleeding disorders. Transfus Med Rev. 1993;7:78–83. doi: 10.1016/s0887-7963(93)70126-1. [DOI] [PubMed] [Google Scholar]
- 2.Lusher J, Roberts H, Davignon G, et al. A randomized, double-blind comparison of two dosage levels of recombinant factor VIIa in the treatment of joint, muscle and mucocutaneous haemorrhages in persons with haemophilia A and B, with and without inhibitors. rFVIIa Study Group. Haemophilia. 1998;4:790–8. doi: 10.1046/j.1365-2516.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- 3.Hedner U. Dosing and monitoring NovoSeven treatment. Haemostasis. 1996;26(Suppl 1):102–8. doi: 10.1159/000217250. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M, Monroe DI, Roberts H. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis. 1998;9(Suppl 1):S61–5. [PubMed] [Google Scholar]
- 5.Roberts HR. Clinical experience with activated factor VII: focus on safety aspects. Blood Coagul Fibrinolysis. 1998;9(Suppl 1):S115–8. [PubMed] [Google Scholar]
- 6.Hedner U, Erhardtsen E. Potential role for rFVIIa in transfusion medicine. Transfusion. 2002;42:114–24. doi: 10.1046/j.1537-2995.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 7.Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354:1879. doi: 10.1016/S0140-6736(99)05155-7. [DOI] [PubMed] [Google Scholar]
- 8.Phillips L, Zatta A, Schembri N, et al. Uncontrolled bleeding in surgical patients: the role of recombinant activated factor VIIa. Curr Drug Targets. 2009;10:744–70. doi: 10.2174/138945009788982469. [DOI] [PubMed] [Google Scholar]
- 9.Simpson E, Lin Y, Stanworth S, et al. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2012;2012:1–121. doi: 10.1002/14651858.CD005011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boffard KD, Riou B, Warren B, et al. Recombinant Factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–18. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- 11.Hauser CJ, Boffard K, Dutton R, et al. Results of the CONTROL trial: efficacy and safety of Recombinant Activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma Acute Care Surg. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Stanworth S, Birchall J, et al. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. Can Med Assoc J. 2011;183:E9–19. doi: 10.1503/cmaj.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connell KA, Wood JJ, Wise RO, et al. Thromboembolic adverse events after use of recombinant human coagulation Factor VIIa. JAMA. 2006;295:293–8. doi: 10.1001/jama.295.3.293. [DOI] [PubMed] [Google Scholar]
- 14.Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154:529–40. doi: 10.7326/0003-4819-154-8-201104190-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzik W, Blajchman M, Fergusson D, et al. Clinical review: Canadian National Advisory Committee on Blood and Blood Products - Massive Transfusion Consensus Conference 2011: report of the panel. Crit Care. 2011;15:242. doi: 10.1186/cc10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health and Medical Research Council (NHMRC) Critical Bleeding and Massive Transfusion. Canberra: National Blood Authority; 2011. Patient Blood Management Guidelines: Module 1. [Google Scholar]
- 17.Willis C, Jolley D, McNeil J, et al. Identifying and improving unreliable items in registries through data auditing. Int J Qual Health Care. 2011;23:317–23. doi: 10.1093/intqhc/mzr004. [DOI] [PubMed] [Google Scholar]
- 18.Willis C, Cameron P, Phillips L. Clinical guidelines and off-license recombinant activated factor VII: content, use, and association with patient outcomes. J Thromb Haemost. 2009;7:2016–22. doi: 10.1111/j.1538-7836.2009.03632.x. [DOI] [PubMed] [Google Scholar]
- 19.McQuilten Z, Barnes C, Zatta A, et al. Off-label use of recombinant factor VIIa in pediatric patients. Pediatrics. 2012;129:e1533–40. doi: 10.1542/peds.2011-2561. [DOI] [PubMed] [Google Scholar]
- 20.Dutton RP, Parr M, Tortella BJ, et al. Recombinant activated Factor VII safety in trauma patients: results from the CONTROL trial. J Trauma. 2011;71:12–9. doi: 10.1097/TA.0b013e31821a42cf. [DOI] [PubMed] [Google Scholar]
- 21.CRASH-2 collaborators. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 22.Gill R, Herbertson M, Vuylsteke A, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–7. doi: 10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- 23.Ponschab M, Landoni G, Biondi-Zoccai G, et al. Recombinant activated factor VII increases stroke in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2011;25:804–10. doi: 10.1053/j.jvca.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Phillips LE, McLintock C, Pollock W, et al. Recombinant activated factor VII in obstetric hemorrhage: experiences from the Australian and New Zealand Haemostasis Registry. Anesth Analg. 2009;109:1908–15. doi: 10.1213/ANE.0b013e3181c039e6. [DOI] [PubMed] [Google Scholar]
- 25.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 26.Mayer SA, Brun NC, Broderick J, et al. Safety and feasibility of recombinant factor VIIa for acute intracerebral hemorrhage. Stroke. 2005;36:74–9. doi: 10.1161/01.STR.0000149628.80251.b8. [DOI] [PubMed] [Google Scholar]
- 27.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 28.Diringer M, Skolnick B, Mayer S, et al. Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2010;41:48–53. doi: 10.1161/STROKEAHA.109.561712. [DOI] [PubMed] [Google Scholar]
- 29.Ranucci M, Isgrò G, Soro G, et al. Efficacy and safety of recombinant activated factor VII in major surgical procedures: systematic review and meta-analysis of randomized clinical trials. Arch Surg. 2008;143:296–304. doi: 10.1001/archsurg.2007.66. [DOI] [PubMed] [Google Scholar]
- 30.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 31.Mitra B, Phillips L, Cameron P, et al. The safety of recombinant factor VIIa in cardiac surgery. Anaesth Intensive Care. 2010;38:671–7. doi: 10.1177/0310057X1003800409. [DOI] [PubMed] [Google Scholar]
- 32.Willis C, Bird R, Mullany D, et al. Use of rFVIIa for critical bleeding in cardiac surgery: dose variation and patient outcomes. Vox Sang. 2010;98:531–7. doi: 10.1111/j.1423-0410.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- 33.Thomas GOR, Dutton RP, Hemlock B, et al. Thromboembolic complications associated with factor VIIa administration. J Trauma. 2007;62:564–9. doi: 10.1097/TA.0b013e318031afc2. [DOI] [PubMed] [Google Scholar]
- 34.Leighton BL, Wall MH, Lockhart EM, et al. Use of recombinant factor VIIa in patients with amniotic fluid embolism: a systematic review of case reports. Anesthesiology. 2011;115:1201–8. doi: 10.1097/ALN.0b013e31821bdcfd. [DOI] [PubMed] [Google Scholar]
- 35.Meng ZH, Wolberg AS, Monroe DMI, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high-dose Factor VIIa in hypothermic and acidotic patients. J Trauma. 2003;55:886–91. doi: 10.1097/01.TA.0000066184.20808.A5. [DOI] [PubMed] [Google Scholar]
- 36.Cameron P, Phillips L, Balogh Z, et al. The use of recombinant activated factor VII in trauma patients: experience from the Australian and New Zealand haemostasis registry. Injury. 2007;38:1030–8. doi: 10.1016/j.injury.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Dunkley S, Phillips L, McCall P, et al. Recombinant activated factor VII in cardiac surgery: Experience from the Australian and New Zealand Haemostasis Registry. Ann Thorac Surg. 2008;85:836–44. doi: 10.1016/j.athoracsur.2007.06.076. [DOI] [PubMed] [Google Scholar]
- 38.Mitra B, Cameron PA, Parr MJ, Phillips L. Recombinant factor VIIa in trauma patients with the ‘triad of death’. Injury. 2012;43:1409–14. doi: 10.1016/j.injury.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Massive Transfusion Protocol (MTP) Template. 2013. [Accessed on 24/09/2013]. Available at: http://www.blood.gov.au/sites/default/files/documents/pbm-mtp-template_0.ppt.
- 40.Flower O, Phillips L, Cameron P, et al. Recombinant activated factor VII in liver patients: a retrospective cohort study from Australia and New Zealand. Blood Coagul Fibrinolysis. 2010;21:207–15. doi: 10.1097/MBC.0b013e3283333589. [DOI] [PubMed] [Google Scholar]