Abstract
Compared to deep brain electrical stimulation, which has been applied to treating pathological brain diseases, little work has been done on the effect of deep brain light stimulation. A fiber-coupled laser stimulator at 840 nm wavelength and 130 Hz pulse repetition rate is developed in this work for deep brain light stimulation in a rat model. Concentration changes in glutamate and dopamine in the striatum are observed using a microdialysis probe when the subthalamic nucleus (STN) is stimulated at various optical power levels. Experimental results show that light stimulation causes the concentration of glutamate to decrease while that of dopamine is increased. This suggests that deep brain light stimulation of the STN is a promising therapeutic strategy for dopamine-related diseases such as Parkinson’s disease. The stimulator developed for this work is useful for deep brain light stimulation in biomedical research.
OCIS codes: (170.5180) Photodynamic therapy, (170.3660) Light propagation in tissues, (170.3890) Medical optics instrumentation, (170.1420) Biology, (170.1610) Clinical applications, (170.0170) Medical optics and biotechnology
1. Introduction
Light stimulation has attracted continuous research interest and has been shown to have varying efficacy on a variety of organisms and cell types [1–4]. Many research studies have shown that infrared light stimulation has a positive effect [5] on traumatic brain injury [6] and stroke [7]. Absorbed photons can be converted to useful energy for an organism: During mitochondrial respiration, cytochrome c oxidase (COX) is used as a light acceptor to transform light energy into useful energy in the form of ATP for a cell. The converted energy is stored in mitochondria to promote an increase in the secretion of many molecules used to accelerate cell repair. This mechanism is well known for low-level light therapy [8, 9]. Most modern instruments currently used for light stimulation, such as the light stimulation instrument used to treat traumatic brain injury, are usually designed to illuminate the surface of an organism but are unable to probe beyond surface level. To investigate responses inside the brain, we developed an instrument coupled with stereotactic surgery, which deeply penetrates the brain in vivo to precisely stimulate the target region. For deep brain light stimulation therapy, this is a powerful method and an excellent candidate for further development because it enables the experimenter to specifically target a deep brain region for localized light stimulation.
Developing a treatment for Parkinson’s disease has been an important subject in medicine. The neurotransmitter dopamine is essential in the pathophysiology of Parkinson’s disease [10]. Patients with Parkinson’s disease have a decreased level of dopamine in the striatum [11]. The cortico-striato-thalamocortical loop (CSTC) is considered to be the circuit associated with Parkinson’s disease, and dopamine and glutamate play important roles in the loop [12, 13]. The subthalamic nucleus (STN) sends glutamatergic projections to the globus pallidus pars internalis (GPi) and substantia nigra pars reticulata (SNr) to control the release of γ-Aminobutyric acid (GABA) from the GPi and the SNr. Moreover, glutamate in the cortex is modulated by glutamate produced in the thalamus, which is also influenced by GABA from the GPi and SNr. Cortical neurons send glutamatergic projections to the striatum, forming glutamatergic cortico-striatal synapses. The striatum then sends GABAergic projections to the substantia nigra pars compacta (SNc) to control dopamine release from the SNc to the striatum. An indirect pathway also connects the STN and the striatum: GABAergic cells in the striatum project to the globus pallidus pars externalis (GPe), which then sends GABAergic projections to the STN. Consequently, it is important to study changes in striatal glutamate and dopamine concentration when the STN is stimulated.
Currently, drug administration is the most widely used treatment for Parkinson’s disease [14]. Dopamine pills cannot be directly taken due to the blood–brain barrier. Levodopa is widely known for treatment of Parkinson’s disease because it can cross the blood–brain barrier to increase the dopamine level in the central nervous system [15]. Other drugs, such as amantadine, are also used [16]. These drugs temporarily alleviate the symptoms of Parkinson’s disease, but their efficacy decreases over time. In addition, the drug treatment causes many side effects such as hypotension, arrhythmias, nausea, disturbed respiration, and auditory and visual hallucinations. For an effective treatment, it is important to maintain drug efficacy while reducing any side effects.
Deep brain electrical stimulation is an effective treatment for Parkinson’s disease, but it is only used for patients in the advanced stages of the disease. Release of striatal dopamine is enhanced by stimulating the STN to reduce the symptoms of Parkinson’s disease [17–22]. In this treatment, electrodes are implanted in the STN and the patient is able to control a switch that generates current stimulation at a frequency of 130 Hz. Although deep brain electrical stimulation can reduce symptoms, it cannot repair the striatal neurons or prevent them from further degeneration.
In our study, deep brain light stimulation is achieved using an optical fiber-coupled laser stimulating system that we developed. To verify the feasibility of our instrument for biomedical research, the light stimulator was used on adult male Sprague-Dawley rats. We recorded changes in the concentration of striatal glutamate and dopamine caused by stimulation to the STN with different optical power levels. The results were used to evaluate whether it is possible to use deep brain light stimulation to treat Parkinson’s disease. Since this technique applies low-level light stimulation, this method is relatively mild compared to electrical stimulation and could possibly activate these striatal neurons. Deep brain light stimulation has fewer side effects on the body; it also avoids the risk of encountering electronic current instability during treatment.
2. Materials and methods
2.1 Deep brain light stimulator
A schematic of the deep brain light stimulator is shown in Fig. 1(a) , and its overall external appearance is shown in Fig. 1(b). To comply with the wavelength band of the tissue optical window (650 nm – 1200 nm) [8], the original light source is a collimated continuous-wave (CW) laser diode at a peak wavelength of 840 nm. The laser light is coupled to and guided by an optical fiber so that it can be delivered to the deep brain target location through the optical fiber at minimal injury to the brain tissue.
Fig. 1.
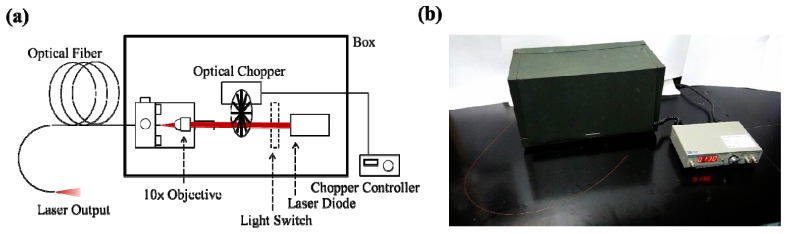
(a) Schematic of deep brain laser-light stimulator. (b) Overall external appearance of the system.
Shown in Fig. 2 is the optical spectrum of the light stimulator measured with an optical spectral analyzer (Anritsu MS9740A). Stable optical pulses are produced using an optical chopper (Standford Research SR540) to modulate the CW laser light; the chopping frequency can be varied from 4 Hz to 3.7 kHz. The optical chopper has a periodic structure, as shown in Fig. 3(a) , which alternately transmits or blocks the laser light to produce repetitive amplitude-modulated laser pulses when its wheel spins. The pulse train is recorded with a silicon photodetector (HINDS DET-200-002) of 1 MHz bandwidth and displayed on a real-time oscilloscope (Agilent 54622A) of 100 MHz bandwidth. The repetition rate can be arbitrarily controlled by varying the modulation frequency of the chopper. The waveforms of the amplitude-modulated laser light at three modulation frequencies of 0 Hz, 50 Hz, and 130 Hz are displayed in Fig. 3(b). Note that a modulation frequency of 0 Hz yields the CW output that is not modulated by the optical chopper.
Fig. 2.
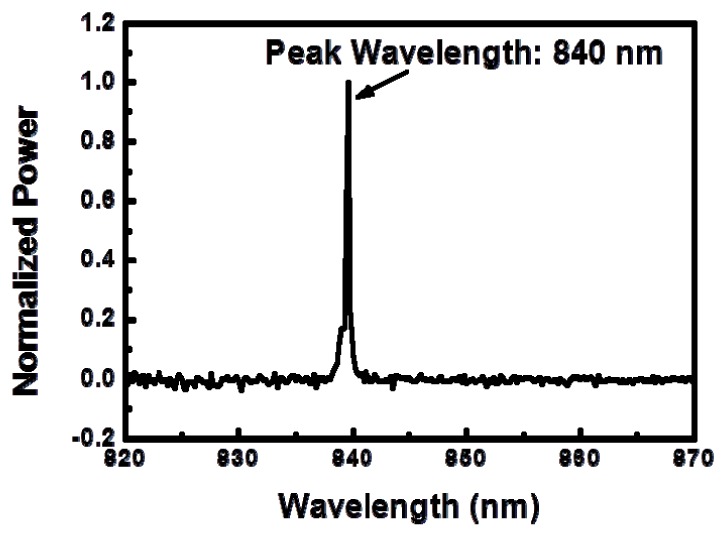
Optical spectrum of the light stimulator showing 840 nm peak wavelength.
Fig. 3.
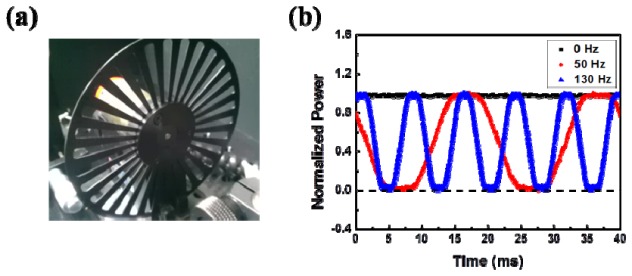
(a) Periodic structure of the optical chopper. (b) Temporal waveforms of the amplitude-modulated laser light at three modulation frequencies of 0 Hz (black), 50 Hz (red), and 130 Hz (blue). Note that a modulation frequency of 0 Hz yields the unmodulated CW output.
To transmit the laser light into the brain, the modulated laser light is coupled to a multimode optical fiber using a microscope objective (10 × ). An optical fiber is a cylindrical waveguide of a circular cross section, in which a high-index light-guiding core is surrounded by a low-index cladding and a protecting layer. The irradiated dimension at the output end of the optical fiber is determined by the core area of the optical fiber; therefore, the fiber core diameter is one of the parameters to be considered in designing the light stimulator. One of the most commonly chosen fiber core diameters is 50 μm. Once the optical fiber is chosen, the optical intensity on the illuminated target is tuned with the average output power of the laser. The optical power is calibrated using an optical power meter with a silicon photodiode sensor (Newport 918-SL-OD3). To confirm the stability of the illuminating optical power, the optical power of the laser is always monitored before and after each light stimulation experiment.
2.2. Parameters of light stimulation
The parameters of light stimulation include the wavelength, the modulation frequency, and the optical power. The 840 nm peak wavelength of the light source used in this stimulator is in the near-infrared spectral region. The repetition rate of the optical pulses produced by modulating the CW laser light with the optical chopper is set at 130 Hz. The blue curve in Fig. 3(b) shows the temporal waveform of these pulses. Three optical power levels of 2 mW, 5 mW, and 10 mW are used, which are calibrated with an optical power meter at the terminal end of the optical fiber. The core and cladding diameters of the multimode optical fiber are 50 µm and 125 µm, respectively, and the overall diameter of the fiber including its outside protecting layer is 250 µm. Therefore, for laser powers of 2 mW, 5 mW, and 10 mW, the averaged optical intensity at the illuminated spot are 102 W/cm2, 255 W/cm2, and 510 W/cm2, respectively, and the cumulative optical energies of light stimulation over a time duration of 60 s are 0.12 J, 0.3 J, and 0.6 J, respectively. The above parameters are summarized in Table 1 .
Table 1. Deep Brain Light Stimulation.
| Optical Parameters
|
|
Effect
|
|||
|---|---|---|---|---|---|
| Optical Power (mW) | Optical Intensity (W/cm2) | Optical Energy (J) | Glutamate Concentration Change | Dopamine Concentration Change | |
| 2 | 102 | 0.12 | S | NS | |
| 5 | 255 | 0.3 | S | NS | |
| 10 | 510 | 0.6 | S | S | |
NS: No statistical difference.
S: Group significantly different from sham group.
2.3 Light stimulation experiment on rats
Adult male Sprague-Dawley rats weighing 370 ± 30 g were used in the following experiments. All of the animals were kept on a 12-hour light/dark cycle and were allowed free access to food and water. All experimental procedures conformed to the guidelines of the National Institute of Health, Taiwan and were approved by the Animal Care and Use Committee of Chi-Mei Medical Center to minimize discomfort to animals during surgery and recovery.
Rats were anesthetized using sodium pentobarbital before the experiment. The animals were head-fixed with a stereotactic apparatus, which allowed for the brain to be positioned correctly in order to locate the STN and striatum. As shown in Fig. 4(a) , the terminal end of the optical fiber from the light stimulator was precisely located at the STN (anterior−posterior −3 mm, left lateral + 0.5 mm, and height −7 mm from the bregma) and the microdialysis probe was placed in the striatum (anterior−posterior + 1 mm, lateral + 2.9 mm, and height −7 mm from the bregma). Using the microdialysis probe to extract cerebrospinal fluid (CSF), cortical responses to light stimulation with different optical parameters can be studied by analyzing the concentration changes, caused by light stimulation, of the chemical ingredients in the extracted CSF.
Fig. 4.
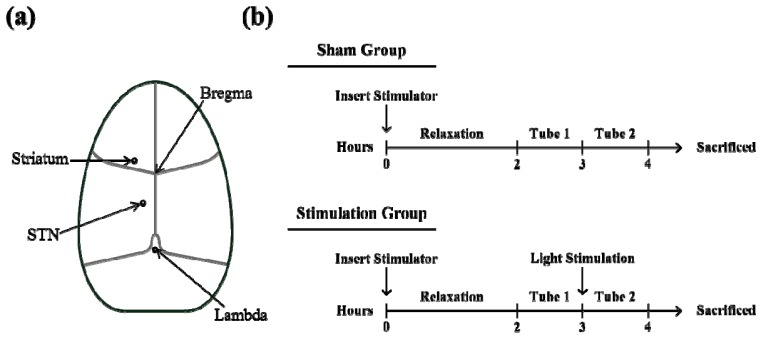
(a) Schematic top view of the rat brain. A rat is fixed on a stereotactic apparatus, which is used to accurately locate the stimulus fiber in the targeted STN and the microdialysis probe in the striatum. (b) Flow diagram of the experimental protocol.
The experimental protocol is shown as a flow diagram in Fig. 4(b); the unit time of extracting CSF is 1 hour. The intracranial insertion of an optical fiber and a chemical probe caused large initial fluctuations in the chemical composition in the CSF of the rat. Because the CSF chemical composition returned to its baseline 2 hours post-insertion, there was a 2-hour buffer period following the insertion of the fiber and probe to allow the CSF chemical composition to stabilize. After this rest period, a tube of 72 μL CSF was collected through the microdialysis probe at a rate of 1.2 μL/min over the unit collection time. This initial collection served as the sampling reference tube and all subsequent collections were normalized to this baseline. The brain was then stimulated with one of three different power levels of laser light for 1 minute and another tube (Tube 2) of 72 μL CSF was collected. This second collection was the affected variation. The sham group did not receive light stimulation, but the rest of the experimental protocol was precisely followed, including the intracranial insertion of the optical fiber and the extraction of CSF. A total of 20 rats were used in this experiment: 5 for each stimulation group of the three different stimulation power levels and 5 for the sham group. To study the effect of light stimulation on the striatum, changes in the striatal secretion of both dopamine and glutamate were analyzed [23]. Aliquots of the CSF were injected onto a CMA 600 microdialysis analyzer (Carnegie Medicine) for the measurement of glutamate. The concentration of dopamine in CSF was measured by high-performance liquid chromatography using a two-channel electrochemical detector (LC-4C; Bioanalytical Systems, West Lafayette, IN).
2.4 Statistical analysis
The experimental data of physiological responses are analyzed using analysis of variation (ANOVA) to find statistically significant responses caused by light simulation at different power levels compared to the sham group. The statistical value of each set of data is expressed in the form of , i.e., mean ± standard deviation. The statistical significance level is set at a p-value < 0.05; data of such significance level are marked with * in the figures presented in the following section. Data of even higher statistical significance are marked with ** for a p-value < 0.01 and with *** for a p-value <0.005.
3. Experimental results
The effects of light stimulation were quantified by the light-induced changes in the concentration of glutamate and dopamine following extraction of CSF from the striatum. In order to properly evaluate the impact of light stimulation on changes in the concentration of these neurotransmitters, we defined the normalized concentration of each neurotransmitter as the ratio of its concentration in the variation tube (Tube 2) to that in the reference tube (Tube 1). For the sham group, the variation tube was collected without light illumination.
As shown in Fig. 5 , normalized glutamate concentrations of the three stimulation groups were 1.27 ± 0.26 (p = 0.0041), 1.00 ± 0.59 (p = 0.0151), and 1.31 ± 0.36 (p = 0.0025) for stimulation with optical powers of 2 mW, 5 mW, and 10 mW, respectively, compared to 2.37 ± 0.27 for the sham. The corresponding p-values for the three illumination groups were determined with respect to the sham. These data show that light stimulation at all three power levels causes a clear decrease in glutamate concentration.
Fig. 5.
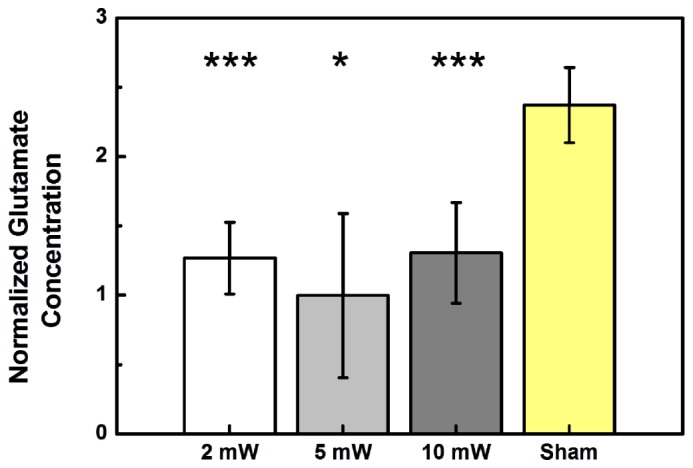
Effects of light stimulation on glutamate in the striatum at three different optical power levels (2 mW, 5 mW, and 10 mW). The normalized glutamate concentration varies with the stimulating optical power. Sham animals were not optically stimulated. (N = 5 in each column.)
Concentration changes in striatal dopamine were also analyzed. As shown in Fig. 6 , the normalized dopamine concentrations of the groups stimulated with optical powers of 2mW, 5mW, and 10 mW were 0.89 ± 0.34 (p = 0.1241), 0.91 ± 0.21 (p = 0.0787), and 1.35 ± 0.37 (p = 0.0349), respectively, while that of the sham group was 0.64 ± 0.21. The corresponding p-values for the three illumination groups were determined with respect to the sham. The increased secretion of dopamine caused by light illumination at 10 mW power was statistically significant, but the increases caused by illumination at 2 mW and 5 mW were not significant with respect to the sham. By comparing Fig. 6 with Fig. 5, it is clear that the response of the dopamine concentration to light stimulation is opposite to that of the glutamate concentration; light stimulation promotes dopamine secretion but suppresses glutamate secretion.
Fig. 6.
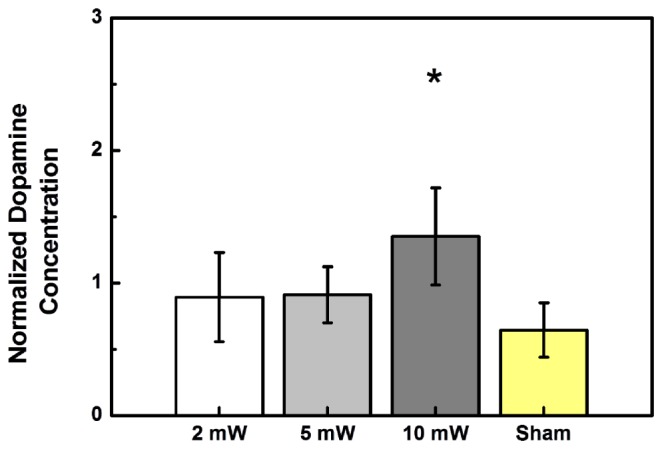
Effects of light stimulation on dopamine in the striatum at three different optical power levels (2 mW, 5 mW, and 10 mW). The normalized dopamine concentration varies with the stimulating optical power. Sham animals were not optically stimulated. (N = 5 in each column.)
4. Discussion
This is the first study to investigate concentration changes in dopamine and glutamate in response to deep brain light stimulation in vivo by using an optical fiber located in the STN while collecting the secreted CSF to measure chemical changes in the striatum. The light stimulator developed in this work is useful for precise execution of light stimulation with minimal injury even at deep brain locations that cannot be optically reached by external illumination [24]. The results presented above show that this device can serve as an important foundation for future studies on laser light therapy in dopamine-related diseases, such as Parkinson’s disease.
Deep brain light stimulation is distinct from deep brain electrical stimulation. Cells stimulated by light at a proper wavelength and power are able to convert the photon energy into a usable substance, such as ATP, so that cell function can be activated or improved. Whereas traditional light stimulation methods such as percutaneous laser ablation for osteoid osteoma destroy cells by thermo-accumulation, laser therapy attempts to stimulate the cells instead of destroying them [25]. Deep brain light stimulation will likely play an important role in the future development of biomedical research and treatment.
By using a properly chosen light-guiding optical fiber, the light stimulator greatly reduced the intracranial lesion to a small diameter of only 250 µm, and the tunneling path was precisely controlled and guided by using the stereotactic apparatus to minimize brain injury. The light-guiding structure of the fiber chosen for our system consists of only the core and the cladding within a diameter of 125 µm. The protecting layer of 250 µm outside diameter only serves to protect the fiber. This diameter is necessary to preserve fiber strength for insertion into deep regions in the brain; stripping off the protecting layer could cause the fiber to break during insertion. Here, CSF was extracted by a microdialysis probe so that the response to light stimulation could be analyzed. For clinical practice, the microdialysis probe is not necessary if the light stimulation mechanism and the parameters for the stimulation are well known from the preclinical experiments that establish the practice. Thus, brain lesion and injury resulting from the microdialysis probe insertion can be eliminated in clinical practice. Compared to deep brain electrical stimulation, deep brain light stimulation in clinical applications is minimally invasive with only a very small lesion created by the thin optical fiber.
Concentration changes in striatal glutamate and dopamine were studied when the STN is stimulated. Glutamate concentration is higher without light stimulation as in the sham group and is suppressed after light stimulation for 1 minute at each of the three power levels, as seen in Fig. 5. In all three experimental conditions, changes in glutamate concentration in response to light stimulation are statistically significant, indicating that there was a wide optical power range for this effect. By contrast, increase in dopamine concentration in response to light stimulation was statistically significant only at an optical power of 10 mW, but not at the other two lower powers, as seen in Fig. 6. The effective power level for dopamine response is clearly more specific than that for glutamate. It is clear that a proper optical power level has to be found for a different light stimulation effect and the proper power level for a combination of effects has to be found by considering the effective power range of each effect. The experimental data shown in Figs. 5 and 6 indicate that the optical power of 10 mW is the best choice to simultaneously suppress glutamate secretion and enhance dopamine secretion in the striatum, triggered by light stimulation to the STN. Our results are consistent with previous studies on electrical therapy of Parkinson’s disease: the release of dopamine to the striatum is enhanced by stimulating the STN with high-frequency electrical stimulation [20, 21]. This finding suggests that the deep brain light stimulation method can potentially be applied to controlling or treating dopamine-related diseases, such as Parkinson’s disease.
5. Conclusions
Deep brain light stimulation can be implemented with minimal invasion or injury to the brain for light therapy of neurological diseases using the fiber-coupled laser stimulator developed for this study. This method differs from traditional light therapy on the surface of an organism and it provides a way to directly study the intracranial response to light stimulation. By using a flexible optical fiber with a small diameter of 250 µm, the stimulator head can be deeply inserted into the brain in vivo without causing severe damage along the tunneling path. Deep brain light stimulation experiments performed on adult male rats show that stimulating the STN with light can significantly suppress the secretion of glutamate while promoting the secretion of dopamine that are projected to the striatum. These results suggest that deep brain light stimulation of the STN is a promising therapeutic strategy for neurological diseases by controlling the secretion of glutamate and dopamine to maintain the balance of the CSF chemical composition. The stimulator developed for this work is also useful for deep brain light stimulation in other biomedical research.
Acknowledgments
The authors would like to acknowledge the financial support of the Chi-Mei Medical Center (Grant No. CMCT10007 and CMCT10107).
References and links
- 1.Sousa F. F. A., Andraus R. A. C., Barbieri C. H., Mazzer N., “Influence of laser radiation in nerve regeneration in different treatment sites,” Acta Ortop. Bras. 17(6), 331–335 (2009). [Google Scholar]
- 2.Rochkind S., El-Ani D., Nevo Z., Shahar A., “Increase of neuronal sprouting and migration using 780 nm laser phototherapy as procedure for cell therapy,” Lasers Surg. Med. 41(4), 277–281 (2009). 10.1002/lsm.20757 [DOI] [PubMed] [Google Scholar]
- 3.Dittami G. M., Rajguru S. M., Lasher R. A., Hitchcock R. W., Rabbitt R. D., “Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes,” J. Physiol. 589(6), 1295–1306 (2011). 10.1113/jphysiol.2010.198804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong W.-K., Chen H.-F., Tsai C.-T., Fu Y.-J., Wong Y.-S., Yen D.-J., Chang T.-H., Huang H.-D., Lee O. K., Chien S., Ho J. H., “The activation of directional stem cell motility by green light-emitting diode irradiation,” Biomaterials 34(8), 1911–1920 (2013). 10.1016/j.biomaterials.2012.11.065 [DOI] [PubMed] [Google Scholar]
- 5.Hashmi J. T., Huang Y. Y., Osmani B. Z., Sharma S. K., Naeser M. A., Hamblin M. R., “Role of low-level laser therapy in neurorehabilitation,” Am. Acad. Phys. Med. Rehabilitation 2(12Suppl 2), S292–S305 (2010). 10.1016/j.pmrj.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oron A., Oron U., Streeter J., de Taboada L., Alexandrovich A., Trembovler V., Shohami E., “Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits,” J. Neurotrauma 24(4), 651–656 (2007). 10.1089/neu.2006.0198 [DOI] [PubMed] [Google Scholar]
- 7.Lampl Y., Zivin J. A., Fisher M., Lew R., Welin L., Dahlof B., Borenstein P., Andersson B., Perez J., Caparo C., Ilic S., Oron U., “Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1),” Stroke 38(6), 1843–1849 (2007). 10.1161/STROKEAHA.106.478230 [DOI] [PubMed] [Google Scholar]
- 8.Huang Y. Y., Chen A. C., Carroll J. D., Hamblin M. R., “Biphasic dose response in low level light therapy,” Dose Response 7(4), 358–383 (2009). 10.2203/dose-response.09-027.Hamblin [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tafur J., Mills P. J., “Low-intensity light therapy: exploring the role of redox mechanisms,” Photomed. Laser Surg. 26(4), 323–328 (2008). 10.1089/pho.2007.2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drukarch B., Jongenelen C. A. M., Schepens E., Langeveld C. H., Stoof J. C., “Glutathione is involved in the granular storage of dopamine in rat PC 12 pheochromocytoma cells: implications for the pathogenesis of Parkinson’s disease,” J. Neurosci. 16(19), 6038–6045 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrag A., Jahanshahi M., Quinn N., “What contributes to quality of life in patients with Parkinson’s disease?” J. Neurol. Neurosurg. Psychiatry 69(3), 308–312 (2000). 10.1136/jnnp.69.3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ting J. T., Feng G., “Glutamatergic synaptic dysfunction and obsessive-compulsive disorder,” Curr Chem Genomics 2, 62–75 (2008). 10.2174/1875397300802010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange K. W., Kornhuber J., Riederer P., “Dopamine/glutamate interactions in Parkinson’s disease,” Neurosci. Biobehav. Rev. 21(4), 393–400 (1997). 10.1016/S0149-7634(96)00043-7 [DOI] [PubMed] [Google Scholar]
- 14.Rascol O., Brooks D. J., Korczyn A. D., De Deyn P. P., Clarke C. E., Lang A. E., “A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group,” N. Engl. J. Med. 342(20), 1484–1491 (2000). 10.1056/NEJM200005183422004 [DOI] [PubMed] [Google Scholar]
- 15.LeWitt P. A., “Levodopa for the treatment of Parkinson’s Disease,” N. Engl. J. Med. 359(23), 2468–2476 (2008). 10.1056/NEJMct0800326 [DOI] [PubMed] [Google Scholar]
- 16.Goetz C. G., Poewe W., Rascol O., Sampaio C., “Evidence-based medical review update: pharmacological and surgical treatments of Parkinson’s disease: 2001 to 2004,” Mov. Disord. 20(5), 523–539 (2005). 10.1002/mds.20464 [DOI] [PubMed] [Google Scholar]
- 17.Greenberg B. D., Malone D. A., Friehs G. M., Rezai A. R., Kubu C. S., Malloy P. F., Salloway S. P., Okun M. S., Goodman W. K., Rasmussen S. A., “Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder,” Neuropsychopharmacology 31(11), 2384–2393 (2006). 10.1038/sj.npp.1301165 [DOI] [PubMed] [Google Scholar]
- 18.Nuttin B. J., Gabriëls L. A., Cosyns P. R., Meyerson B. A., Andréewitch S., Sunaert S. G., Maes A. F., Dupont P. J., Gybels J. M., Gielen F., Demeulemeester H. G., “Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder,” Neurosurgery 62(6Suppl 3), 966–977 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Limousin P., Krack P., Pollak P., Benazzouz A., Ardouin C., Hoffmann D., Benabid A.-L., “Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease,” N. Engl. J. Med. 339(16), 1105–1111 (1998). 10.1056/NEJM199810153391603 [DOI] [PubMed] [Google Scholar]
- 20.Pazo J. H., Höcht C., Barceló A. C., Fillipini B., Lomastro M. J., “Effect of electrical and chemical stimulation of the subthalamic nucleus on the release of striatal dopamine,” Synapse 64(12), 905–915 (2010). 10.1002/syn.20809 [DOI] [PubMed] [Google Scholar]
- 21.Shon Y. M., Lee K. H., Goerss S. J., Kim I. Y., Kimble C., Van Gompel J. J., Bennet K., Blaha C. D., Chang S. Y., “High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery,” Neurosci. Lett. 475(3), 136–140 (2010). 10.1016/j.neulet.2010.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallet L., Polosan M., Jaafari N., Baup N., Welter M. L., Fontaine D., du Montcel S. T., Yelnik J., Chéreau I., Arbus C., Raoul S., Aouizerate B., Damier P., Chabardès S., Czernecki V., Ardouin C., Krebs M. O., Bardinet E., Chaynes P., Burbaud P., Cornu P., Derost P., Bougerol T., Bataille B., Mattei V., Dormont D., Devaux B., Vérin M., Houeto J. L., Pollak P., Benabid A. L., Agid Y., Krack P., Millet B., Pelissolo A.,“Subthalamic nucleus stimulation in severe obsessive-compulsive disorder,” N. Engl. J. Med. 359(20), 2121–2134 (2008). 10.1056/NEJMoa0708514 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto B. K., Davy S., “Dopaminergic modulation of glutamate release in striatum as measured by microdialysis,” J. Neurochem. 58(5), 1736–1742 (1992). 10.1111/j.1471-4159.1992.tb10048.x [DOI] [PubMed] [Google Scholar]
- 24.Abdo A., Sahin M., “NIR light penetration depth in the rat peripheral nerve and brain cortex,” in Proceedings of IEEE Conference on Engineering in Medicine and Biology Society (IEEE, 2007), pp. 1723–1725 10.1109/IEMBS.2007.4352642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangi A., Alizadeh H., Wong L., Buy X., Dietemann J.-L., Roy C., “Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients,” Radiology 242(1), 293–301 (2007). 10.1148/radiol.2421041404 [DOI] [PubMed] [Google Scholar]


