Abstract
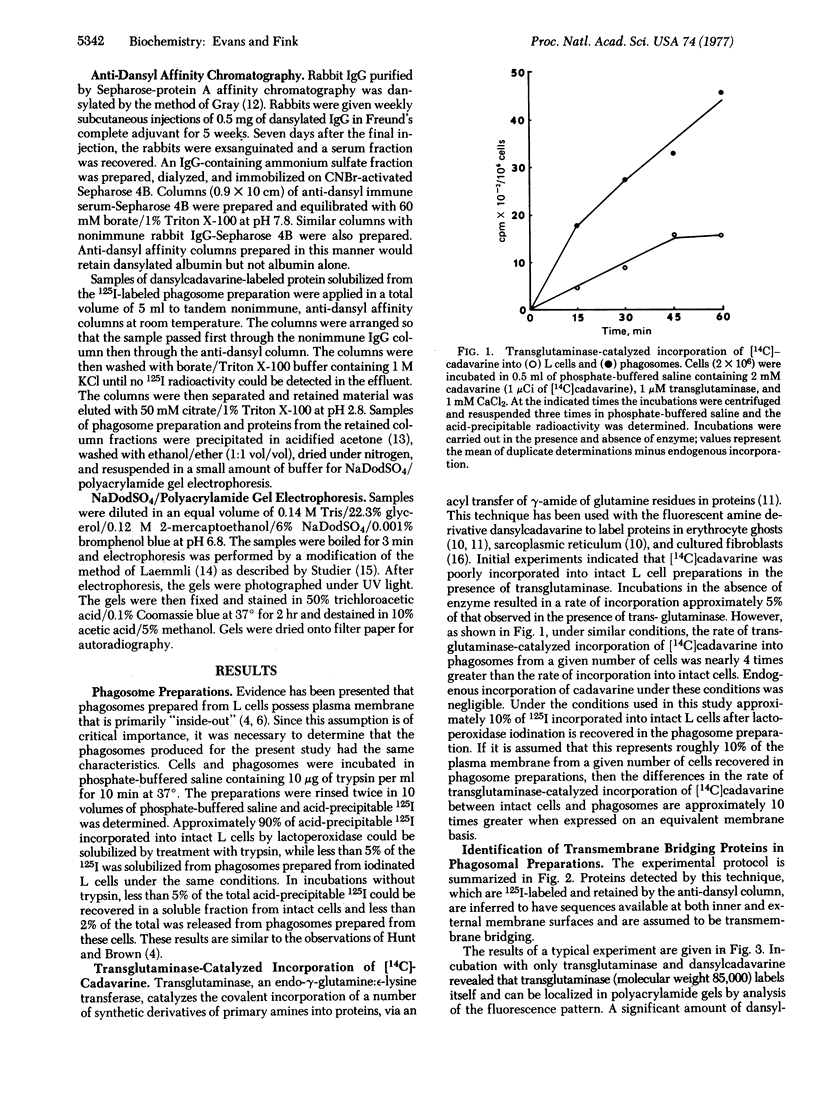
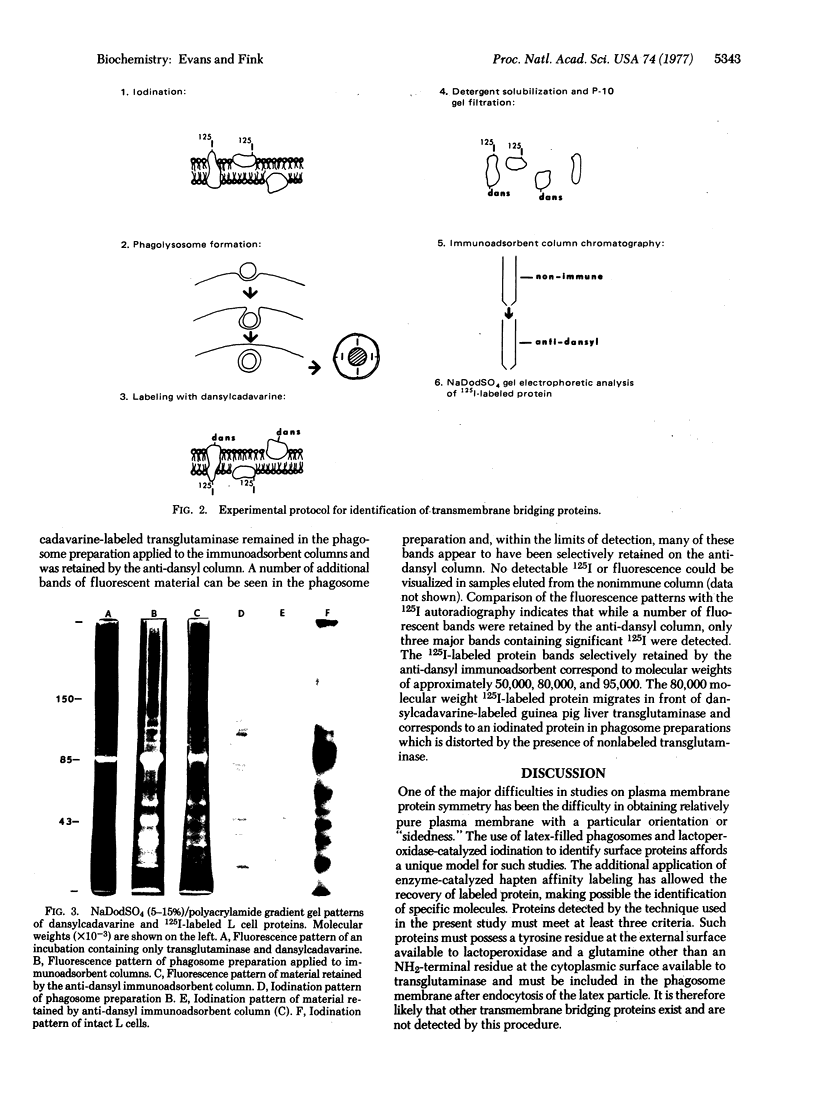
Studies were carried out to identify transmembrane bridging proteins in the plasma membrane of mouse L-929 cells. Cells grown in suspension culture were 125I-labeled by lactoperoxidase and allowed to ingest latex particles to produce inside-out membrane phagosome preparations. Phagosomes were isolated and the inner membrane surface was labeled with N-(5'-aminopentyl)-5-dimethylamino-1-naphthalenesulfonamide (dansylcadavarine) by a transglutaminase-catalyzed reaction. The phagosome membrane proteins were solubilized and dansylcadavarine-labeled proteins were isolated by anti-dansyl immunoadsorbent affinity chromatography. Dansylcadavarine-labeled proteins were analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and autoradiography for the presence of 125I-labeled material. By this technique, two iodinated proteins with molecular weights of approximately 50,000 and 80,000 appear to be selectively retained by the anti-dansyl immunoadsorbent, suggesting that these proteins span the plasma membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birckbichler P. J., Dowben R. M., Matacic S., Loewy A. G. Isopeptide bonds in membrane proteins from eukaryotic cells. Biochim Biophys Acta. 1973 Jan 2;291(1):149–155. doi: 10.1016/0005-2736(73)90070-9. [DOI] [PubMed] [Google Scholar]
- Connellan J. M., Chung S. I., Whetzel N. K., Bradley L. M., Folk J. E. Structural properties of guinea pig liver transglutaminase. J Biol Chem. 1971 Feb 25;246(4):1093–1098. [PubMed] [Google Scholar]
- Dutton A., Rees E. D., Singer S. J. An experiment eliminating the rotating carrier mechanism for the active transport of Ca ion in sarcoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1532–1536. doi: 10.1073/pnas.73.5.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Singer S. J. Crosslinking and labeling of membrane proteins by transglutaminase-catalyzed reactions. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2568–2571. doi: 10.1073/pnas.72.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. II. Metabolic fate of iodinated polypeptides of mouse L cells. J Cell Biol. 1975 Feb;64(2):461–479. doi: 10.1083/jcb.64.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. C., Brown J. C. Identification of a high molecular weight trans-membrane protein in mouse L cells. J Mol Biol. 1975 Oct 5;97(4):413–422. doi: 10.1016/s0022-2836(75)80051-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. Structural studies of sodium and potassium ion-activated adenosine triphosphatase. The relationship between molecular structure and the mechanism of active transport. J Biol Chem. 1975 Sep 25;250(18):7443–7449. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Shishido R., Parameswaran K. N., Steck T. L. Modification of human erythrocyte ghosts with transglutaminase. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1158–1166. doi: 10.1016/0006-291x(75)90795-0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Wetzel M. G., Korn E. D. Phagocytosis of latex beads by Acahamoeba castellanii (Neff). 3. Isolation of the phagocytic vesicles and their membranes. J Cell Biol. 1969 Oct;43(1):90–104. doi: 10.1083/jcb.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]



