Introduction
ABO blood group polymorphisms of humans and other primates are determined by the expression of A, B, or H antigens, which are terminal neutral glycan sequences that are abundant in glycoproteins and glycolipids. H substance is a precursor which, under the influence of genes A and B, is converted into blood group substance A or B, respectively. Given that the inactive O allele does not alter H substance, the presence of unchanged H determinants is characteristic of blood group O individuals.
ABH blood group antigens in human erythrocyte membranes occur as glycolipids and glycoproteins. Among blood group ABH glycolipids on erythrocytes, substances with type 2 chains are predominant. Glycolipids with type 3 and 4 chains are present only in insignificant amounts and type 1 chain glycolipids are adsorbed from the plasma because they are not endogenous erythrocyte substances. Blood group H active antigens have been found on O-linked and N-linked carbohydrate chains1–3. The H-specific oligosaccharide unit has been identified as the trisaccharide Fucα1-2Galβ1-3GalNAc-. In the majority of these H chains, N-acetylgalactosamine carries an α2-6 neuraminic acid residue, but this structure does not seem to have any serological activity4.
The blood group H determinant in human erythrocytes is carried mainly by a lacto-series type 2 chain5. Type 3 chain glycolipids have been found only in group A erythrocytes. Type 3 substance is characteristic of group A1 erythrocytes and is present in only trace amounts in A2 cells. Precursor H type 3 occurs in greater quantities in A2 erythrocytes than in A1 erythrocytes, but is absent in O and B cells.
As early as the mid-20th century, studies showed that some micro-organisms have enzymatic activities that can modify ABO type6. However, it was not until the 1980s that the pioneering work of Goldstein and Lenny (New York Blood Center, NY, USA) discovered technology for enzymatic conversion of A and B antigens which could be used in blood transfusion7. The general name used for the technological concept is “Enzyme-Converted group O Red Blood Cells” (ECO-RBC).
Based on the ECO-RBC concept, glycosidase and glycosyltransferase were used to modify the structure of blood group H antigens. α-1,2-L-fucosidase is a highly specific exoglycosidase that catalyses the hydrolysis of α1-2 linked L-fucopyranosyl residues from oligosaccharides, whereas plasma-derived N-acetylgalactosaminyltransferases (A-transferase) transfer N-acetylgalactosamine residues onto galactose residues in H determinants in the presence of UDP-N-acetylgalactosamine.
In this study, we used α-1,2-L-fucosidase to modify the structure of type 2 chain H. The modified antigens did not show type 2 chain H antigen activity. The erythrocytes treated by fucosidase are called FM-erythrocytes. We used the A-transferase from A1 plasma to modify the structure of type 3 chain H antigens. The erythrocytes treated by A-transferase are called TM-erythrocytes.
Materials and methods
Red blood cells from normal blood donors with ABO phenotype were obtained from the Shanghai Blood Centre and washed three times with phosphate-buffered saline (PBS). Type 2 chain H was defined by a monoclonal antibody from our laboratory, and type 3 chain H was defined by the monoclonal antibody MBr1, which identifies the type 3 chain H structure Fucα1-2Galβ1-3GalNAcα1-3[Fucα1-2] Galβ1-4GlcNAcβ and globo-H structure Fucα1-2Galβ1-3GalNAcβ1-3Galα.
Establishment of optimal fucosidase conditions
To establish the optimum conditions for fucosidase activity, the erythrocytes were first treated with α-1,2-L-fucosidase at a constant temperature for the same incubation period but at different pH levels (5.6, 6.0, 6.5 and 6.9). Next, erythrocytes were treated with α-1,2-L-fucosidase at a constant temperature and pH level, but for different incubation periods (1, 2, 3 and 4 hours). DG gel neutral was used to analyse the amount of type 2 chain H antigen in the erythrocytes.
Incubation conditions for fucosidase treatment of erythrocytes
α-1,2-L-fucosidase was added at a final concentration of 10,000 U/mL to a mixture of erythrocytes and 1×G4 reaction buffer (50 mM sodium citrate, 100 mM NaCl). Bovine serum albumin was then added at a final concentration of 100 μg/mL. The mixture was incubated for 4 hours at 27 °C and a pH of 5.6 to 6.0. The control sample contained only PBS (at a volume equal to that of the α-1,2-L-fucosidase) and reaction buffer and then the added bovine serum albumin.
Analysis of type 2 chain H structure
DG gel neutral analysis
Up to 50 μL of 1% red blood cell suspension was dispensed and combined with 25 μL of IgM anti-H type 2 structure antibody. The mixture was then incubated at room temperature for 5 minutes and centrifuged for the DG gel card.
Flow cytometry analysis
IgG anti-H type 2 structure was used as the first antibody, whereas anti-mouse IgG and fluorescein isothiocyanate (FITC) conjugate were used as the second antibody. Para-Bombay cells were used as a negative control. About 50 μL of 2% red blood cell saline suspension were incubated with 50 μL of the first antibody for 30 minutes at 37 °C, and subsequently washed twice with PBS, and then incubated again with 50 μL of a 1:60 dilution of the second antibody for 30 minutes at 37 °C. The red blood cells were washed twice, resuspended in PBS, and analysed using FACSCalibur™ (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Analysis of Fucα1-2Galβ1-3GalNAc structure (type 3/4 chain H)
MBr1 was used as the first antibody and anti-mouse IgM and FITC conjugate as the second antibody to detect the number of Fucα1-2Galβ1-3GalNAc structures on A2 erythrocytes. The negative control was O cells. The analysis was performed using a FACSCalibur™ flow cytometer (Becton, Dickinson and Company).
Analysis of α1-2 fucose-related structures
Leb specificity is determined by a precursor chain substituted with an Lea-specific α1-4 fucose and a blood group H determinant known as α1-2 fucose. This antigen represents a hybrid epitope composed of Lewis and H determinant structures.
I antigen is the core structure of H antigen. Most substances carrying α1-2 fucose exhibit only reduced I and i activities; this finding is in accordance with the increased I and i reactivity in “Bombay” erythrocytes.
The A specific structures are represented by α1-3 N-acetylgalactosamine residues linked to the subterminal β-galactose unit of the H determinant.
DG gel neutral was used to analyse the Leb and I antigens, whereas flow cytometry was utilised for A antigen analysis.
Analysis of blood group antigen-active glycolipids and glycoproteins
Antigens of the Rh, MNS, Kell, Duffy and Kidd systems are important and clinically significant8. The specificity of blood group N is determined by the amino-terminal end of glycophorin A, which is a sialoglycoprotein of the human erythrocyte membrane. Rh antigens are carried by integral membrane proteins that are neither phosphorylated nor glycosylated. The carriers of the Duffy antigens are glycoproteins.
DG gel neutral was used to analyse C, N and JKa antigens, whereas flow cytometry was used to analyse Fya, k and D antigens.
Effect on absolute membrane potential and shape of erythrocytes
The absolute membrane potential of erythrocytes was determined by flow cytometry9. Bis(1,3-dibutylbarbituric acid[5]) trimethine oxonol (diBA-C4-[3]) dye was dissolved in dimethylsulphoxide, and then 2% glutaraldehyde was slowly added to equal the volume of cells (at 2×106/mL concentration). The solution was kept at 4 °C for 60 minutes. About 1 mL of fixed and non-fixed erythrocyte suspension (4×105 cells in PBS) containing 3,000 nM of diBA-C4-(3) was incubated at room temperature for 30 minutes. The analysis was performed using a FACSCalibur™ flow cytometer (Becton, Dickinson and Company).
Analysis of the effect of A-transferase treatment on the type 3 chain H structure of erythrocytes
Up to 5 μL of packed erythrocytes were washed three times with 0.16 M NaCl, and then added to a 45 μL reaction mixture (pH 6.5) containing 0.15 M NaCl, 0.05 M Tris, 15 mM MnCl2 buffer, 0.5 mM UDP-N-acetylgalactosamine and A1 serum (the source of A-transferase) for a total volume of 90 μL. The erythrocyte suspension was incubated for 24 hours at 37 °C.
MBr1 was used to detect the effect of A-transferase on type 3 chain H structure.
Results
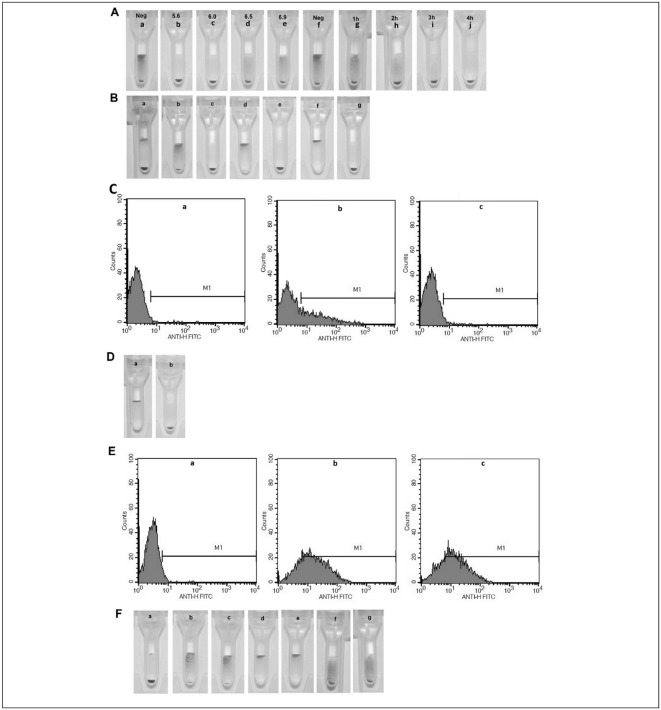
Figure 1 suggests that α-1,2-L-fucosidase demonstrates the greatest catalytic activity in the hydrolysis of α-1,2 linked L-fucopyranosyl residues from type 2 chain H structure on A1 erythrocytes at pH 5.6 (Figure 1A, panel b) to 6.0 (Figure 1A, panel c). In pH 6.5 (Figure 1A, panel d) and 6.9 (Figure 1A, panel e), the catalytic activity of α-1,2-L-fucosidase decreased. In addition, α-1,2-L-fucosidase completely releases α-1,2-fucose on A1 erythrocytes after 4 hours (Figure 1A, panel j). α-1,2-L-fucosidase specifically hydrolyses the terminal α-(1-2)-L-fucosidic linkage to D-galactose in 2′-fucosyllactose. Cells treated with α-1,2-L-fucosidase were no longer reactive to mouse monoclonal antibodies against type 2 chain H (Figure 1B, panels c, e and g, and Figure 1C). The α-1,2-L-fucosidase successfully modified the type 2 chain H structure on the surface of erythrocytes. The enzyme-treated A2 erythrocytes did not agglutinate with commercial IgM anti-H type 2 monoclonal antibodies after 7 hours of incubation (Figure 1D, panel b). The type 2 chain H structures were completely modified. The erythrocytes still reacted with MBr1 monoclonal after 24 hours of incubation and the strength of agglutination was not weakened (Figure 1E). α-1,2-L-fucosidase treatment of erythrocytes did not affect I (Figure 1F, panel c) or Leb antigen structure on the surface of erythrocytes (Figure 1F, panels e and g).
Figure 1.
The detection of type 2 chain H (A, B, C and D), type 3 chain H (E), I antigen (F, panel a, b and c), and Leb antigen (F, panel d, e, f and g) structure on erythrocytes.
For experiment details see Materials and methods section. A, the number of type 2 chain H structure on A1 erythrocytes. The location of erythrocytes shows the structure site density on native A1, pH 5.6, 6.0, 6.5 and 6.9, FM-A1 erythrocytes; native A1, 1h, 2h, 3h and 4h, FM-A1 erythrocytes, sedimentation of erythrocytes in the test tube means no agglutination. B, the number of type 2 chain H structure on erythrocytes. The location of erythrocytes shows the site density in Para-Bombay erythrocytes (panel a), native B (panel b) and FM-B erythrocytes (panel c), native A1 (panel d) and FM-A1 erythrocytes (panel e), native O (panel f) and FM-O erythrocytes (panel g). C, the number of type 2 chain H structure on A1 erythrocyte. The FACS histograms show the site density on para-Bombay (a), native A1 (b) and FM-A1 erythrocytes (c). The x-axis represents the fluorescence intensity on a logarithmic scale, whereas the y-axis shows the number of erythrocytes evaluated. M1 is the gate in flow cytometry. The erythrocyte in M1 area is positive. D, the number of Fucα1-2Galβ (type 2 chain H) structure on A2 erythrocytes. The type 2 chain H structure site density on native A2 (panel a) and FM-A2 erythrocytes (panel b); E, the Fucα1-2Galβ1-3GalNAc (type 3\4 chain H) structure site density on negative O (a), native A2 (b) and FM-A2 erythrocytes (c). F, the number of I and Leb antigen structure on erythrocytes. The I antigen structure site density in newborns (panel a), native (panel b) and FM-erythrocytes (panel c); the Leb antigen structure on the native (panel d) and FM-erythrocytes (panel e); when the anti-Leb antibody diluting concentration was 1:4, Leb antigen structure on the native (panel f) and FM-erythrocytes (panel g).
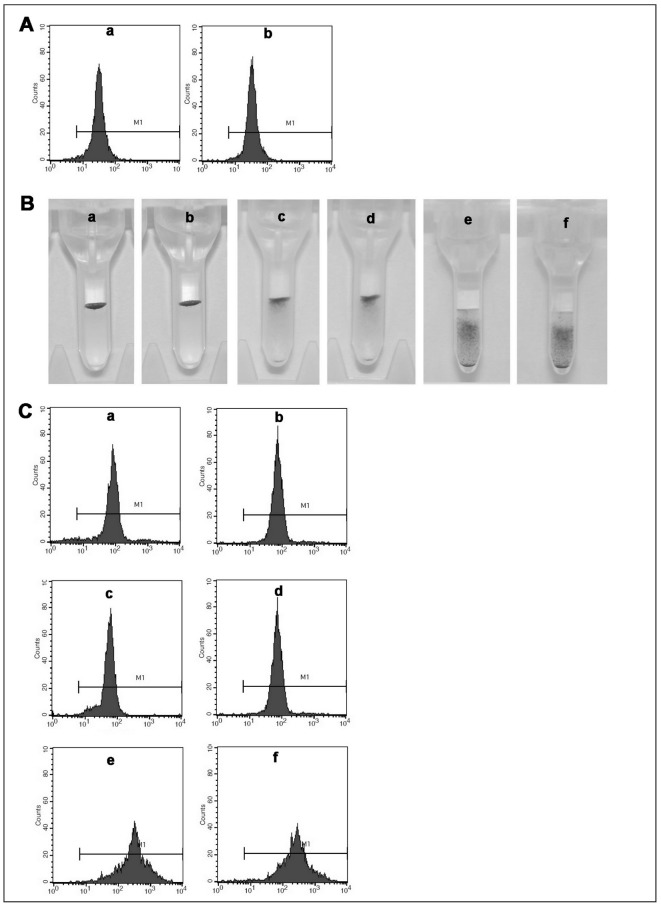
Figure 2 suggests that α-1,2-L-fucosidase treatment of erythrocytes does not affect A antigen structure on the surface of erythrocytes (Figure 2A, panel b). The treatment did not affect the expression of Rh, MNS, Kell, Lutheran, Duffy or Kidd blood group systems antigens, such as C (Figure 2B, panel b), N (Figure 2B, panel d), Jka (Figure 2B, panel f), Fya (Figure 2C, panel b), k (Figure 2C, panel d) and D (Figure 2C, panel f) antigens, because their antigenicity does not depend on the terminal fucose residue.
Figure 2.
The detection of A antigen (A, a and b), C antigen (B, panel a and b), N antigen (B, panel c and d), JKa antigen (B, panel e and f) and Fya antigen (C, a and b), k antigen (C, c and d), D antigen (C, e and f) structure on erythrocytes.
A, the number of A antigen structure on erythrocytes. The A antigen structure site density on native A1 (a) and FM-A1 erythrocytes (b). B, the number of C, N and JKa antigen structure on erythrocytes. The C antigen structure site density of the native (panel a) and FM-erythrocytes (panel b); the N antigen structure site density on the native (panel c) and FM-erythrocytes (panel d); the JKa antigen structure site density on the native (panel e) and FM-erythrocytes (panel f). C, the number of Fya, k and D antigen structure on erythrocytes. The Fya antigen structure site density on native A1 (a) and FM-A1 erythrocytes (b); the k antigen structure site density on native A1 (c) and FM-A1 erythrocytes (d); the D antigen structure site density on native A1 (e) and FM-A1 erythrocytes (f).
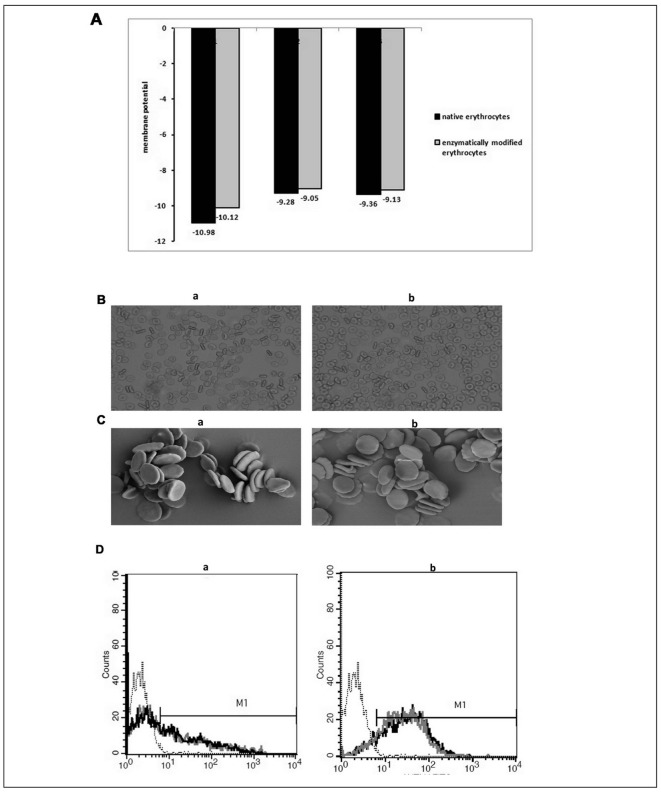
Figure 3A suggests that the absolute membrane potential of the FM-erythrocytes is a little lower than that of native erythrocytes. Erythrocyte membrane potential changes in altered pH buffers, with the membrane potential in low pH buffers being lower than that in high pH buffers10. In our experiments, the enzyme-treated erythrocytes were in pH 5.6 to 6.0 buffers, whereas the native erythrocytes were in PBS. The fucose residue does not influence membrane potential, so the membrane potential of fucosidase-treated erythrocytes is a little lower than that of native erythrocytes because of the pH).
Figure 3.
The detection of absolute membrane potential (A), shape of erythrocytes (B and C) and type 3 chain H structure (D).
A, the three values of absolute membrane potential of erythrocytes. B, the shape of erythrocytes captured by optical microscope (100×). Native erythrocytes (a) and FM-erythrocytes (b). C, erythrocyte images from scanning electron microscopy (SEM). Native erythrocytes (a) and FM-erythrocytes (b). D, the number of type 3 chain H structure on A1 (a) and A2 (b) erythrocytes. The type 3 chain H structure site density on negative O (⋯⋯, black dotted thread), native A1 (–, black solid thread) and A2 (–, black solid thread), TM-A1 (–, grey solid thread) and TM-A2 erythrocytes (–, grey solid thread).
The treatment of the erythrocytes did not affect the shapes of the cells (Figure 3B and C). The A-transferase from A1 serum had no effect on the type 3 chain H structure on A1 (Figure 3D, panel b) or A2 erythrocytes (Figure 3D, panel a). In addition, the antigenic properties of A1 and A2 erythrocytes were unchanged.
Discussion
In 1952 the first H deficient variant, the “Bombay” phenotype, was described by Bhende et al. This blood group phenotype was defined by a total lack of ABH activity on erythrocytes and in secretions. In the years since, a series of further examples of H deficient variants have been discovered. The “Bombay” phenotype is extremely rare in Europids. In the Marathi population around Bombay, however, it occurs at a frequency of about one in 7,600. Bombay individuals are characterised by the complete absence of ABH activity both on erythrocytes and in secretions. Their red blood cells are not agglutinated by anti-A, anti-B, or anti-H reagents under the conditions normally used in blood group serology. The Bombay blood group is the rarest blood group; new facilities for donor units should, therefore, be developed. α-1,2-L-fucosidase can successfully modify the type 2 chain H structure on the surface of erythrocytes, thereby altering its antigenic properties to enable transfusion in individuals with the “Bombay” phenotype.
A previous study made a number of findings on anti-carbohydrate antibodies by profiling and evaluating carbohydrate-binding antibodies in a cohort of 106 healthy donors using a glycochip. The glycan type 2 chain H is extensively present on erythrocytes. Surprisingly, four of 106 sera contained corresponding auto-antibodies: three at moderate levels and one at a rather high level11. At the same time, more individual sera contain glycan Fucα1-2Galβ1-3GalNAcα-sp auto-antibody structure.
When human erythrocytes were incubated with α-1,2-L-fucosidase, the cells could not be further agglutinated by anti-type 2 chain H antibody, but the strength of agglutination caused by MBr1 remained. The modification by α-1,2-L-fucosidase did not affect other related antigens, thus confirming that α-1,2-L-fucosidase can safely modify the type 2 chain H antigen. However, the type 3 chain H antigen was unaffected.
Blood group O and A2 erythrocytes were incubated with A-transferase isolated from group A1 individuals in the presence of UDP-N-acetylgalactosamine. O cells agglutinate in anti-A serum, whereas A2 cells become agglutinable through anti-A112. Thus, A-transferase can transfer N-acetylgalactosamine residues onto the H structure in the presence of UDP-N-acetylgalactosamine. In our A-transferase reaction system, the type 2 chain H structure on O erythrocytes was converted into type 2 chain A antigen, thus reducing or eliminating type 2 chain H antigenicity. A-transferase treatment of A1 and A2 erythrocytes did not change type 3 chain H antigenicity, a fact which may be attributed to the low concentration of A-transferase in the serum, although the difference between the structure of type 2 chain H and type 3 chain H may be another possible reason. The branched chain in type 3 chain H may be a steric hindrance to the enzymatic modification reaction. The α-L-Fuc residue in type 3 chain H is not completely accessible to α-1,2-L-fucosidase and A-transferase, so modification of the Fucα1-2Galβ1-3GalNAcα1-3[Fucα1-2]Galβ 1-4GlcNAcβ structure on A erythrocytes requires further investigation.
Acknowledgments
This work was supported by National Health Standard Project of China (20141601) and Shanghai Technology Standards Project (14DZ0502300).
Footnotes
Authorship contributions
Zhang Wei and Zhu Zi-yan contibuted equally to the paper.
The Authors declare no conflicts of interest.
References
- 1.Morgan WT. A contribution to human biochemical genetics the chemical basis of blood-group specificity. Proc R Soc Lond B. 1960;151:308–47. doi: 10.1098/rspb.1960.0002. [DOI] [PubMed] [Google Scholar]
- 2.Watkins WM. Biochemistry and genetics of the ABO, Lewis and P blood group systems. In: Harris H, Hirschhorn K, editors. Advances in Human Genetics. Vol. 10. New York: Plenum Press; 1981. pp. 1–136. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda M, Fukuda MN. Changes in cell surface glycoproteins and carbohydrate structures during the development and differentiation of human erythroid cells. J Supramol Struct. 1981;17:313–24. doi: 10.1002/jsscb.380170403. [DOI] [PubMed] [Google Scholar]
- 4.Schenkel-Brunner H. Human Blood Groups: Chemical and Biochemical Basis of Antigen Specificity. 2nd ed. Vienna: Springer Verlag; 2000. pp. 67–75. [Google Scholar]
- 5.Hakomori SI. Blood group ABH and Ii antigens of human erythrocytes: chemistry, polymorphism, and their developmental change. Sem Hematol. 1981;18:39–44. [PubMed] [Google Scholar]
- 6.Goldstein J. Conversion of ABO blood groups. Transfus Med Rev. 1989;3:206–12. doi: 10.1016/s0887-7963(89)70080-8. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein J, Siviglia G, Hurst R. Group B erythrocytes enzymatically converted to group O survive normally in A, B, and O individuals. Science. 1982;215:168–70. doi: 10.1126/science.6274021. [DOI] [PubMed] [Google Scholar]
- 8.Krasznai Z, Marian T, Balkay L, et al. Flow cytometric determination of absolute membrane potential of cells. J Photochem Photobiol B. 1995;28:93–9. doi: 10.1016/1011-1344(94)07099-a. [DOI] [PubMed] [Google Scholar]
- 9.Poole J, Daniels G. Blood group antibodies and their significance in transfusion medicine. Transfus Med Rev. 2007;21:58–71. doi: 10.1016/j.tmrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Gedde MM, Huestis WH. Membrane potential and human erythrocyte shape. Biophys J. 1997;72:1220–33. doi: 10.1016/S0006-3495(97)78769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huflejt ME, Vuskovic M. Anti-carbohydrate antibodies of normal sera: findings, surprises and challenges. Mol Immunol. 2009;46:3037–49. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Schenkel-Brunner H. Studies on blood-group A1 and A2 - further evidence for the predominant influence of quantitative differences in the number of A antigenic sites present on A1 and A2 erythrocytes. Eur J Biochem. 1982;122:511–4. [PubMed] [Google Scholar]