Highlights
-
•
Urban residence and history of TB contact/disease were associated with increased risk of latent TB infection at age five years.
-
•
BCG vaccine strain, LTBI, HIV and malaria infections, and anthropometry predict anti-mycobacterial immune responses.
-
•
Helminth infections do not influence response to BCG vaccination.
-
•
Cytokine responses at one year were not associated with LTBI at age five years.
Keywords: Tuberculosis, HIV, Helminth, Pregnancy, Bacille Calmette–Guerin, Crude culture filtrate protein
Abstract
Background
BCG is used widely as the sole licensed vaccine against tuberculosis, but it has variable efficacy and the reasons for this are still unclear. No reliable biomarkers to predict future protection against, or acquisition of, TB infection following immunisation have been identified. Lessons from BCG could be valuable in the development of effective tuberculosis vaccines.
Objectives
Within the Entebbe Mother and Baby Study birth cohort in Uganda, infants received BCG at birth. We investigated factors associated with latent tuberculosis infection (LTBI) and with cytokine response to mycobacterial antigen at age five years. We also investigated whether cytokine responses at one year were associated with LTBI at five years of age.
Methods
Blood samples from age one and five years were stimulated using crude culture filtrates of Mycobacterium tuberculosis in a six-day whole blood assay. IFN-γ, IL-5, IL-13 and IL-10 production was measured. LTBI at five years was determined using T-SPOT.TB® assay. Associations with LTBI at five years were assessed using multivariable logistic regression. Multiple linear regression with bootstrapping was used to determine factors associated with cytokine responses at age five years.
Results
LTBI prevalence was 9% at age five years. Only urban residence and history of TB contact/disease were positively associated with LTBI. BCG vaccine strain, LTBI, HIV infection, asymptomatic malaria, growth z-scores, childhood anthelminthic treatment and maternal BCG scar were associated with cytokine responses at age five. Cytokine responses at one year were not associated with acquisition of LTBI by five years of age.
Conclusion
Although multiple factors influenced anti-myocbacterial immune responses at age five, factors likely to be associated with exposure to infectious cases (history of household contact, and urban residence) dominated the risk of LTBI.
1. Introduction
Latent tuberculosis infection (LTBI) is an important reservoir from which new tuberculosis (TB) cases arise. In children, active TB disease results either from reactivation of LTBI or from primary infection acquired from contact with an infectious individual [1]. Lifetime risk of LTBI reactivation is approximately 10% [2]. In endemic settings, Bacille Calmette–Guerin (BCG) is routinely offered to protect infants from TB [3]. Recently, evidence has accrued that BCG may protect against infection with Mycobacterium tuberculosis, as well as against TB disease [4,5]. However, the protective efficacy of BCG varies widely [6–10]. Despite advances in immunology, there are no sufficiently validated immune correlates of BCG-induced protection [11] and mechanisms of protection are poorly understood. Accurate information is lacking on risk factors and immunological markers associated with subsequent TB infection or disease among children from developing countries.
Proposed explanations for variation in BCG efficacy include population genetics [10,12], BCG vaccine strains [13–15], environmental mycobacteria exposure [8,14,16], strains of Mycobacterium tuberculosis (M.tb) [17], exposure to chronic helminth infections [9,12,18] and nutritional status [10,12].
BCG induces strong interferon gamma (IFN-γ) production, a type 1 immune response essential for protection against M.tb [19,20]. IFN-γ alone may not be sufficient for disease prevention [20], but excessive production of type 2 cytokines may be detrimental [21]. Interleukin-10 (IL-10) is associated with suppression of protective responses [22]. Although BCG induces only minor type 2 responses, these may be sufficient in some individuals to undermine the efficacy of type 1-mediated immunity and cause immune-pathology [23]. Differences predetermined in utero, or during the first months of life, may explain the variable protection induced by BCG [8].
In this cohort of children who received BCG at birth, we investigated factors associated with LTBI at age five, factors associated with cytokine responses to BCG at age five and whether cytokine responses at one year were associated with subsequent LTBI. Understanding these relationships may aid the development of new vaccines against tuberculosis, several of which are either recombinant forms of BCG or designed to boost responses primed by BCG [24].
2. Methods
2.1. Study design and setting
We analysed data from the Entebbe Mother and Baby Study (EMaBS), a randomised double-blinded placebo-controlled trial of anthelminthic treatment in pregnancy and early childhood, conducted in a peri-urban and rural setting by Lake Victoria, Uganda (ISRCTN32849447). EMaBS was established to investigate effects of helminths and their treatment on immune responses to vaccines and on susceptibility to infectious and allergy-related diseases. The design and results of the trial have been reported [25–27]. Briefly, women attending antenatal care at Entebbe hospital were enrolled between April 2003 and November 2005, and randomised to receive single dose albendazole (400 mg) or placebo and praziquantel (40 mg/kg) or placebo in a 2 × 2 factorial design. At age fifteen months, their children were randomised to receive albendazole or placebo quarterly until age five years. In 2008, additional funding was awarded which allowed us to assess children for LTBI at age five.
2.2. Study objectives
For this analysis, our primary objectives were to investigate (1) factors associated with LTBI at age five years, (2) factors associated with cytokine response to BCG at age five years, and (3) whether cytokine responses at one year of age were associated with acquisition of LTBI by age five years, among children with documented BCG immunisation in infancy.
2.3. Study procedures
Socio-demographic data, blood and stool samples were obtained at enrolment during pregnancy. Children received routine BCG immunisation (with polio immunisation) at birth. The three BCG vaccine strains used were provided by the National Medical Stores according to availability: BCG-Russia (BCG-I Moscow strain, Serum Institute of India, India); BCG-Bulgaria (BCG-SL 222 Sofia strain, BB-NCIPD Ltd., Bulgaria); and BCG-Danish (BCG-SSI 1331, Statens Seruminstitut, Denmark) [13]. Participants were seen at the clinic for annual visits (at which they gave blood and stool samples and were weighed and measured) and when ill.
Maternal history of TB exposure and disease was ascertained during pregnancy. History of TB exposure and disease in the child was ascertained at annual visits and through illness visits. At one and five years of age, cytokine responses to Mycobacterium tuberculosis crude culture filtrate protein (M.tb-cCFP) were assessed. LTBI at age five was assessed using the Interferon gamma release assay (IGRA), T-SPOT.TB® (Oxford Immunotec, Abingdon, UK), for all children in the cohort who turned age five from March 2009 onwards (when the TB sub-study began). Z-scores for weight-for-age, height-for-age and weight-for-height at age five were calculated from World Health Organisation (WHO) growth standards, using WHO Anthro and AnthroPlus macros.
The trial was approved by the Science and Ethics Committee of the Uganda Virus Research Institute, Uganda National Council for Science and Technology, and London School of Hygiene and Tropical Medicine. During pregnancy, women gave written, informed consent for their and their child's participation. The mother, father or guardian gave written informed consent for additional procedures in this study.
2.4. Immunological assays
Cytokine responses were assessed among children who had documented BCG immunisation in infancy and who provided a blood sample at five years of age, using a whole blood assay [12,18]. Briefly, unseparated heparinised blood was diluted to a final concentration of one-in-four (RPMI supplemented with penicillin, streptomycin and glutamine), plated in 96-well plates and stimulated with M.tb-cCFP (10 μg/ml; kindly provided by John Belisle, University of Colorado, Fort Collins, USA), tetanus toxoid (TT) (12 Lf/ml; Statens Seruminstitut, Denmark), phytohaemagglutinin (10 μg/ml; Sigma, UK), or left unstimulated. Supernatants were harvested on day six and frozen at −80 °C until analysed. Supernatant cytokine concentrations were measured by Enzyme Linked Immunosorbent Assay (ELISA) (Becton Dickinson, UK). Cytokine production in unstimulated wells was subtracted from concentrations produced in response to stimulation. Cytokine responses were regarded as positive if greater than the higher of the mean plus two standard deviations of the negative control for all assays and the lowest standard in the assay (IFN-γ > 73 pg/ml; IL-5 > 34 pg/ml; IL-13 > 18 pg/ml; IL-10 > 48 pg/ml at one year [18] and IFN-γ >9 pg/ml; IL-5 > 8 pg/ml; IL-13 > 16 pg/ml; IL-10 > 8 pg/ml at five years). Values below the cut-off were set to zero.
Age of infection with cytomegalovirus (CMV) and herpes simplex virus (HSV) were determined by examining for Immunoglobulin G responses by ELISA (DiaSorin, Saluggia, Italy).
To avoid confounding of secular trends with assay performance variability, assays were performed in a randomised sequence after completion of sample collection.
2.5. Parasitology and haematology
Stool was examined for helminth ova and Strongyloides larvae using Kato-Katz [28] and charcoal culture [27] methods, respectively. Blood was examined for Mansonella perstans using modified Knott's method [29] and for malaria by thick blood film and Leishman's stain. HIV status was determined in mothers and children ≥18 months by rapid antibody test algorithm, and in younger children by polymerase chain reaction [27].
2.6. Statistical methods
Data were double-entered into Microsoft Access (Redmond, WA, USA) and analysed using Stata v11 (College Station, TX, USA). The sample size was determined for the trial objectives; from the planned enrolment of 2500 women we expected to retain 1046 children in follow-up at age five [25]. Assuming standard deviation of 0.8log10 this sample size would give 80% power to detect a difference in mean cytokine response of 0.14log10 for an exposure with prevalence 50%, at 5% significance level.
Outcomes for this analysis were LTBI at age five years determined by T-SPOT.TB® result, and cytokine responses (IFN-γ, IL-5, IL-13 and IL-10) to M.tb-cCFP at age five. Variables considered as exposures were maternal and childhood anthelminthic treatment, maternal socio-demographic characteristics and helminth infections at enrolment; child sex, birth weight, HIV status, illness history, TB exposure/disease, childhood helminth infections, and anthropometry at age five; BCG vaccine strain used for immunisation. In addition, T-SPOT.TB® status was considered as an exposure for age five cytokine responses.
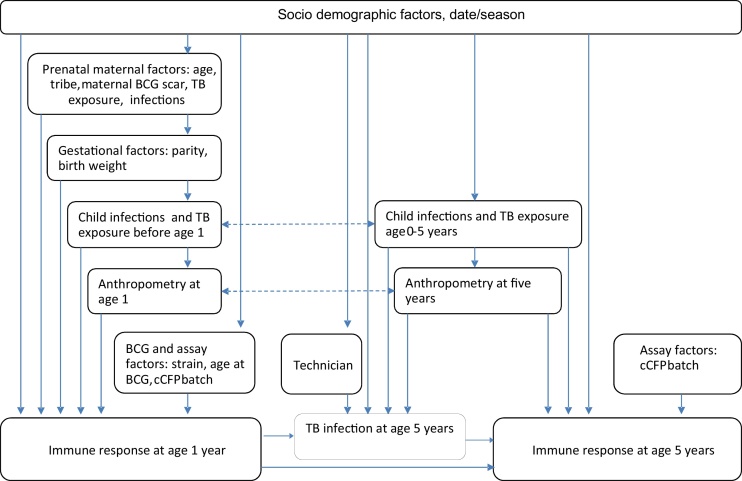
Analyses followed a hierarchical causal diagram approach (Fig. 1) [30]: factors at the same level were considered as potential confounders for each other and for proximal factors. Crude associations were examined and a 15% significance level used to decide which factors to consider in multivariable analyses. Anthropometry variables were not included together in multivariable models to avoid collinearity. Unadjusted effects of trial interventions are reported.
Fig. 1.
Conceptual framework.
Logistic regression was used to examine associations with LTBI at age five. Cytokine responses were transformed to log10 (concentration + 1) and then analysed using linear regression with bootstrapping to estimate bias corrected accelerated confidence intervals [31]; results were back-transformed to give geometric mean ratios.
Spearman's correlation coefficients between cytokines responses at one and five years were calculated. Logistic regression was used to investigate whether cytokine responses at one year were associated with LTBI at five years, restricting to children with history of TB exposure or disease (which implies exposure) between one and five years.
3. Results
We enrolled 2507 women resulting in 2345 live births, with 1474 children seen at age five years. Full details on participant enrolment and follow-up are described elsewhere [13,18,26,27]. Of the 1474 children seen at age five, 1191 had documented BCG immunisation in infancy, and cytokine responses to mycobacterial antigen at age five were assessed among this group. For these children, mean maternal age at enrolment was 24 years, 805 (68%) were born to women who were urban residents at enrolment. Ten percent of women reported having been exposed to TB. Hookworm was the most prevalent helminth at 42%; 10% of mothers had asymptomatic malaria. Of the children, 606 (51%) were female; 22 (1.9%) were HIV-infected; 638 (54%) had received BCG-Russia, 445 (37%) BCG-Bulgaria and 107 (9%) BCG-Danish. At age five, mean (SD) weight-for-age, height-for-age and weight-for-height z-scores were −0.9 (0.9), −1.3 (1.2) and −0.1 (1.2), respectively; 63 (5.5%) had asymptomatic malaria, 639 (80.5%) and 691 (98.0%) children had been infected with HSV or CMV, respectively, and 142 (5.9%) had a history of tuberculosis exposure and, or, disease (12 had probable TB disease, as reported by a parent).
At age five, T-SPOT.TB® results were available from 886 children who reached age five years after the TB sub-study began, with 75 infected with tuberculosis (prevalence 8.5%, 95% CI: 6.7–10.5). Of the 1191 children with cytokine results, 1024 (86.0%), 575 (48.3%), 1067 (89.7%) and 849 (71.3%) had detectable IFN-γ, IL-5, IL-10 and IL-13 responses to M.tb-cCFP.
3.1. Factors associated with LTBI at age five years
Table 1 shows associations between each exposure and LTBI. Rural residence was associated with reduced odds of LTBI, while history of TB contact or disease was associated with increased odds of LTBI. The odds of LTBI increased with the closeness of the TB contact: compared to contact outside the household, ORs (95% CI) of LTBI for children sharing a living room, a bedroom, and a bed with the contact, were 0.54 (0.13–2.36), 1.79 (0.27–11.86) and 8.33 (1.33–52.03), respectively (trend p-value =0.02). There was no evidence for association with LTBI for any other exposure.
Table 1.
Factors associated with T-SPOT. TB among five year old children vaccinated with BCG in infancy.
| Factora |
TB infection |
Crude OR (95% CI) |
P |
Adjusted OR (95% CI)b,c |
P |
|
| Positive (%) |
Negative (%) |
|||||
| Socio-demographic characteristics | ||||||
| Maternal age at enrolment | 1.02 (0.98–1.06) | 0.27 | ||||
| Parity | 1.11 (0.99–1.26) | 0.08 | 1.10 (0.95–1.27) | 0.21 | ||
| Maternal education | ||||||
| None/primary | 34 (7.4) | 425 (92.6) | 1 | |||
| Secondary/tertiary | 41 (9.7) | 384 (90.4) | 1.33 (0.83–2.15) | 0.23 | ||
| Household SES | 0.92 (0.76–1.12) | 0.41 | ||||
| Household crowding | 1.14 (0.96–1.36) | 0.14 | 1.03 (0.83–1.27) | 0.80 | ||
| Location of residence | ||||||
| Urban | 59 (10.5) | 505 (89.5) | 1 | 1 | ||
| Rural | 16 (5.1) | 300 (94.9) | 0.46 (0.26–0.81) | 0.01 | 0.39 (0.19–0.78) | 0.01 |
| Maternal characteristics | ||||||
| Schistosoma mansoni | ||||||
| No | 58 (8.3) | 645 (91.8) | 1 | |||
| Yes | 17 (9.6) | 161 (90.5) | 1.17 (0.67–2.07) | 0.58 | ||
| Hookworm | ||||||
| No | 45 (8.6) | 478 (91.4) | 1 | |||
| Yes | 30 (8.4) | 328 (91.6) | 0.97 (0.60–1.57) | 0.91 | ||
| Mansonella perstans | ||||||
| No | 59 (8.4) | 644 (91.6) | 1 | |||
| Yes | 16 (8.7) | 167 (91.3) | 1.05 (0.59–1.86) | 0.88 | ||
| Malaria | ||||||
| No | 70 (8.9) | 717 (91.1) | 1 | |||
| Yes | 4 (4.8) | 79 (95.2) | 0.52 (0.18–1.46) | 0.21 | ||
| Maternal TB exposure | ||||||
| No | 65 (8.2) | 723 (91.8) | 1 | |||
| Yes | 9 (9.8) | 83 (90.2) | 1.21 (0.58–2.51) | 0.62 | ||
| Maternal BCG scar | ||||||
| No | 27 (7.5) | 332 (92.5) | 1 | |||
| Yes | 46 (9.3) | 449 (90.7) | 1.26 (0.77–2.07) | 0.36 | ||
| Child characteristics | ||||||
| Sex | ||||||
| Male | 30 (7.9) | 350 (92.1) | 1 | |||
| Female | 36 (9.9) | 328 (90.1) | 1.28 (0.77–2.13) | 0.34 | ||
| Birth weight | 1.03 (0.59–1.82) | 0.91 | ||||
| HIV status | ||||||
| Unexposed | 64 (8.0) | 737 (92.0) | 1 | |||
| Exposed-uninfected | 9 (12.9) | 61 (87.1) | 1.70 (0.81–3.58) | |||
| Infected | 2 (15.4) | 11 (84.6) | 2.09 (0.45–9.65) | 0.30 | ||
| BCG scar | ||||||
| No | 29 (8.6) | 307 (91.4) | 1 | |||
| Yes | 41 (9.5) | 391 (90.5) | 1.11 (0.67–1.83) | 0.68 | ||
| BCG vaccine strain | ||||||
| Russia | 53 (10.1) | 471 (89.9) | 1 | 1 | ||
| Bulgaria | 13 (5.2) | 237 (94.8) | 0.49 (0.26–0.91) | 0.73 (0.35–1.51) | ||
| Danish | 6 (6.7) | 84 (93.3) | 0.63 (0.26–1.52) | 0.05 | 2.25 (0.82–6.22) | 0.17 |
| History of TB contact/disease | ||||||
| No | 60 (7.5) | 740 (92.5) | 1 | 1 | ||
| Yes | 15 (17.4) | 71 (82.6) | 2.61 (1.41–4.82) | <0.01 | 2.16 (1.02–4.55) | 0.04 |
| Any helminth infection ≤5 years | ||||||
| No | 63 (8.8) | 652 (91.2) | 1 | |||
| Yes | 12 (7.0) | 159 (93.0) | 0.78 (0.41–1.48) | 0.45 | ||
| WHZ score, age 5 | 0.95 (0.77–1.18) | 0.67 | ||||
| HAZ score, age 5 | 1.19 (0.96–1.49) | 0.11 | 1.10 (0.86–1.40) | 0.44 | ||
| WAZ score, age 5 | 1.15 (0.86–1.53) | 0.36 | ||||
| Asymptomatic malaria, age 5 | ||||||
| No | 73 (8.9) | 752 (91.1) | 1 | |||
| Yes | 1 (2.9) | 34 (97.1) | 0.30 (0.04–2.25) | 0.24 | ||
| Number of malaria events | 0.96 (0.86–1.07) | 0.46 | ||||
| Number of diarrhoea events | 1.03 (0.96–1.12) | 0.40 | ||||
| Number of LRTI events | 1.12 (0.92–1.36) | 0.26 | ||||
| Age of infection with CMV | 0.73 (0.43–1.23) | 0.23 | ||||
| Age of infection with HSV | 1.19 (0.91–1.55) | 0.22 | ||||
| Trial interventions | ||||||
| Maternal albendazole treatment | ||||||
| No | 39 (8.8) | 406 (91.2) | 1 | |||
| Yes | 36 (8.2) | 405 (91.8) | 0.93 (0.57–1.49) | 0.75 | ||
| Maternal praziquantel treatment | ||||||
| No | 35 (7.6) | 426 (92.4) | 1 | |||
| Yes | 40 (9.4) | 385 (90.6) | 1.26 (0.79–2.03) | 0.33 | ||
| Childhood albendazole treatment | ||||||
| No | 37 (8.2) | 414 (91.8) | 1 | |||
| Yes | 38 (8.8) | 395 (91.2) | 1.08 (0.67–1.73) | 0.76 | ||
Missing values: maternal education 2; household socioeconomic status 16; crowding 2; location of residence 6; Schistosoma mansoni 5; hookworm 5; maternal malaria 16; maternal TB contact 6; maternal BCG scar 36; birth weight 146; HIV status 2; child's BCG scar 118; BCG strain 2; WHZ score 104; HAZ score 111; WAZ score 101; asymptomatic malaria at five years 6; illness episodes 2.
Adjusted for each other and for technician.
Adjusted ORs for which 95% CIs exclude 1 are highlighted in bold.
3.2. Factors associated with cytokine response at age five years
There was moderate positive correlation between cytokine responses at age one and five years for IFN-γ, IL-5 and IL-13 (coefficients 0.20, 0.20 and 0.26, respectively; p < 0.001), but no correlation between IL-10 responses (coefficient 0.00, p = 0.95).
Crude associations between exposures and cytokine response at age five are shown in Table 2 and exposures remaining associated after multivariable analysis in Table 3. HIV-infected children showed lower IFN-γ and IL-13 responses, compared to HIV-unexposed infants. Asymptomatic malaria at age five was associated with reduced IFN-γ production. There were no consistent associations with maternal helminths or with maternal anthelminthic treatment. In multivariable analyses, childhood helminth infection at any annual visit was not associated with cytokine responses, however, quarterly albendazole treatment during childhood was associated with reduced IFN-γ and IL-13 responses to M.tb-cCFP. Maternal BCG scar was associated with higher IL-10 response to M.tb-cCFP. Greater height-for age was associated with increased responses for all cytokines.
Table 2.
Crude associations with cytokine response to crude culture filtrate proteins of Mycobacterium tuberculosis in five year olds who received BCG immunisation at birth.
| Factor1 | IFN-γ |
IL-5 |
IL-13 |
IL-10 |
||||
|---|---|---|---|---|---|---|---|---|
| Geometric mean2 | Crude GMR (95%CI)3 | Geometric mean2 | Crude GMR (95%CI)3 | Geometric mean2 | Crude GMR (95%CI)3 | Geometric mean2 | Crude GMR (95%CI)3 | |
| Socio-demographic characteristics | ||||||||
| Maternal age at enrolment | 1.00 (0.98–1.03) | 1.02 (1.00–1.04) | 1.01 (0.99–1.04) | 1.01 (0.99–1.02) | ||||
| Parity | 0.98 (0.90–1.05) | 1.03 (0.97–1.09) | 1.00 (0.93–1.07) | 0.98 (0.93–1.03) | ||||
| Maternal education | ||||||||
| None/primary | 169.0 | 1 | 5.7 | 1 | 25.7 | 1 | 50.7 | 1 |
| Secondary/tertiary | 163.3 | 0.97 (0.74–1.29) | 5.7 | 0.99 (0.79–1.24) | 25.5 | 0.99 (0.76–1.28) | 50.6 | 1.00 (0.84–1.21) |
| Household SES | 0.99 (0.90–1.12) | 0.94 (0.86–1.03) | 0.97 (0.87–1.07) | 0.97 (0.90–1.05) | ||||
| Household crowding | 1.05 (0.94–1.16) | 1.03 (0.94–1.12) | 1.06 (0.95–1.17) | 1.03 (0.95–1.10) | ||||
| Location of residence | ||||||||
| Urban | 197.7 | 1 | 6.3 | 1 | 29.2 | 1 | 51.6 | 1 |
| Rural | 114.3 | 0.58 (0.42–0.76) | 4.7 | 0.75 (0.60–0.95) | 19.4 | 0.66 (0.50–0.87) | 48.0 | 0.93 (0.75–1.11) |
| Maternal characteristics | ||||||||
| Schistosoma mansoni | ||||||||
| No | 171.2 | 1 | 5.7 | 1 | 25.5 | 1 | 51.0 | 1 |
| Yes | 146.4 | 0.86 (0.58–1.20) | 5.9 | 1.03 (0.97–1.09) | 26.9 | 1.06 (0.77–1.44) | 48.7 | 0.95 (0.73–1.18) |
| Hookworm | ||||||||
| No | 159.2 | 1 | 5.9 | 1 | 25.8 | 1 | 48.9 | 1 |
| Yes | 176.7 | 1.11 (0.84–1.46) | 5.6 | 0.96 (0.77–1.21) | 25.6 | 0.99 (0.78–1.29) | 53.1 | 1.09 (0.89–1.30) |
| Mansonella perstans | ||||||||
| No | 174.4 | 1 | 6.2 | 1 | 26.8 | 1 | 52.3 | 1 |
| Yes | 143.1 | 0.82 (0.57–1.12) | 4.6 | 0.74 (0.58–0.95) | 22.4 | 0.83 (0.62–1.16) | 43.9 | 0.84 (0.66–1.04) |
| Malaria | ||||||||
| No | 171.5 | 1 | 5.8 | 1 | 26.1 | 1 | 50.7 | 1 |
| Yes | 119.1 | 0.69 (0.42–1.06) | 5.7 | 0.98 (0.67–1.41) | 23.4 | 0.90 (0.56–1.39) | 49.9 | 0.98 (0.72–1.29) |
| Maternal TB exposure | ||||||||
| No | 162.3 | 1 | 5.6 | 1 | 25.2 | 1 | 51.3 | 1 |
| Yes | 200.2 | 1.23 (0.77–1.86) | 7.3 | 1.30 (0.89–1.89) | 29.7 | 1.18 (0.77–1.78) | 44.7 | 0.87 (0.64–1.18) |
| Maternal BCG scar | ||||||||
| No | 153.8 | 1 | 6.0 | 1 | 24.0 | 1 | 42.8 | 1 |
| Yes | 177.8 | 1.16 (0.88–1.53) | 5.7 | 0.95 (0.75–1.21) | 27.6 | 1.15 (0.86–1.49) | 55.9 | 1.31 (1.06–1.61) |
| Child characteristics | ||||||||
| Sex | ||||||||
| Male | 151.4 | 1 | 5.6 | 1 | 26.0 | 1 | 52.1 | 1 |
| Female | 184.9 | 1.22 (0.92–1.61) | 5.9 | 1.07 (0.86–1.32) | 25.6 | 0.98 (0.75–1.27) | 49.0 | 0.94 (0.78–1.14) |
| Birth weight | 1.25 (0.92–1.63) | 1.29 (1.00–1.63) | 1.15 (0.85–1.56) | 0.95 (0.77–1.18) | ||||
| HIV status | ||||||||
| Unexposed | 170.6 | 1 | 5.8 | 1 | 25.8 | 1 | 50.3 | 1 |
| Exposed, uninfected | 250.9 | 1.47 (0.92–2.16) | 6.9 | 1.19 (0.84–1.86) | 41.1 | 1.59 (1.09–2.27) | 49.5 | 0.98 (0.67–1.31) |
| Infected | 10.9 | 0.06 (0.03–0.22) | 2.0 | 0.34 (0.22–0.76) | 2.3 | 0.09 (0.05–0.26) | 65.5 | 1.30 (0.65-2.25) |
| BCG scar | ||||||||
| No | 170.6 | 1 | 5.3 | 1 | 23.2 | 1 | 59.3 | 1 |
| Yes | 183.3 | 1.07 (0.79–1.44) | 6.5 | 1.21 (0.96–1.55) | 30.0 | 1.29 (1.01–1.72) | 46.9 | 0.79 (0.65–0.97) |
| BCG vaccine strain | ||||||||
| Russia | 266.9 | 1 | 6.6 | 1 | 34.9 | 1 | 52.0 | 1 |
| Bulgaria | 122.6 | 0.46 (0.35–0.60) | 5.5 | 0.84 (0.66–1.07) | 22.6 | 0.65 (0.51–0.85) | 63.2 | 1.22 (1.01–1.46) |
| Danish | 36.2 | 0.14 (0.08–0.23) | 3.3 | 0.50 (0.36–0.76) | 7.3 | 0.21 (0.13–0.34) | 16.8 | 0.32 (0.32–0.48) |
| History of TB contact/disease | ||||||||
| No | 168.7 | 1 | 5.7 | 1 | 25.6 | 1 | 51.3 | 1 |
| Yes | 147.9 | 0.88 (0.48–1.43) | 6.2 | 1.08 (0.71–1.60) | 28.5 | 1.12 (0.70–1.76) | 43.0 | 0.84 (0.58–1.17) |
| Any helminth infection ≤5 years | ||||||||
| No | 179.3 | 1 | 5.7 | 1 | 26.0 | 1 | 52.3 | 1 |
| Yes | 122.6 | 0.68 (0.45–0.96) | 6.0 | 1.06 (0.82–1.43) | 25.1 | 0.96 (0.69–1.36) | 43.7 | 0.84 (0.64–1.09) |
| WHZ score, age 5 | 0.84 (0.75–0.95) | 0.97 (0.87–1.07) | 0.88 (0.78–0.98) | 0.86 (0.80–0.93) | ||||
| HAZ score, age 5 | 1.37 (1.20–1.54) | 1.21 (1.10–1.34) | 1.28 (1.14–1.43) | 1.24 (1.14–1.34) | ||||
| WAZ score, age 5 | 1.17 (1.00–1.39) | 1.20 (1.05–1.36) | 1.14 (0.97–1.33) | 1.08 (0.96–1.20) | ||||
| Asymptomatic malaria, age 5 | ||||||||
| No | 174.3 | 1 | 6.0 | 1 | 26.1 | 1 | 49.3 | 1 |
| Yes | 82.2 | 0.47 (0.23–0.82) | 3.8 | 0.63 (0.41–1.03) | 21.1 | 0.81 (0.45–1.37) | 66.9 | 1.36 (0.84–1.94) |
| Number of malaria events up to age 5 | 0.95 (0.89–1.01) | 1.01 (0.96–1.06) | 1.00 (0.95–1.05) | 1.03 (1.00–1.07) | ||||
| Number of diarrhea events up to age 5 | 1.00 (0.95–1.04) | 1.03 (0.99–1.07) | 1.02 (0.98–1.07) | 1.02 (0.99–1.06) | ||||
| Number of LRTI events up to age 5 | 1.02 (0.88–1.15) | 1.03 (0.92–1.15) | 1.01 (0.88–1.15) | 1.02 (0.92–1.11) | ||||
| Age of infection with CMV | 1.17 (0.95–1.37) | 1.17 (1.00–1.38) | 1.17 (0.97–1.37) | 1.09 (0.96–1.20) | ||||
| Age of infection with HSV | 0.99 (0.84–1.13) | 0.94 (0.84–1.05) | 1.00 (0.87–1.14) | 1.00 (0.90–1.10) | ||||
| T-spot TB | ||||||||
| Negative | 140.0 | 1 | 4.8 | 1 | 18.8 | 1 | 40.2 | 1 |
| Positive | 380.3 | 2.72 (1.51–4.35) | 9.4 | 1.97 (1.14–3.41) | 48.7 | 2.59 (1.46–4.14) | 43.7 | 1.09 (0.70–1.62) |
| Trial interventions | ||||||||
| Maternal albendazole treatment | ||||||||
| No | 153.8 | 1 | 5.7 | 1 | 25.8 | 1 | 50.2 | 1 |
| Yes | 180.6 | 1.17 (0.89–1.54) | 5.8 | 1.03 (0.82–1.28) | 25.8 | 1.00 (0.79–1.32) | 50.9 | 1.01 (0.83–1.19) |
| Maternal praziquantel treatment | ||||||||
| No | 171.3 | 1 | 5.7 | 1 | 26.2 | 1 | 49.3 | 1 |
| Yes | 162.2 | 0.95 (0.72–1.23) | 5.8 | 1.01 (0.79–1.22) | 25.4 | 0.97 (0.75–1.26) | 51.9 | 1.05 (0.87–1.25) |
| Childhood albendazole treatment | ||||||||
| No | 194.8 | 1 | 6.2 | 1 | 30.5 | 1 | 50.6 | 1 |
| Yes | 141.3 | 0.73 (0.55–0.98) | 5.3 | 0.86 (0.68–1.05) | 21.6 | 0.71 (0.56–0.93) | 50.5 | 1.00 (0.83–1.20) |
1 Missing values: maternal education 3; household socioeconomic status 22; crowding 4; location of residence 9; Schistosoma mansoni 2; hookworm 2; Mansonella perstans 2; maternal malaria 19; maternal TB contact 6; maternal BCG scar 10; birth weight 180; HIV status 3; child's BCG scar 157; WHZ score 158; HAZ score 163; WAZ score 155; asymptomatic malaria at five years 36; illness episodes 3.
2 Geometric mean of response concentration + 1.
3 GMR = geometric mean ratio, confidence intervals not including one are highlighted in bold.
Table 3.
Factors associated with the cytokine response to crude culture filtrate proteins of Mycobacterium tuberculosis in five year olds who received BCG immunisation at birth, after adjustment for potential confounders.
| Cytokine/Factor | Adjusted GMR (95% CI)a |
|---|---|
| Interferon-γ | |
| Child's HIV status | |
| Unexposed | 1 |
| Exposed, uninfected | 1.43 (0.88–2.17) |
| Infected | 0.16 (0.05–0.51) |
| BCG vaccine strain | |
| Russia | 1 |
| Bulgaria | 0.46 (0.33–0.63) |
| Danish | 0.19 (0.10–0.36) |
| Height-for-age z-score, age 5 years | 1.21 (1.04–1.41) |
| Asymptomatic malaria, age 5 years | |
| No | 1 |
| Yes | 0.50 (0.22–0.96) |
| T-spot TB | |
| Negative | 1 |
| Positive | 2.05 (1.08–3.41) |
| Childhood albendazole treatment | |
| No | 1 |
| Yes | 0.73 (0.55–0.98) |
| Interleukin-5 | |
| BCG vaccine strain | |
| Russia | 1 |
| Bulgaria | 0.89 (0.68–1.15) |
| Danish | 0.59 (0.40–0.91) |
| Height-for-age z-score, age 5 years | 1.15 (1.03–1.29) |
| Interleukin-13 | |
| Child's HIV status | |
| Unexposed | 1 |
| Exposed, uninfected | 1.58 (1.03–2.32) |
| Infected | 0.13 (0.06–0.55) |
| BCG vaccine strain | |
| Russia | 1 |
| Bulgaria | 0.66 (0.50–0.89) |
| Danish | 0.24 (0.15–0.41) |
| Height-for-age z-score, age 5 years | 1.14 (1.01–1.27) |
| T-spot TB | |
| Negative | 1 |
| Positive | 2.10 (1.17–3.63) |
| Childhood albendazole treatment | |
| No | 1 |
| Yes | 0.71 (0.56–0.93) |
| Interleukin-10 | |
| Maternal BCG scar | |
| No | 1 |
| Yes | 1.24 (1.01–1.56) |
| BCG vaccine strain | |
| Russia | 1 |
| Bulgaria | 1.27 (1.01–1.58) |
| Danish | 0.45 (0.28–0.73) |
| Height-for-age z-score, age 5 years | 1.22 (1.11–1.32) |
Figures are geometric mean ratios of cytokine production, with bootstrap 95% confidence intervals.
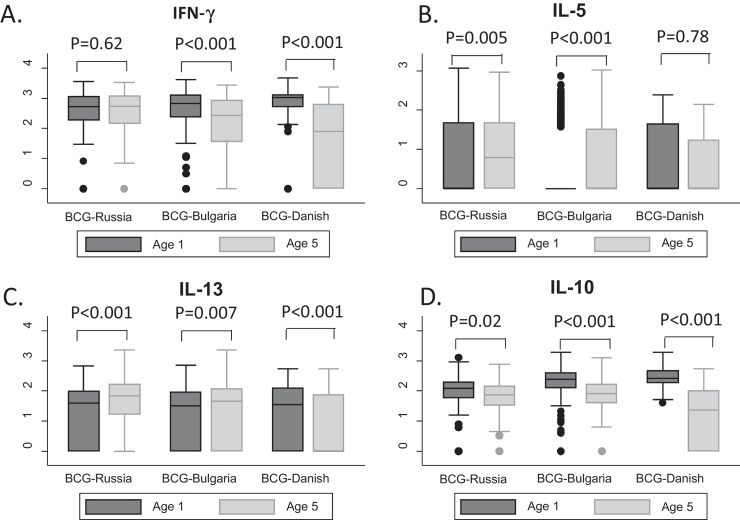
Vaccination with BCG-Danish was associated with lower responses to M.tb-cCFP for all cytokines and BCG-Bulgaria with lower IFN-γ and IL-13 responses, compared to BCG-Russia. IFN-γ responses had waned between age one and five years for children who received BCG-Bulgaria or BCG-Danish, but had remained similar for children who received BCG-Russia (Fig. 2). For the type 2 cytokines, responses among children who received BCG-Russia or BCG-Bulgaria increased between one and five years, while among children who received BCG-Danish they had stayed the same (IL-5) or waned (IL-13).Responses to IL-10 had decreased substantially among children receiving BCG-Bulgaria or BCG-Danish, with a smaller reduction seen among children receiving BCG-Russia (Fig. 2).
Fig. 2.
Distribution of cytokine responses to Mycobacterium tuberculosis crude culture filtrate protein at age one and five years, by BCG vaccine strain received in infancy. Graphs show distributions of cytokine responses (panel A: IFN-γ, panel B: IL-5, panel C: IL-13, panel D: IL-10) to Mycobacterium tuberculosis crude culture filtrate protein at age one year (indicated in blue) and age five years (indicated in pink), separately for children who received BCG-Russia, BCG-Bulgaria and BCG-Danish-values for comparison of responses at age one with responses at age five for each BCG strain and cytokine, were calculated using Wilcoxon signed rank test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Positive T-SPOT.TB® at age five years was associated with higher IFN-γ and IL-13 response to M.tb-cCFP.
Among 58 children with a history of TB contact who had cytokine and T-SPOT.TB® data available, there was no evidence of association between cytokine responses to M.tb-cCFP at one year and odds of LTBI at five years of age (Table 4). We also found no evidence of a relationship between child's BCG scar and T-SPOT.TB® in this subgroup (OR = 1.08, 95% CI: 0.34-3.42, p = 0.89).
Table 4.
Associations between cytokine responses to mycobacterial antigens at age one and odds of subsequent infection with M. tuberculosis infection by five years of age, among 58 children with a history of tuberculosis exposure.
| Cytokine response to cCFP at age 1 | Crude odds ratio | P | Adjusted odds ratioa | P |
|---|---|---|---|---|
| IFN-γ | 1.10 (0.58–2.09) | 0.76 | 1.14 (0.55–2.37) | 0.72 |
| IL-5 | 1.56 (0.80–3.05) | 0.20 | 1.88 (0.90–3.93) | 0.09 |
| IL-13 | 1.45 (0.72–2.94) | 0.28 | 1.73 (0.76–3.96) | 0.19 |
| IL-10 | 1.23 (0.48–3.16) | 0.65 | 1.21 (0.45–3.26) | 0.71 |
Adjusted for zone of residence.
4. Discussion
In this study, using data from a large birth cohort in Entebbe, Uganda, only urban location of residence and history of TB contact or disease during childhood were associated with LTBI at five years. Children testing positive for LTBI at five years had increased type 1 and type 2 cytokine responses to mycobacterial antigens. Other determinants for cytokine response at five years were HIV infection, anthropometry, malaria infection, quarterly albendazole treatment during childhood, maternal BCG scar and the BCG vaccine strain used to immunise the child during infancy.
LTBI prevalence among Ugandan children aged five years was 9% using T-SPOT.TB®, comparable with the 12% prevalence among children from Tanzania assessed by QuantiFERON(R)-TB assay [32] but less than the 21% prevalence reported among Ethiopian children [33]. Urban location of residence and history of TB contact/disease were positively associated with LTBI, with odds of LTBI increasing with the closeness of the TB contact. In contrast to findings from Ethiopia, we did not find that helminth exposure, either prenatally, or in childhood, was associated with LTBI [33].
In the same cohort, we have previously investigated factors associated with cytokine responses to M.tb-cCFP at age one [13,18]. Consistent with these findings, HIV infection remained associated with large reductions in IFN-γ and IL-13 responses at age five, and concurrent asymptomatic malaria infection remained associated with reduced IFN-γ response. These results emphasise the importance of HIV infection as a suppressor of immune responses. Since there were no consistent patterns for HIV-exposed-uninfected children, it appears that exposure to HIV in utero has no long-term impact on subsequent immune response, as long as the child remains uninfected (despite short-term differences observed in other studies [34,35]).
Anthropometry measures were consistently associated with higher cytokine responses, and these associations were stronger than those observed at one year, indicating that poor growth may have increasing importance among older children.
Consistent with our findings at one year [18], maternal helminth infections were not associated with cytokine responses at age five, suggesting that contrary to some hypotheses [36], maternal helminth infection during pregnancy does not explain poor BCG efficacy in the tropics.
As at one year, maternal BCG scar was associated with cytokine responses at age five, although the association pattern had changed: maternal BCG scar was associated with reduced Th2 cytokine responses at age one, but increased IL-10 responses at age five. These findings support the idea that some aspect of maternal exposure, or of maternal immune response, to mycobacteria may influence the infant immune response to mycobacterial antigen.
Cohort children were randomised to quarterly albendazole or placebo between 15 months and five years. Those receiving albendazole had reduced IFN-γ and IL-13 responses compared to those receiving placebo, as previously reported [26]. This is unlikely to be due to worm removal since childhood helminth infection was not associated with increased cytokine responses (in crude analyses, it was associated with reduced responses). These may be chance findings, since no effects were observed on the response to other antigens tested [26] and given the large number of statistical tests undertaken. Indeed, we did not formally adjust for multiplicity, but instead focus on patterns and consistency of results, when discussing our findings.
At five years, BCG vaccine strain was associated with cytokine production, with children who received BCG-Danish showing consistently reduced responses compared to children who received BCG-Russia, and children who received BCG-Bulgaria showing reduced type 1 and 2 responses, but increased regulatory (IL-10) responses. This implies that the choice of BCG vaccine has a direct effect on the immune response elicited, a hypothesis that is also supported by findings from other studies which have examined the association between BCG strain and immune response during infancy [37,38]. Differences in vaccines are due to evolution from the original BCG vaccine [14,39], for example BCG-Bulgaria is genetically derived from BCG-Russia. “Early” BCG vaccines, such as BCG-Russia, have been hypothesised to have better efficacy than later vaccines [39], although we saw little evidence for this in relation to LTBI, and a recent systematic review found little evidence of a relationship between BCG strain and subsequent efficacy [40].
These findings contrast with those we reported at one year, where infants who received BCG-Danish had markedly higher responses [13]. One possible explanation is that BCG-Danish, initially more immunogenic, could protect against TB infection and possibly against other non-TB mycobacteria, thus children immunised with BCG-Danish are now less likely to have acquired the infections that are the strongest inducers of responses at five years. However, we found no evidence that LTBI status modified the association between BCG vaccine strain and cytokine response to support this hypothesis. Second, studies have shown that the magnitude of cytokine response to mycobacterial antigen gradually reduces over time [3,41]. We found that the decline in cytokine response varied with BCG vaccine strain, with IFN-γ and IL-10 responses among children who received BCG-Danish or BCG-Bulgaria waning faster than among children who received BCG-Russia. Type 2 responses increased among children who received BCG-Russia or BCG-Bulgaria, but stayed the same or decreased among children who received BCG-Danish.
We found no evidence that immune response to mycobacterial antigen at one year was associated with subsequent LTBI, among those with documented exposure to TB disease, although power for this analysis was limited due to the relatively low LTBI prevalence. Further follow-up of the cohort is planned to investigate this. It remains a priority to identify reliable bio-markers of protection following immunisation, if we are to develop a better vaccine against TB.
Our study population exhibited high-prevalence, low-intensity helminth burden in mothers, but low prevalence in children, presumably related to mass drug administration and urbanisation. Findings might differ in populations with continuing high helminth prevalence and in non-TB endemic areas.
In conclusion, prenatal exposure to maternal helminth and malaria infections are unlikely to explain poor BCG vaccine efficacy in the tropics. Concurrent HIV or malaria infection and poor growth are important predictors of immune response. Policies aimed at prevention of mother-to-child HIV transmission and poor growth may contribute to effective immunisation programmes in the tropics. Only urban location of residence and having a history of TB contact/disease were associated with LTBI at five years in this cohort, and it remains to be seen whether the factors we have identified as important for cytokine responses at one and five years will be associated with TB infection at a later age.
Funding
This work was supported by the Wellcome Trust (063693 and 079110); mycobacterial antigens were provided through the National Institutes of Health contract NOI-AI-25147; albendazole and matching placebo were provided by GlaxoSmithKline. The funders had no further role in the conduct of the research, the preparation of this article, or the decision to submit for publication.
Contributors
A. Elliott conceived and designed the study. S. Lule conducted the statistical analysis under the supervision of E. Webb. P. Mawa and D. Kizito contributed to sample processing and conducted the cytokine assays. S. Lule, M. Nampijja and F. Akello contributed to recruitment and follow-up of participants and to clinical care. G. Nkurunungi conducted T-SPOT.TB® assays. L. Muhangi was responsible for data management. S. Lule, A. Elliott and E. Webb drafted the report with contributions from G. Nkurunungi, M. Nampijja and L. Muhangi. All authors have read and approved the final article.
Conflicts of interest statement
The authors have no associations that might pose a conflict of interest.
Acknowledgements
We thank all staff and participants of the Entebbe Mother and Baby Study, the midwives of the Entebbe Hospital Maternity Department, the community field team in Entebbe and Katabi, and the staff of the Clinical Diagnostic Services Laboratory at the MRC/UVRI Uganda Research Unit on AIDS.
References
- 1.Banfield S., Pascoe E., Thambiran A., Siafarikas A., Burgner D. Factors associated with the performance of a blood-based interferon-gamma release assay in diagnosing tuberculosis. PLoS One. 2012;7:e38556. doi: 10.1371/journal.pone.0038556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kus J., Demkow U., Lewandowska K., Korzeniewska-Kosela M., Rabczenko D., Siemion-Szczesniak I. Prevalence of latent infection with Mycobacterium tuberculosis in Mazovia Region using interferon gamma release assay after stimulation with specific antigens ESAT-6 and CFP-10. Pneumonol Alergol Pol. 2011;79:407–418. [PubMed] [Google Scholar]
- 3.Weir R.E., Gorak-Stolinska P., Floyd S., Lalor M.K., Stenson S., Branson K. Persistence of the immune response induced by BCG vaccination. BMC Infect Dis. 2008;8:9. doi: 10.1186/1471-2334-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soysal A., Millington K.A., Bakir M., Dosanjh D., Aslan Y., Deeks J.J. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005;366:1443–1451. doi: 10.1016/S0140-6736(05)67534-4. [DOI] [PubMed] [Google Scholar]
- 5.Zelner J.L., Murray M.B., Becerra M.C., Galea J., Lecca L., Calderon R. Bacillus Calmette–Guerin and isoniazid preventive therapy protect contacts of patients with tuberculosis. Am J Respir Crit Care Med. 2014;189:853–859. doi: 10.1164/rccm.201310-1896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Packe G.E., Innes J.A. Protective effect of BCG vaccination in infant Asians: a case-control study. Arch Dis Child. 1988;63:277–281. doi: 10.1136/adc.63.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatherill M. Prospects for elimination of childhood tuberculosis: the role of new vaccines. Arch Dis Child. 2011;96:851–856. doi: 10.1136/adc.2011.214494. [DOI] [PubMed] [Google Scholar]
- 8.Lalor M.K., Ben-Smith A., Gorak-Stolinska P., Weir R.E., Floyd S., Blitz R. Population differences in immune responses to Bacille Calmette–Guerin vaccination in infancy. J Infect Dis. 2009;199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine P.E. Bacille Calmette–Guerin vaccines: a rough guide. Clin Infect Dis. 1995;20:11–14. doi: 10.1093/clinids/20.1.11. (an official publication of the Infectious Diseases Society of America) [DOI] [PubMed] [Google Scholar]
- 10.Wilson M.E., Fineberg H.V., Colditz G.A. Geographic latitude and the efficacy of Bacillus Calmette–Guerin vaccine. Clin Infect Dis. 1995;20:982–991. doi: 10.1093/clinids/20.4.982. [DOI] [PubMed] [Google Scholar]
- 11.Thakur A., Pedersen L.E., Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine. 2012;30:4907–4920. doi: 10.1016/j.vaccine.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 12.Elliott A.M., Hurst T.J., Balyeku M.N., Quigley M.A., Kaleebu P., French N. The immune response to Mycobacterium tuberculosis in HIV-infected and uninfected adults in Uganda: application of a whole blood cytokine assay in an epidemiological study. Int J Tuberc Lung Dis. 1999;3:239–247. (the official journal of the International Union against Tuberculosis and Lung Disease) [PubMed] [Google Scholar]
- 13.Anderson E.J., Webb E.L., Mawa P.A., Kizza M., Lyadda N., Nampijja M. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine. 2012;30:2083–2089. doi: 10.1016/j.vaccine.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosch R., Gordon S.V., Garnier T., Eiglmeier K., Frigui W., Valenti P. Genome plasticity of BCG and impact on vaccine efficacy. Proc Nat Acad Sci USA. 2007;104:5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer T.F., Colditz G.A. Bacille Calmette–Guerin vaccination for the prevention of tuberculosis in health care workers. Clin Infect Dis. 1995;20:136–142. doi: 10.1093/clinids/20.1.136. (an official publication of the Infectious Diseases Society of America) [DOI] [PubMed] [Google Scholar]
- 16.Haile M., Kallenius G. Recent developments in tuberculosis vaccines. Curr Opin Infect Dis. 2005;18:211–215. doi: 10.1097/01.qco.0000168380.08895.9a. [DOI] [PubMed] [Google Scholar]
- 17.Fine P.E. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 18.Elliott A.M., Mawa P.A., Webb E.L., Nampijja M., Lyadda N., Bukusuba J. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine. 2010;29:247–255. doi: 10.1016/j.vaccine.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalor M.K., Floyd S., Gorak-Stolinska P., Ben-Smith A., Weir R.E., Smith S.G. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis. 2011;204:1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher H.A. Correlates of immune protection from tuberculosis. Curr Mol Med. 2007;7:319–325. doi: 10.2174/156652407780598520. [DOI] [PubMed] [Google Scholar]
- 21.Rook G.A., Hernandez-Pando R., Zumla A. Tuberculosis due to high-dose challenge in partially immune individuals: a problem for vaccination. J Infect Dis. 2009;199:613–618. doi: 10.1086/596654. [DOI] [PubMed] [Google Scholar]
- 22.Higgins D.M., Sanchez-Campillo J., Rosas-Taraco A.G., Lee E.J., Orme I.M., Gonzalez-Juarrero M. Lack of IL-10 alters inflammatory and immune responses during pulmonary Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2009;89:149–157. doi: 10.1016/j.tube.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Rook G.A., Dheda K., Zumla A. Do successful tuberculosis vaccines need to be immunoregulatory rather than merely Th1-boosting. Vaccine. 2005;23:2115–2120. doi: 10.1016/j.vaccine.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 24.Weiner J., 3rd., Kaufmann S.H. Recent advances towards tuberculosis control: vaccines and biomarkers. J Intern Med. 2014;275:467–480. doi: 10.1111/joim.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott A.M., Kizza M., Quigley M.A., Ndibazza J., Nampijja M., Muhangi L. The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double-blind, placebo-controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood [ISRCTN32849447] Clin Trials. 2007;4:42–57. doi: 10.1177/1740774506075248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndibazza J., Mpairwe H., Webb E.L., Mawa P.A., Nampijja M., Muhangi L. Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial. PLoS One. 2012;7:e50325. doi: 10.1371/journal.pone.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb E.L., Mawa P.A., Ndibazza J., Kizito D., Namatovu A., Kyosiimire-Lugemwa J. Effect of single-dose anthelmintic treatment during pregnancy on an infant's response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:52–62. doi: 10.1016/S0140-6736(10)61457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz N., Chaves A., Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 29.Melrose W.D., Turner P.F., Pisters P., Turner B. An improved Knott's concentration test for the detection of microfilariae. Trans R Soc Trop Med Hyg. 2000;94:176. doi: 10.1016/s0035-9203(00)90266-9. [DOI] [PubMed] [Google Scholar]
- 30.Victora C.G., Huttly S.R., Fuchs S.C., Olinto M.T. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224–227. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 31.McGuinness D., Bennett S., Riley E. Statistical analysis of highly skewed immune response data. J Immunol Methods. 1997;201:99–114. doi: 10.1016/s0022-1759(96)00216-5. [DOI] [PubMed] [Google Scholar]
- 32.Rose M.V., Kimaro G., Nissen T.N., Kroidl I., Hoelscher M., Bygbjerg I.C. QuantiFERON(R)-TB gold in-tube performance for diagnosing active tuberculosis in children and adults in a high burden setting. PLoS One. 2012;7:e37851. doi: 10.1371/journal.pone.0037851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wassie L., Aseffa A., Abebe M., Gebeyehu M.Z., Zewdie M., Mihret A. Parasitic infection may be associated with discordant responses to QuantiFERON and tuberculin skin test in apparently healthy children and adolescents in a tuberculosis endemic setting, Ethiopia. BMC Infect Dis. 2013;13:265. doi: 10.1186/1471-2334-13-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidzeru E.B., Hesseling A.C., Passmore J.A., Myer L., Gamieldien H., Tchakoute C.T. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS. 2014;28:1421–1430. doi: 10.1097/QAD.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzola T.N., da Silva M.T., Abramczuk B.M., Moreno Y.M., Lima S.C., Zorzeto T.Q. Impaired Bacillus Calmette–Guerin cellular immune response in HIV-exposed, uninfected infants. AIDS. 2011;25:2079–2087. doi: 10.1097/QAD.0b013e32834bba0a. [DOI] [PubMed] [Google Scholar]
- 36.Elliott A.M., Namujju P.B., Mawa P.A., Quigley M.A., Nampijja M., Nkurunziza P.M. A randomised controlled trial of the effects of albendazole in pregnancy on maternal responses to mycobacterial antigens and infant responses to Bacille Calmette–Guerin (BCG) immunisation [ISRCTN32849447] BMC Infect Dis. 2005;5:115. doi: 10.1186/1471-2334-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davids V., Hanekom W.A., Mansoor N., Gamieldien H., Gelderbloem S.J., Hawkridge A. The effect of Bacille Calmette–Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J Infect Dis. 2006;193:531–536. doi: 10.1086/499825. [DOI] [PubMed] [Google Scholar]
- 38.Ritz N., Dutta B., Donath S., Casalaz D., Connell T.G., Tebruegge M. The influence of Bacille Calmette–Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med. 2012;185:213–222. doi: 10.1164/rccm.201104-0714OC. [DOI] [PubMed] [Google Scholar]
- 39.Behr M.A. BCG—different strains, different vaccines. Lancet Infect Dis. 2002;2:86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 40.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. (an official publication of the Infectious Diseases Society of America) [DOI] [PubMed] [Google Scholar]
- 41.Bennett A.R., Gorak-Stolinska P., Ben-Smith A., Floyd S., de Lara C.M., Weir R.E. The PPD-specific T-cell clonal response in UK and Malawian subjects following BCG vaccination: a new repertoire evolves over 12 months. Vaccine. 2006;24:2617–2626. doi: 10.1016/j.vaccine.2005.12.011. [DOI] [PubMed] [Google Scholar]