Abstract
The crystal structure of a 14-kDa bovine spleen S-lectin complexed with the disaccharide N-acetyllactosamine at 1.9-A resolution reveals a surprising structural relationship to legume lectins, despite the lack of sequence homology. Two monomers associate to form an extended beta-sandwich, each with the same jelly roll topology typical of legume lectins but with dramatically trimmed loops and with different dimer association. Each monomer binds one N-acetyllactosamine molecule in a topologically and spatially different site than that of legume lectins. The carbohydrate-binding site provides an unprecedented paradigm for carbohydrate binding, with a unique network of salt bridges. The specificity for beta-galactose arises from intricate interactions that constrain the position of the O4 atom.
Full text
PDF
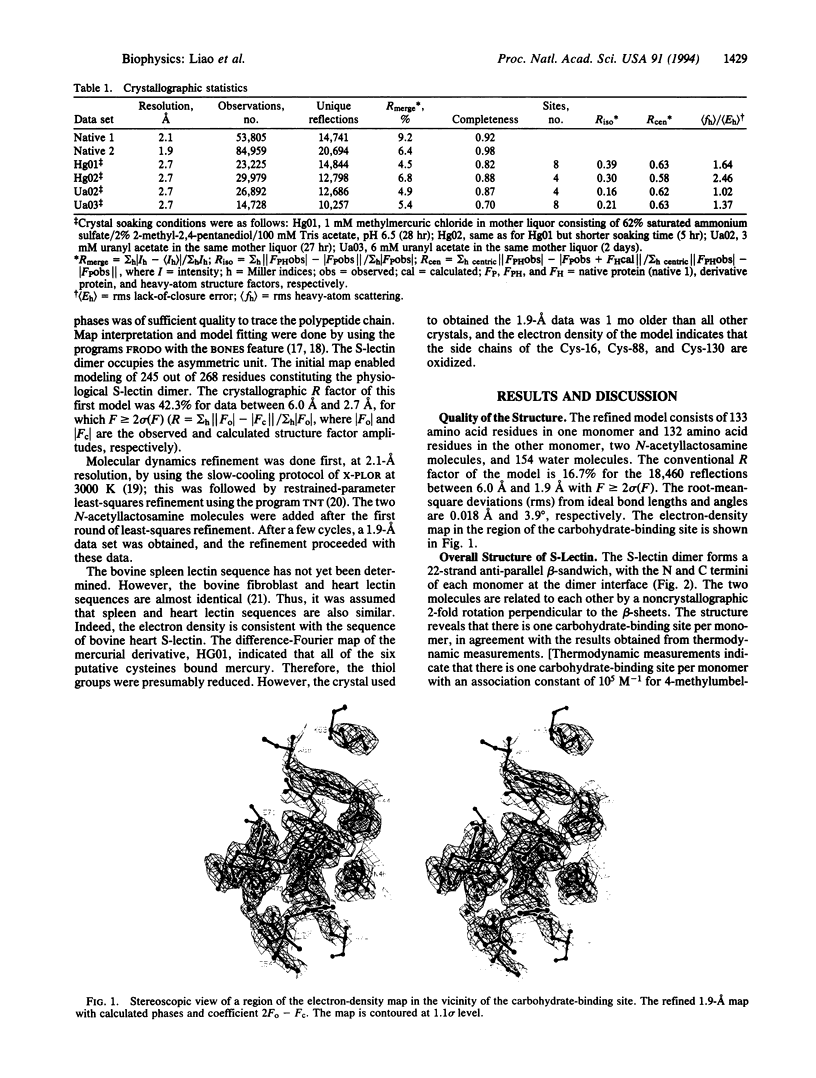
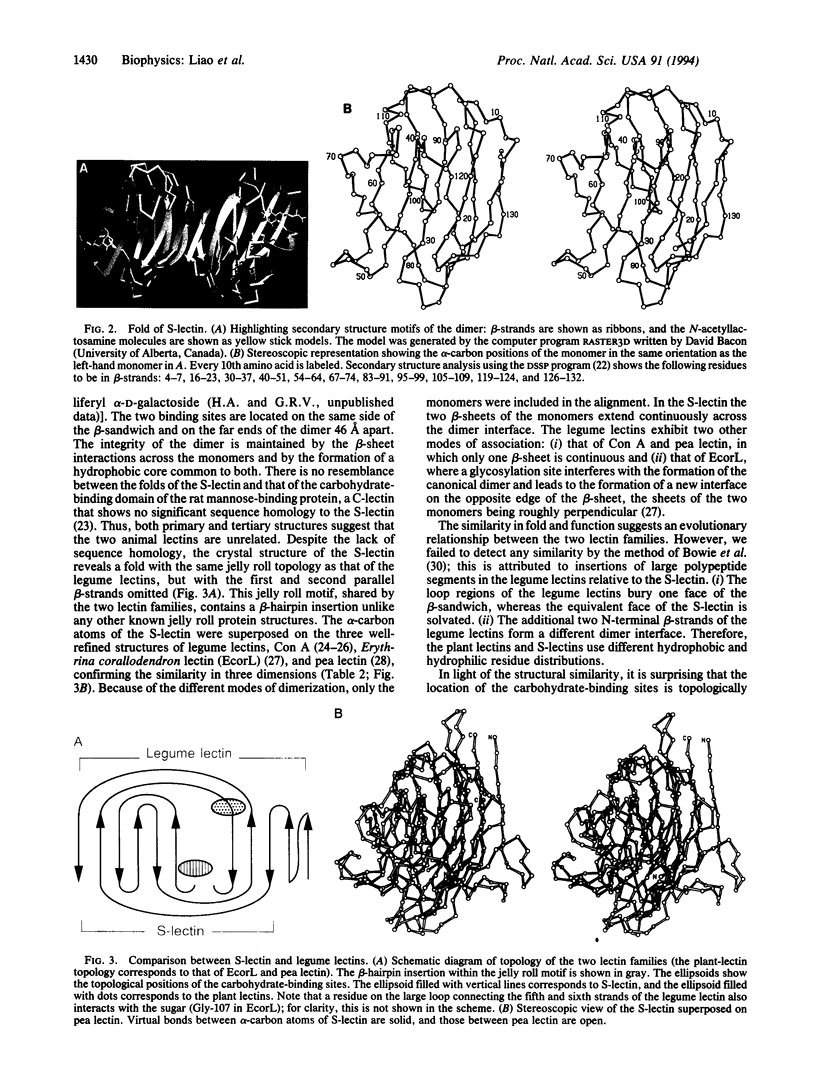

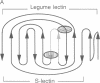
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. M., Feizi T. Soluble 14-kDa beta-galactoside-specific bovine lectin. Evidence from mutagenesis and proteolysis that almost the complete polypeptide chain is necessary for integrity of the carbohydrate recognition domain. J Biol Chem. 1991 Mar 25;266(9):5552–5557. [PubMed] [Google Scholar]
- Abbott W. M., Mellor A., Edwards Y., Feizi T. Soluble bovine galactose-binding lectin. cDNA cloning reveals the complete amino acid sequence and an antigenic relationship with the major encephalitogenic domain of myelin basic protein. Biochem J. 1989 Apr 1;259(1):283–290. doi: 10.1042/bj2590283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H., Allen H. J., Sharma A., Matta K. L. Human splenic galaptin: carbohydrate-binding specificity and characterization of the combining site. Biochemistry. 1990 Jun 5;29(22):5315–5319. doi: 10.1021/bi00474a015. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr, Wang J. L., Cunningham B. A., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. III. Structure of the monomer and its interactions with metals and saccharides. J Biol Chem. 1975 Feb 25;250(4):1513–1524. [PubMed] [Google Scholar]
- Bowie J. U., Lüthy R., Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991 Jul 12;253(5016):164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Clerch L. B., Whitney P., Hass M., Brew K., Miller T., Werner R., Massaro D. Sequence of a full-length cDNA for rat lung beta-galactoside-binding protein: primary and secondary structure of the lectin. Biochemistry. 1988 Jan 26;27(2):692–699. doi: 10.1021/bi00402a030. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Barondes S. H. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990 May;110(5):1681–1691. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Massa S. M., Barondes S. H. Endogenous muscle lectin inhibits myoblast adhesion to laminin. J Cell Biol. 1991 Dec;115(5):1437–1448. doi: 10.1083/jcb.115.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K. Y., Smith D. F., Cummings R. D. LAMP-1 in CHO cells is a primary carrier of poly-N-acetyllactosamine chains and is bound preferentially by a mammalian S-type lectin. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1123–1128. doi: 10.1016/s0006-291x(05)80902-7. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Einspahr H., Parks E. H., Suguna K., Subramanian E., Suddath F. L. The crystal structure of pea lectin at 3.0-A resolution. J Biol Chem. 1986 Dec 15;261(35):16518–16527. [PubMed] [Google Scholar]
- Feizi T., Childs R. A. Carbohydrates as antigenic determinants of glycoproteins. Biochem J. 1987 Jul 1;245(1):1–11. doi: 10.1042/bj2450001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman K. D., Agarwal R. C., Freiser M. J. Manganese and calcium binding sites of concanavalin A. J Mol Biol. 1982 May 5;157(1):69–86. doi: 10.1016/0022-2836(82)90513-7. [DOI] [PubMed] [Google Scholar]
- Harrison F. L. Soluble vertebrate lectins: ubiquitous but inscrutable proteins. J Cell Sci. 1991 Sep;100(Pt 1):9–14. doi: 10.1242/jcs.100.1.9. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J., Kasai K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J Biol Chem. 1991 Dec 15;266(35):23648–23653. [PubMed] [Google Scholar]
- Hughes R. C. Role of glycosylation in cell interactions with extracellular matrix. Biochem Soc Trans. 1992 May;20(2):279–284. doi: 10.1042/bst0200279. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Leffler H., Barondes S. H. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian beta-galactosides. J Biol Chem. 1986 Aug 5;261(22):10119–10126. [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. IV. Atomic coordinates, hydrogen bonding, and quaternary structure. J Biol Chem. 1975 Feb 25;250(4):1525–1547. [PubMed] [Google Scholar]
- Sato S., Hughes R. C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992 Apr 5;267(10):6983–6990. [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Shaanan B., Lis H., Sharon N. Structure of a legume lectin with an ordered N-linked carbohydrate in complex with lactose. Science. 1991 Nov 8;254(5033):862–866. doi: 10.1126/science.1948067. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991 Dec 13;254(5038):1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- Wells V., Mallucci L. Identification of an autocrine negative growth factor: mouse beta-galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell. 1991 Jan 11;64(1):91–97. doi: 10.1016/0092-8674(91)90211-g. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Cummings R. D. The S-type lectin from calf heart tissue binds selectively to the carbohydrate chains of laminin. Arch Biochem Biophys. 1990 Aug 15;281(1):27–35. doi: 10.1016/0003-9861(90)90408-q. [DOI] [PubMed] [Google Scholar]