Abstract
Background:
The diagnosis of pulmonary alveolar proteinosis (PAP) is based on computed tomography, histology, and antibodies to granulocyte-macrophage colony-stimulating factor. The role of a novel technique for imaging cells and elastin during endoscopy, probe-based confocal laser endomicroscopy (pCLE), has not yet been investigated in PAP patients. The aim of the present study was to estimate the value of pCLE in the PAP diagnosis and treatment in comparison with the findings of high-resolution computed tomography (HRCT) before and after whole-lung lavage.
Methods:
In vivo pCLE was performed during bronchoscopy in 6 male patients with PAP before and after whole-lung lavage. In certain lung segments, pCLE was followed by HRCT.
Results:
During the in vivo pCLE, we found characteristic signs of PAP: a fluorescent floating amorphous substance in the alveoli lumen sticking to conglomerates along with alveolar macrophages. These features were present to a lesser extent after a whole-lung lavage. pCLE revealed specific PAP features not only in segments with crazy-paving and ground-glass opacity, but also in segments without HRCT findings.
Conclusions:
The alveolar imaging in PAP patients is able to reveal characteristic changes, both in the presence and in the absence of HRCT findings. Therefore, pCLE may be a helpful tool for the diagnosis and whole-lung lavage therapy. Our data prove that accumulation of lipoproteinaceous substances within the alveoli at PAP is a diffuse but not a patchy process.
Key Words: pulmonary alveolar proteinosis, diagnosis, confocal laser endomicroscopy, pCLE, computed tomography
Pulmonary alveolar proteinosis (PAP) is a rare lung disease characterized by alveolar accumulation of lipoproteinaceous material resulting from a decreased clearance of surfactant components from the air spaces by the alveolar macrophages and type II epithelial cells.1–3 The prevalence is approximately 0.5 to 1 per 1 million per year, and the peak incidence of the disease occurs at middle age.4 Among men, the relative risk for cigarette smokers is approximately 2.6.2
The diagnosis of PAP is based on computed tomography, bronchoalveolar lavage, and histology. Antibodies to granulocyte-macrophage colony-stimulating factor (GM-CSF) may also be helpful in making the diagnosis.5 The treatment of PAP includes whole-lung lavage, application of a recombinant GM-CSF, and lung transplantation.6
A relatively new technology in pulmonary medicine, probe-based confocal laser endomicroscopy (pCLE) allows for real-time minimally invasive intra-acinar imaging. In the pCLE technology, a specially designed optical probe can be inserted into the distal airway through the working channel of the bronchoscope to provide optical imaging. The confocal microscopy system enables illumination and detection from a single plane of imaging in the tissue to provide a gray-scale video sequence. The imaging system produced 9 frames-1 for a 8966640 pixel image and a circular 600 mm diameter field of view. The lateral resolution and depth of focus of the probe were 3.5 and 0–50 mm, respectively. The lateral resolution was 3.5 μm. The images are produced through thousands of compacted microfibers, which allow flexibility through our bronchoscopes into the distal lung.7–9
The role of this new method in diagnosing and monitoring PAP patients after the treatment has yet to be investigated. To date, pulmonary pCLE has demonstrated the ability to detect the elastin scaffold of central and peripheral airways, the structure of alveoli, blood vessels, and alveolar macrophages.7–10 Several studies summarizing the pCLE criteria of different inflammatory and oncologic lung diseases have been conducted.11–14 Only 1 case report is devoted to pCLE imaging in a patient with PAP.15 The purpose of our study was to estimate the role of pCLE in diagnostics and treatment of PAP compared with high-resolution computed tomography (HRCT) findings, before and after whole-lung lavage.
PATIENTS AND METHODS
This prospective study was approved by the Ethics committee at the Federal Research Clinical Center FMBA (Moscow, Russia). The study participants gave their informed consent for the participation in the study.
Patients
From 2010 to 2013, 6 patients with PAP had been admitted to our clinic. They were 34- to 59-year-old men, of which 5 had a long-term smoking history and 1 never smoked (Table 1). Each patient had progressive exertion dyspnea, nonproductive cough, and hypoxemia. HRCT revealed typical bilateral diffuse ground-glass opacities with interlobular septal thickening in a crazy-paving pattern. The diagnosis of autoimmune PAP was proved by the transbronchial lung biopsy or the video-assisted thoracoscopic surgical lung biopsy and also by detecting high levels of anti-GM-CSF serum antibodies.
TABLE 1.
Patient Characteristics
Whole-Lung Lavage
Under general anesthesia, first, the right and then the left lung was washed using 37°C saline in 1 L aliquots with a 1-week interval in between. Single-lung ventilation was used for the nonlavaged lung. The lavage was repeated until the drained fluid was clear.16
Five patients underwent whole-lung lavage of both the lungs, whereas patient no. 1 refused the second procedure, and therefore only the right lung was lavaged. The lavage fluid from the first and the last portions of the whole-lung lavage was subjected to the cytologic examination.
pCLE
pCLE was performed under topical lidocaine anesthesia using a 5.9 mm flexible bronchoscope (EB-530T model; Fujinon, Japan). pCLE was performed using the commercial Cellvizio system (Mauna Kea Technologies, Paris, France). We used a 1.4 mm diameter x-ray contrast miniprobe (Alveoflex; Mauna Kea Technologies). This probe uses a laser (wavelength 488 nm) directed into the alveolar space, generating real-time moving images with an optical area of 600 μm at a video frame rate of 12 images per second and a focus depth of 50 μm. The probe was introduced through the working channel of the bronchoscope and gently pushed down to the alveolar ducts and sacs. We did not use any exogenous fluorophores. All segments, or subsegments if available, were sequentially evaluated on both sides of the lung, excluding RB1 and LB1+2 in patients no. 1, 2, 3, and 5. On average, 1 pCLE examination lasted 14.1±5.3 minutes. The video sequences were analyzed with the built-in software (Cellvizio viewer, version 1.6.0; Mauna Kea Technologies). A total of 113,522 confocal images from 262 locations were analyzed prospectively. For each location (segment or subsegment), 10.3±2.1 images were informative and diagnostically valuable. Finally, we analyzed only clear images of different fields of vision. Images with the same alveoli in 1 video sequence were not counted.
The individuals scoring the pCLE images had both HRCT data and information about the performance of whole-lung lavage. Researches were not blinded.
pCLE was performed once before and then on the second day after the whole-lung lavage.
The subsequent pCLE was performed at different times after the whole-lung lavage to control the long-term results in 4 patients: patient no. 1, 18 months; patient no. 3, 6 months, and patients no. 4 and 5, 3 months. We were unable to examine patient no. 2 after he was discharged from the hospital because he relocated to a different area.
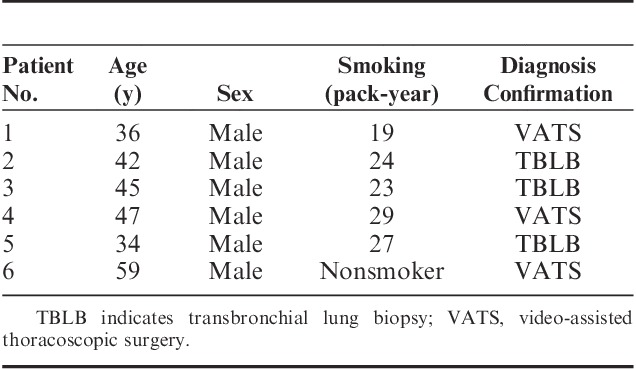
The amount of the floating intra-alveolar substances with alveolar macrophages was assessed by 2 independent researchers using a 6-point score, where a zero meant the absence of the feature, and a 5 meant its most noticeable presence (Fig. 1).
FIGURE 1.
Semiquantitative estimation of the amount of floating intra-alveolar complexes: (A) complexes are absent (0 points), (B) single complexes (1 point), (C) less than half of the visual field is covered (2 points), (D) half of the visual field is covered (3 points), (E) more than half of the visual field is covered (4 points), (F) the visual field is completely covered (5 points).
HRCT
HRCT was performed for all the 6 patients along with pCLE before the treatment and on the second day after the whole-lung lavage to estimate the efficacy of the treatment.
All the follow-up HRCT scans were obtained with a 128 Philips Ingenuity CT scanner.
In patients no. 3 and no. 4, we performed 1 pCLE of the distal airways controlled by the chest HRCT.
We used the following HRCT protocol: patient in the supine position; 0.5 to 1 mm slice thickness; cluster scanning of the regions of interest to decrease the radiation exposure; scanning at the end of a deep inspiration; and high spatial resolution reconstruction algorithm.
To ensure that the HRCT scan was visualizing the Alveoflex probe and not an anatomic structure (eg, vessel), we followed up the probe on sequential scans and measured the density of its tip. Because of a metal-containing material of the tip, the density was very high (approximately 3000 HU). Therefore, the tip was very well distinguishable from all the lung structures. The follow-up HRCT scans for the pCLE guidance were conducted in 2 segments with ground-glass opacity and crazy-paving detected earlier and in 2 zones with intact lung parenchyma.
RESULTS
Whole-Lung Lavage Fluid
The first portion of the lavaged fluid was opalescent and milky. The cytologic examination revealed a large amount of amorphous, granular, eosinophilic, periodic acid-Schiff (PAS)-positive material. In the last portion, there were only traces of the homogenous substances.
HRCT
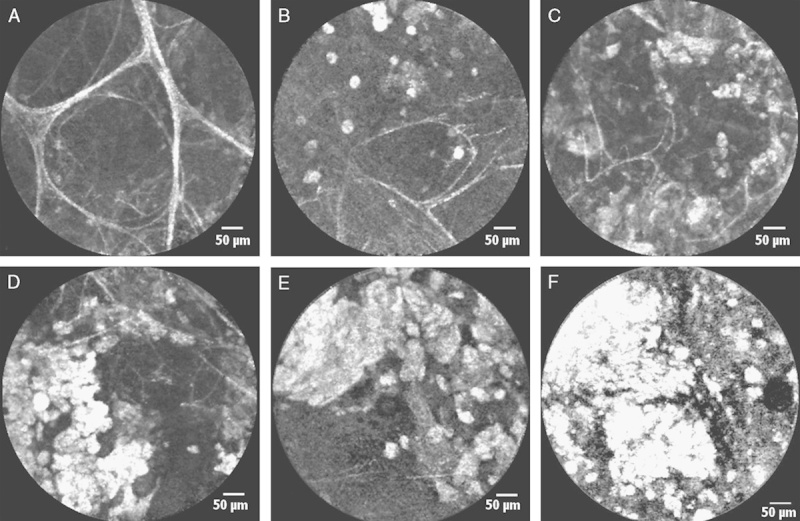
In all the patients, there was no significant clearance of crazy-paving areas on HRCT control performed on the second day after the whole-lung lavage (Figs. 2A, C).
FIGURE 2.
Comparison between HRCT and pCLE imaging in PAP patients before (A, B) and 2 days after (C, D) the whole-lung lavage. Significant reduction in the amount of floating alveolar complexes is shown at the pCLE images with a slight increase in the transparency of the right lung in the corresponding HRCT zones. HRCT indicates high-resolution computed tomography; PAP, pulmonary alveolar proteinosis; pCLE, probe-based confocal laser endoscopy.
The patient no. 1, with the right-sided lung lavage, was examined 1.5 years after the procedure. HRCT revealed only 2 foci of ground-glass areas: 1 in B4 on the right measured 34×38 mm, and 1 in B8 on the left, 12 mm in the diameter.
pCLE in PAP Patients Before Whole-Lung Lavage
Before the whole-lung lavage, we reviewed 121 alveolar areas explored in vivo with pCLE from 6 PAP patients. In 112 of the 121 areas, we found the features distinct for PAP. In 90 (74.4%) of them, we found fluorescent floating intra-alveolar complexes (3 to 5 points according to the score) against the background of the intact alveolar structures (Fig. 3A). Some of them were globular, and others appeared to be highly fluorescent alveolar macrophages, separate or glued together by moderately fluorescent fluid (1 to 3 points) (Fig. 3B). In 22 of the 121 (18.2%) alveolar areas, we only found a moderately fluorescent fluid (1 point) without any additional substances (Fig. 3C). Moreover, such a fluid, which is absent in normal pCLE in vivo imaging, was detected not only in the alveoli lumen but also in the bronchial tubes. In 9 of the 121 (7.4%) alveolar areas, we did not find any changes; the pCLE imaging revealed only macrophages (0 points) (Fig. 3D).
FIGURE 3.
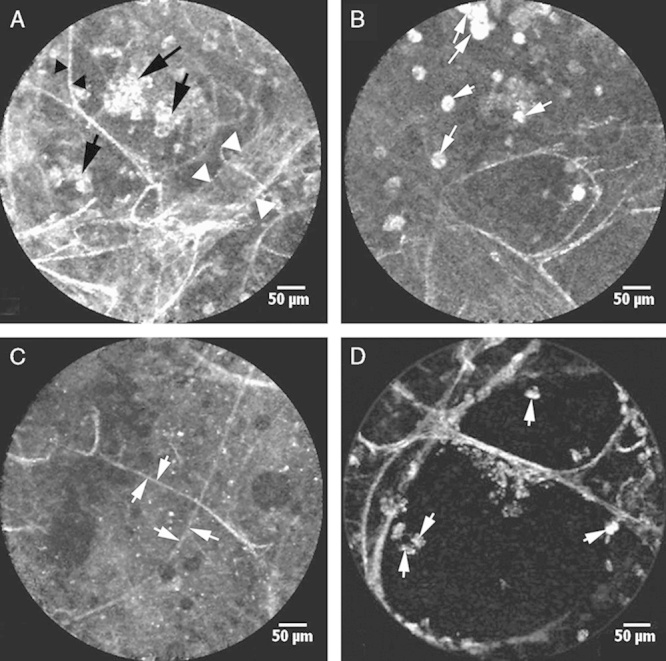
In vivo alveolar pCLE imaging at 488 nm in PAP patients revealed: (A) floating intra-alveolar complexes with high homogenous fluorescence (black arrows) against the background of the preserved alveolar elastin fibers (black arrowheads) and vessels (white arrowheads); (B) alveolar macrophages (arrows) separate or sticking together (some of them are giant); (C) moderately fluorescent fluid in the alveolar lumen (elastin fibers are shown with arrows); (D) normal alveoli with single alveolar macrophages (arrows). PAP indicates pulmonary alveolar proteinosis; pCLE, probe-based confocal laser endoscopy.
pCLE in PAP Patients After the Whole-Lung Lavage
After the whole-lung lavage, during pCLE, we found significant (to 0 to 2 points) reduction in the amount of intra-alveolar floating fluorescent complexes in 63 of the 90 (70.0%) alveolar areas, which were filled with these complexes before the treatment compared with the same regions (Figs. 2B, D).
In the patient no. 1, 1.5 years after the whole-lung lavage, pCLE revealed specific changes not only in the segments with pathologic HRCT findings (2 to 5 points) but also in those (0 to 4 points) where no features of PAP were detected by HRCT. The same findings were obtained for patients no. 3, 4, and 5.
pCLE in PAP Patients Under the Guidance of the Chest HRCT
To prove the hypothesis of the higher pCLE reliability as compared with HRCT, we performed pCLE under the HRCT control in patients no. 3 and 4 at 3 and 6 months after the whole-lung lavage, respectively.
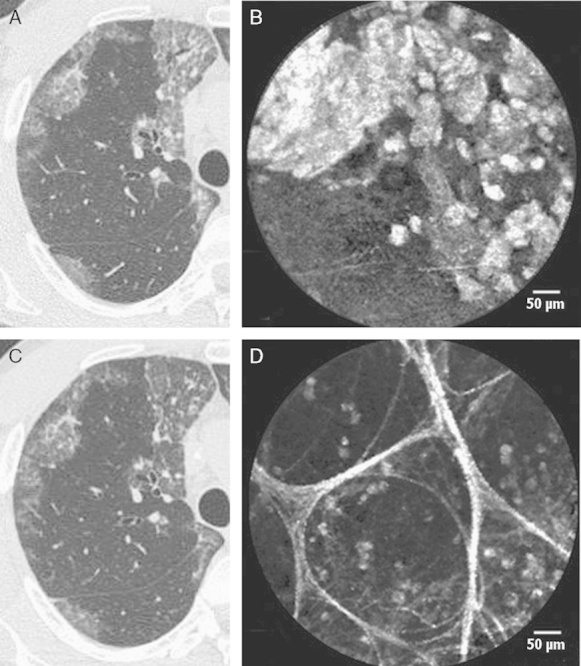
We compared the HRCT and endomicroscopic images. It was observed that in the lung area with no changes according to the HRCT data, there were fluorescent complexes at different degrees (0 to 3 points) filling the alveoli (Fig. 4). They were more pronounced in the areas with HRCT-defined PAP features (2 to 5 points) (Fig. 5).
FIGURE 4.
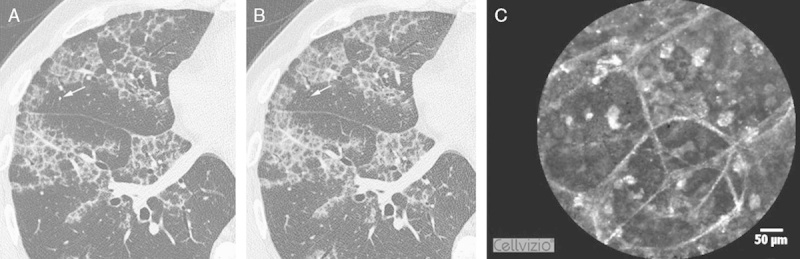
The correspondence between HRCT and pCLE imaging. The last scan (A) of the RS4, where the Alveoflex probe (arrow) is still seen and the next 1.0 mm step without the probe (arrow) (B), demonstrates the focus of the unaffected lung parenchyma. pCLE (C) displays fluorescent floating complexes in the preserved alveolar structures that are less pronounced than those in Figure 4. HRCT indicates high-resolution computed tomography; pCLE, probe-based confocal laser endoscopy.
FIGURE 5.
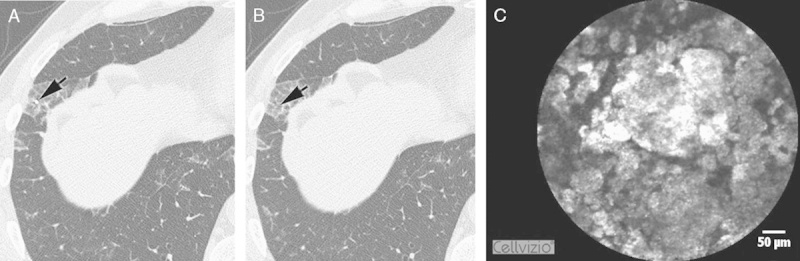
The correspondence between HRCT and pCLE imaging. The last scan (A) of the RS8, where the Alveoflex probe (arrow) is still seen and the next 1.0 mm step without the probe (arrow) (B), demonstrates the HRCT signs typical for PAP. pCLE (C) shows a large amount of fluorescent floating intra-alveolar complexes. HRCT indicates high-resolution computed tomography; PAP, pulmonary alveolar proteinosis; pCLE, probe-based confocal laser endoscopy.
Interobserver scoring agreement was 81.23.
DISCUSSION
PAP is a rare lung disease characterized by accumulation of a lipoproteinaceous surfactant material inside the alveolar airspaces, leading to clinical manifestations ranging from asymptomatic to severe respiratory failure. Without a high clinical suspicion, this diagnosis can easily be missed.17 Therefore, any new diagnostic methods that can give an additional information are useful and valuable.
In vivo pCLE of distal airways, being a new diagnostic method, still requires correlation with the data obtained by reference methods (eg, HRCT or histopathology) for certain diseases.
This study has demonstrated that in vivo pCLE of distal airways in PAP patients indicates specific highly fluorescent complexes (100 to 450 µm) to be the granulated lipoproteinaceous substance. Its appearance is caused by the disorder of the surfactant clearance and alveolar macrophages filling this material because of incomplete phagocytosis. We also detected highly fluorescent giant alveolar macrophages, which are the result of aggregation of several normal size cells for a better disposal of the lipoproteinaceous complexes. In the study by Salaün et al15 describing 1 patient with PAP examined in vivo using confocal endomicroscopy of distal airways, several pCLE diagnostic features of PAP have been reported. Investigators confirmed the correspondence of the fluorescent structures captured in vivo with pCLE with the lipoproteinaceous material by epifluorescence microscopic analysis of the bronchoalveolar lavage fluid. The specificity of the signs was confirmed by the absence of any similar findings in 24 smoking non-PAP subjects, including 9 patients with various interstitial lung diseases and 15 healthy volunteers.15
However, there have been 2 pathologic types in which we found the same giant highly fluorescent alveolar macrophages using in vivo pCLE, namely, invasive pulmonary aspergillosis18 and amiodarone-related pneumonia.14 Our findings correspond to the findings of Morisse et al19 who used fibered confocal fluorescence microscopy for in vivo and in situ imaging of experimental invasive pulmonary aspergillosis in immunosuppressed rats; they describe a specific fibrillar fluorescence and local infiltration of fluorescent alveolar macrophages in the infected zones.
Thus, it is conceivable that in vivo pCLE in PAP patients is a sensitive diagnostic method displaying highly fluorescent intra-alveolar complexes of different sizes along with giant alveolar macrophages.
In patient no. 1, 1.5 years after the whole-lung lavage of the only right lung, HRCT revealed only 2 small foci of ground-glass opacity areas. These results indicate that some mechanisms of the surfactant clearance improvement in the patient’s alveoli could be initiated after only 1-sided whole-lung lavage. However, this fact can also be explained by spontaneous improvement observed in 7.9% of PAP patients without treatment.2 pCLE showed that specific changes were present not only in the ground-glass opacity and crazy-paving pattern segments, but also in those where no HRCT features of PAP were detected.
Using a monitoring HRCT in patients no. 5 and 3 during pCLE, we could verify the miniprobe tip position. The same findings were obtained in the changed and unchanged zones. Different amounts of fluorescent complexes were found in both zones (with the presence and absence of HRCT findings). It means that PAP diffusely affects nearly all the lung segments, and pCLE visualizes minimal alveolar accumulations undetectable by HRCT. We have not found any previously published data describing such observations. In addition, this fact allowed us to conclude that in vivo pCLE in PAP patients might be a more accurate diagnostic method than HRCT.
According to the data of the cytologic analysis of the first and last lavaged fluid, we can conclude that whole-lung lavage was rather effective in the PAP patients. We suppose that HRCT images are formed by the fraction of the lipoproteinaceous material that is fixed to the alveoli walls and is not observed in the alveoli lumen. That is why we did not observe a substantial decrease in the expressiveness of crazy-paving symptoms on the second day after the whole-lung lavage.
Five of the patients were ex-smokers with a long-term smoking history and only 1 never smoked. The in vivo pCLE findings in the nonsmoking PAP patient in general did not differ from the findings for the smoking PAP patients described in our study and in the observations of Salaün et al.15 The only distinct feature was the absence or a very low amount of macrophages in alveoli lumen in those segments where no PAP signs were found by pCLE.
We did not compare our endomicroscopic findings at PAP to other diffuse parenchymal lung diseases, because there are published articles, proving that similar features had not been revealed at alveolar microlithiasis,20 hypersensitivity pneumonitis, cryptogenic organizing pneumonia, bleomycin-induced diffuse lung disease, autoimmune systemic disease, eosinophilic lung, and Pneumocystis jiroveci pneumonia.14
There are limitations to the current study. First, the small number of patients limits the conclusions that can be drawn.
Second, the examined alveolar area in each lung segment and subsegment was not full, and the miniprobe tip was uncontrolled, moving ahead to distal airways based on the path of the minimum resistance. Therefore, accurate statistical analyses on the degree of the amorphous lipoproteinaceous material deposition in the different alveoli were not possible.
Nevertheless, the observed changes within the alveoli of the patients with PAP suggest that pCLE may be helpful in understanding some correlations between the expressiveness of alveolar filling with amorphous masses and the clinical signs of the disease.
This study proves pCLE of distal airways to be a novel unique technique that may provide a valuable diagnostic information and is more accurate than HRCT in the diagnostics of PAP patients.
A future extended study is therefore planned to investigate the use of pCLE in diagnostics and monitoring of PAP. The current study points toward new opportunities in the PAP diagnosis using pCLE, which aids our step-by-step assembly of the jigsaw puzzle of the disease pathogenesis, development, and recovery. A future work is needed to evaluate whether in PAP patients the disease remains uncured even when there are no clinical signs and changes in HRCT.
CONCLUSIONS
In patients with PAP, in vivo confocal endomicroscopy reveals highly fluorescent complexes of granulated lipoproteinaceous material that is distributed from the distal bronchioles to the alveolar lumens. This material was present but less pronounced in the distal lung zones that were aerated on HRCT than in those that were not and was considerably reduced after whole-lung lavage. Apparently PAP diffusely affects lung parenchyma with varying severity of alveolar accumulation of amorphous complexes, and it may proceed not only subclinically but also to be radiologically indistinguishable. pCLE may prove useful for diagnosis of PAP and for evaluating the response to standard or experimental therapy for the disease.
ACKNOWLEDGMENTS
The authors would like to thank the Director General, Kuzovlev Oleg; the Physician-in-Chief, Laktionova Lyudmila; anesthesiologists, Gavrilova Olga and Maslova Natalya; the Head of the Surgical Service, Shablovsky Oleg; the Head ofthe Endoscopy Department, Sazonov Dmitry; the Head of the Pulmonary Department, Turusina Tamara; and a computer technician, Kranov Sergey for their help in the organization of the present study. Finally, the authors would like to express heartfelt thanks to the Head of Endoscopy Department of HELIOS, Klinik Hagen-Ambrock (Hagen, Germany) and Karl-Josef Franke for his invaluable help in the preparation of the manuscript.
Footnotes
Registration number on clinicaltrials.gov NCT02006940.
O.D. is the guarantor of the entire manuscript and contributed to the concept, design, specimen collection, slide preparation, image analysis, interpretation, data analysis, and drafting of the manuscript. A.A. contributed to the patient selection, patient management, concept, design, data analysis, and drafting of the manuscript. V.L. contributed to the patient selection, concept, design, specimen collection, and drafting of the manuscript. A.C. contributed to the specimen collection, image/slide preparation, and data analysis. A.S. contributed to the concept, image preparation, and drafting of the manuscript.
Disclosure: There is no conflict of interest or other disclosures.
REFERENCES
- 1.Borie R, Danel C, Debray M-P, et al. Pulmonary alveolar proteinosis. Eur Respir Rev. 2011;20:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seymour JF, Preseneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–235. [DOI] [PubMed] [Google Scholar]
- 3.Khan A, Agarwal R. Pulmonary alveolar proteinosis. Respir Care. 2011;56:1016–1028. [DOI] [PubMed] [Google Scholar]
- 4.Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of autoimmune pulmonary alveolar proteinosis patients in Japan. Am J Respir Crit Care Med. 2008;177:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhill SR, Kotton DN. Pulmonary alveolar proteinosis: a bench-to-bedside story of granulocyte-macrophage colony-stimulating factor dysfunction. Chest. 2009;136:571–577. [DOI] [PubMed] [Google Scholar]
- 6.Sarac S, Milić R, Zolotarevski L, et al. Primary pulmonary alveolar proteinosis. Vojnosanit Pregl. 2012;69:1005–1008. [PubMed] [Google Scholar]
- 7.Thiberville L, Bourg-Heckly G, Peltier E, et al. Per-endoscopic alveolar imaging using fluorescent confocal fibered microscopy. J Eur Respir. 2006;28suppl 50155s–156s. [Google Scholar]
- 8.Thiberville L, Moreno-Swirc S, Vercauteren T, et al. In vivo imaging of the bronchial wall microstructure using fibered confocal fluorescence microscopy. J Respir Crit Care Med. 2007;175:22–31. [DOI] [PubMed] [Google Scholar]
- 9.Thiberville L, Salaün M, Lachkar S, et al. Human in vivo fluorescence microimaging of the alveolar ducts and sacs during bronchoscopy. J Eur Respir. 2009;33:974–985. [DOI] [PubMed] [Google Scholar]
- 10.Pauly JL, Allison EM, Hurley EL, et al. Fluorescent human lung macrophages analyzed by spectral confocal laser scanning microscopy and multispectral cytometry. J Microsc Res Tech. 2005;67:79–89. [DOI] [PubMed] [Google Scholar]
- 11.Black PN, Ching PS, Beaumont B, et al. Changes in elastic fibers in the small airways and alveoli in COPD. J Eur Respir. 2008;31:998–1004. [DOI] [PubMed] [Google Scholar]
- 12.Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol. 2007;102:459–467. [DOI] [PubMed] [Google Scholar]
- 13.Newton RC, Kemp SV, Yang GZ, et al. Imaging parenchymal lung diseases with confocal endomicroscopy. Respir Med. 2012;106:127–137. [DOI] [PubMed] [Google Scholar]
- 14.Salaün M, Roussel F, Bourg-Heckly G, et al. In vivo probe-based confocal laser endomicroscopy in amiodarone-related pneumonia. Eur Respir J. 2013;42:1646–1658. [DOI] [PubMed] [Google Scholar]
- 15.Salaün M, Roussel F, Hauss P-A, et al. In vivo imaging of pulmonary alveolar proteinosis using confocal endomicroscopy. J Eur Respir. 2010;36:451–453. [DOI] [PubMed] [Google Scholar]
- 16.Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest. 2009;136:1678–1681. [DOI] [PubMed] [Google Scholar]
- 17.McDonnell MJ, Reynolds C, Tormey V, et al. Pulmonary alveolar proteinosis: report of two cases in the West of Ireland with review of current literature. Ir J Med Sci. 2014;183:123–127. [DOI] [PubMed] [Google Scholar]
- 18.Danilevskaya O, Averyanov A, Klimko N, et al. The case of diagnostics of invasive pulmonary aspergillosis by in vivo probe-based confocal laser endomicroscopy of central and distal airways. Med Mycol Case Rep. 2014;5:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morisse H, Heyman L, Salaün M, et al. In vivo and in situ imaging of experimental invasive pulmonary aspergillosis using fibered confocal fluorescence microscopy. Med Mycol. 2012;50:386–395. [DOI] [PubMed] [Google Scholar]
- 20.Yserbyt J, Alamé T, Dooms C, et al. Pulmonary alveolar microlithiasis and probe-based confocal laser endomicroscopy. J Bronchology Interv Pulmonol. 2013;20:159–163. [DOI] [PubMed] [Google Scholar]


