Key Clinical Message
A surgical technique, materials used for abdominal wall reconstruction, and postoperative care are important for patient outcomes. We report the first case of neonate with Cantrell's pentalogy surviving early reconstruction of abdominal, diaphragmal and pericardial defects. Several recent investigations suggest that intraabdominal pressure monitoring may improve outcomes in this patient category.
Keywords: Congenital abnormalities, herniorrhaphy, intensive care, intraabdominal hypertension, mesh repair, monitoring, omphalocele, pentalogy of Cantrell
Introduction
Cantrell–Haller–Ravitch syndrome (Cantrell's pentalogy) was first described in 1958 1. The origin of this rare syndrome is developmental failure of the ventral mesoderm that usually occurs between days 14 and 18 of embryonic development 1. A defect of the cephalic fold results in a wide range of cardiac, thoracic, and abdominal anomalies, occurring in 1 per 65,000 live births and was found to be more common in boys than in girls (1.35:1) 2,3.
Cantrell's pentalogy is characterized by sternal aplasia, anterior abdominal wall defect, diaphragmal aplasia/hypoplasia, defects of the diaphragmatic part of the pericardium with associated cardiac malformations varying in range and severity 1,3. Mortality is high in the early stage, with a survival rate of 37% 2. Predictors of survival are age at operation, complexity of cardiac malformations, and possibility of surgical reconstruction of abdominal wall defects 2,4.
A surgical reconstruction of cardiac malformations may be postponed for several weeks and sometimes months, whereas a defect of abdominal wall with omphalocele requires early reconstruction 4,5. A method of staged repair may significantly reduce postoperative respiratory insufficiency, ventilator dependency, and even decrease mortality 5.
In this report, we present a case of successful early reconstructive surgical treatment in a premature boy with pentalogy of Cantrell. A material used for the reconstruction, timing of procedures, postoperative complications, and outcome are discussed. An importance of intraabdominal pressure monitoring (IAP) is emphasized in the light of new guidelines 6.
Case Report
A 39-year-old parturient with three previous normal pregnancies followed by normal deliveries presented with a fetal omphalocele detected by ultrasound in the 20th week of gestation. Amniocentesis showed a normal male karyotype of 46 XY. A pregnancy was terminated at 35-week gestational age by cesarean section due to the placental abruption.
A boy was delivered with Apgar scores 7 and 8 at 5 and 10 min, birth weight of 2919 g, and birth length of 48 cm. He had major omphalocele, a defect of lower sternum with caudal displacement of the heart and pulsatile heart apex below the skin in the upper abdomen (Fig.1A). A flat nose and bilateral undescended testes were also registered. In the early postnatal period, the newborn was admitted to the neonatal intensive care unit (NICU) with spontaneous ventilation. Early cardiologic examination using ultrasound had revealed an atrial and ventricular septal defect with cardiac ectopy into epigastrium. A liver was in the central position.
Figure 1.
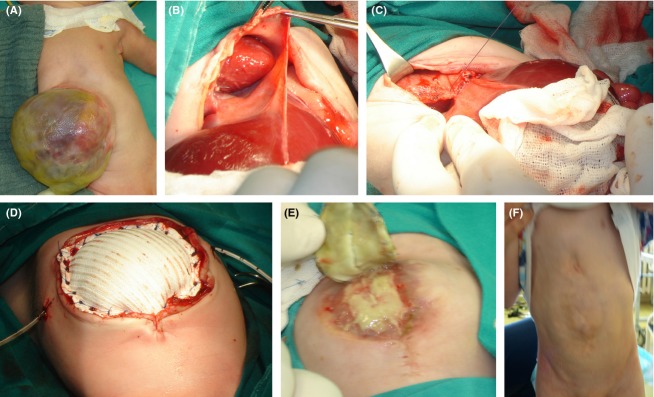
(A) A major omphalocele with liver in the central position. (B) A defect of the ventral part of diaphragm, aplastic lower sternum, defective hypoplastic pericardium, and caudal displacement of heart. (C) A reconstruction of diaphragm with direct stitches after mobilizing the posterior leaf. (D) A polytetrafluoroethylene patch used for the staged abdominal wall reconstruction with pericardial drain and umbilical vein catheter in situ. (E) A scar plate formation below the mesh. (F) A boy at the age of 4. Rectus muscles seem to be retracted with no signs of reherniation.
Following discussion with the family, reconstructive surgery was initiated within 24 h of delivery under general sevoflurane anesthesia. Surgical exploration revealed an omphalocele containing the small bowel and liver in the ventral position. Intestinal malrotation was found with an undeveloped omentum. A defect of the ventral part of diaphragm, aplastic lower half of sternum (Fig.1B), defective hypoplastic pericardium, and caudal displacement of heart was confirmed. During the procedure performed by a pediatric and cardiac surgeon, the heart was moved into the thorax, the pericardium was closed and the ventral part of diaphragm was reconstructed with direct stitches after mobilizing the posterior leaf (Fig.1C). Thoracic and abdominal cavities were separated. An anterior abdominal wall defect was partially repaired by mobilization and minimal stretching of the abdominal muscles. A residual defect of ∼10 cm in transverse diameter was covered by the placement of 2-mm-thick polytetrafluoroethylene (Gore-Tex®; WL Gore and Associates, Flagstaff, AZ) patch (Fig.1D). A patch was sewn using Gore-Tex 3-0 sutures and applied to the full thickness of the abdominal wall. A pericardial drain and umbilical vein catheter were placed (Fig.1D).
During the initial hospitalization, a ventricular septal defect, atrial septal defect type II, and pulmonary artery stenosis of mild grade were verified by MSCT angiography and coronary catheterization. The inferior vena cava was hypoplastic below the renal level with an enlarged azygous vein. Heart rate and blood pressure were within normal range for age. In the NICU, prolonged mechanical ventilation with pressure support ventilation and volume guarantee modes were required due to muscle weakness, recurrent bronchopneumonia, and difficult weaning. Chest radiography did not confirm diaphragmatic paresis. During the period of mechanical ventilation, he developed a cardiac failure, which was successfully treated with noradrenaline and dobutamine, whereas acute renal failure was treated with diuretics. Parenteral nutrition was started immediately and gradually switched to the enteral nutrition via nasogastric tube due to difficulty in swallowing. Enteral feeding with high-energy infant formula (Infatrini®; Nutricia BV Zoetermeer, Zoetermeer, the Netherlands) was continued through the entire hospital stay.
A second surgical correction was carried out on the 20th postnatal day. The mesh showed no signs of infection and was reduced by 50% on the midline. Complete surgical closure whereby the abdominal wall was closed layer by layer was achieved during the third procedure carried out on the 47th day. Even though the mesh was not contaminated, a scar plate formation was registered below the mesh (Fig.1E). Fibrotic scar was partially dissected, and the midline defect covered by rectus muscles. The skin was closed using Gore-tex stitches 3–0. Since intraabdominal (IAP) pressure raised after abdominal closure, 2 weeks later the patient developed bilateral inguinoscrotal hernias. After counseling with the parents, surgical treatment of the hernias was postponed. He was mechanically ventilated for 3 months, and was discharged home at 4.5 months of age with a body weight of 4450 g, which was below the 5th percentile. Enteral feeding via a nasogastric tube was continued at home for 6 weeks subsequently, and replaced gradually with a high-energy oral intake. Following an adequate neurologic outcome at home, walking was commenced at 20 months due to muscle hypotrophy. Both hernias became enlarged within a year after abdominoplasty and a loop of small intestine was seen within the right sac without signs of obstruction. Two asymptomatic gallbladder stones were also detected during routine ultrasound scans.
After 3 years, the parents accepted surgical treatment and the boy was admitted for bilateral inguinoscrotal hernia repair. During the uneventful surgical procedure, the small intestine was pushed back into abdomen, the hernial sac resected; the internal inguinal ring closed with 3–0 vicryl and the left testis was placed into the scrotum. Currently, the boy is 4 years old and normally active. He is still hypotrophic (<5 percentile) and needs high-energy oral supplementation. He has a good cosmetic outcome (Fig.1F), with no signs of reherniation or cardiac dysfunction.
Discussion
The treatment of patients with pentalogy of Cantrell causes serious concern and distress for parents and presents a major challenge for a multidisciplinary team involving a surgeon, anesthetist, and pediatrician. Survival is largely determined by the severity and complexity of cardiac malformations, the efficiency of abdominal wall closure and postoperative complications. A literature review shows that all nonsurvivors had surgery on the first postnatal day 2, whereas the mean age of survivors at operation was 9 months 2,3. Poor surgical survival in the first days of life accounted for a delay in primary surgical repair for weeks or months. Therefore, a conservative approach in the first days of life is routine for Cantrell's syndrome.
The mortality of Cantrell's pentalogy is triphasic. Antenatal loss and early postnatal presurgical loss occur in the majority, although with unknown incidence. Early postoperative death is common in patients with complex cardiac anomalies, or in those having postoperative complications like respiratory insufficiency, bowel dismotility, intraabdominal and pulmonary hypertension 4,7. Late deaths are usually a complication of cardiac dysfunction, infections, or adhesive small bowel obstruction 3,4,7,8.
Postoperative complications vary depending on the timing of surgery and on the materials used for the reconstruction of the defects 7–10. In the patients with diaphragmal hernia who were subjected to early repair of diaphragm, the major mortality risks are respiratory distress, an elevation of the IAP, and infections. All those complications are more common with mesh repair 10, which was avoided in the diaphragmal reconstruction in our patient.
On the contrary, the use of prosthetic patches is efficient for both temporary and definitive repair of abdominal wall defects 5,7,10. The polytetrafluoroethylene in the omphalocele patients gradually stretches abdominal muscles by achieving a low tension midline closure 7. A critical IAP level was earlier defined as >20 mmHg with new organ failure 11–13. Since renal dysfunction shows a good correlation with IAP >20 mmHg, it was widely accepted as a monitoring substitute in the adult population 11,13. In our patient, a transient renal dysfunction correlating with multiple organ failure was registered after mesh reduction, suggesting the increase in the IAP. Lacey and coworkers were able to guide the closure method and timing of silo reductions while maintaining the IAP at levels ≤20 mmHg in five of 14 infants with omphalocele. Although they have not observed renal failure or refractory oliguria, three patients developed necrotizing enterocolitis and one necrotic bowel lesions 11. These results suggested that tension-free condition and normal values of IAP may be somewhat different across various populations, with lower values in the newborns.
The recent guidelines from the World Society of the Abdominal Compartment Syndrome (ACS) have proposed new definitions that are specific for pediatric use. The new proposed IAP level in the critically ill children is between 4 and 10 mmHg. Intraabdominal hypertension (IAH) in children is sustained or repeated pathological elevation in IAP >10 mmHg, whereas ACS is defined as sustained elevation in IAP of greater than 10 mmHg associated with new or worsening organ dysfunction that can be attributed to the elevated IAP 6. Contrary to the earlier opinions, renal function monitoring may not be sensitive enough to recognize IAH in the children 6,11,12.
The prolonged respiratory insufficiency, difficult weaning from mechanical ventilation, and reherniation are usual signs of increased IAP after omphalocele reduction. Respiratory insufficiency occurs as a rule in the Cantrell's pentalogy patients with diaphragmal hernia and omphalocele subjected to primary abdominal wall repair 7,8,10. Typically, requirement for mechanical ventilation is higher and survival poor following early definitive omphalocele repair 5,7,10.
Reherniation is a frequent postoperative complication after diaphragmal hernia repair with patches 10, and after omphalocele repair 14. Reherniation was observed in 41% of survivors after abdominal wall reconstruction and diaphragmatic hernia repair. It typically occurs in bimodal fashion: at median age of 2 months, like in our patient, and at 20 months 6. Although better results were registered in the small studies after delayed abdominal closure, to date, there are no studies investigating the relationship of IAP values and reherniation 15.
A recent study of Shah and coworkers has shown that intestinal dysfunction known as nonocclusive mesenteric hypertension (NMH) may arise as a consequence of mild increase in the mesenteric venous pressure 16. Authors have confirmed a significant intestinal remodeling after the mesenteric venous pressure increased from 4.0 mmHg in sham-treated animals to 7.3 and 9.0 mmHg in nonocclusive group. This study provides evidence that NMH delays intestinal transit, decreases contractility, and increases mucosal permeability with bacterial translocation. Increased smooth-muscle thickness and loss of intestinal length are irreversible structural changes in the treated animals after NMH 16. Intestinal damage may occur along with normal renal function, suggesting that gut function may be affected by the moderate IAP elevation 11. These changes may result in the permanent low weight gain as observed in our patient.
Even though no ischemic intestinal complications were registered in our patent, the recovery of his intestinal function was slow, requiring parenteral supplementation and prolonged feeding via nasogastric tube. Continuous high energy intake was needed, but his body weight is permanently below the 5th centile. Schlatter and coworkers have observed more rapid return of bowel function, fewer ventilator days, fewer complications and better outcomes in the gastroschisis patients after delayed abdominal closure compared with early repair 15. Again, authors did not mention whether IAP measurements were taken.
Routine measurements of IAP were performed by 20% of German pediatric intensivists, and only rarely in less developed countries 14,17. Some pediatric intensivists only measured IAP in the cases of organ dysfunction and failure 14,17. Although awareness among pediatricians on the IAP monitoring has been increasing in the last years 12, guidelines regarding the diagnosis and management of IAH/ACS are not applied widely 14,17. The use of protocolized monitoring and management of IAP in all pediatric surgical units may improve patients' outcome and give new insight in the correlation of IAP and gut function in the complex abdominal wall defects.
Conclusion
A favorable functional and cosmetic outcome was achieved in the reconstruction of major defects in a premature boy with Cantrell pentalogy using Gore-tex patch. The respiratory insufficiency and delayed enteral feeding were registered after early diaphragmal repair and omphalocele reduction. These complications of increased IAP may be prevented by IAP monitoring in the NICU. Clinical studies should be undertaken investigating a critical IAP level in neonates. It may guide stepwise abdominal closure, reduce ventilator dependency, reduce reherniation rate, and permanent gut dysfunction.
Acknowledgments
The authors kindly acknowledge Branko Bulovic, Sydney, Australia, for the language revisions. We gratefully acknowledge the help of Dr. Subhamay Ghosh, Department of Anesthesia, University of Manitoba, Winnipeg, Canada in preparing the final manuscript, and Mr. Ivic who provided technical support during the patient's treatment and who took intraoperative images.
Conflict of Interest
None declared.
References
- Cantrell JR, Haller JA. Ravitch MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium and heart. Surg. Gynecol. Obstet. 1958;107:602–614. [PubMed] [Google Scholar]
- Vazquez-Jimenez JF, Muehler EG, Daebritz S, Keutel J, Nishigaki K, Huegel W, et al. Cantrell's syndrome: a challenge to the surgeon. Ann. Thorac. Surg. 1998;65:1178–1185. doi: 10.1016/s0003-4975(98)00089-7. [DOI] [PubMed] [Google Scholar]
- Engum SA. Embryology, sternal clefts, ectopia cordis, and Cantrell's pentalogy. Semin. Pediatr. Surg. 2008;17:154–160. doi: 10.1053/j.sempedsurg.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wheeler DS. St Louis JD. Pentalogy of Cantrell associated with hypoplastic left heart syndrome. Pediatr. Cardiol. 2007;28:311–313. doi: 10.1007/s00246-007-0016-9. [DOI] [PubMed] [Google Scholar]
- Saxena AK. van Tuil C. Delayed three-stage closure of giant omphalocele using pericard patch. Hernia. 2008;12:201–203. doi: 10.1007/s10029-007-0264-x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn S, Bahr M, Schalamon J. Saxena AK. Single-center 10-year experience in the management of anterior abdominal wall defects. Hernia. 2008;12:345–350. doi: 10.1007/s10029-008-0336-6. [DOI] [PubMed] [Google Scholar]
- Thakur A, Chiu C, Quiros-Tejeira RE, Reyen L, Ament M, Atkinson JB, et al. Morbidity and mortality of short-bowel syndrome in infants with abdominal wall defects. Am. Surg. 2002;68:75–79. [PubMed] [Google Scholar]
- Drury NE, De Silva RJ, Hall RM. Large SR. Congenital defects of the pericardium. Ann. Thorac. Surg. 2007;83:1552–1553. doi: 10.1016/j.athoracsur.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Moss RL, Chen CM. Harrison MR. Prosthetic patch durability in congenital diaphragmatic hernia: a long-term follow-up study. J. Pediatr. Surg. 2001;36:152–154. doi: 10.1053/jpsu.2001.20037. [DOI] [PubMed] [Google Scholar]
- Lacey SR, Carris LA, Beyer AJ, III, Azizkhan RG. Wesley J. Bladder pressure monitoring significantly enhances care of infants with abdominal wall defects: a prospective clinical study. J. Pediatr. Surg. 1993;28:1370–1374. doi: 10.1016/s0022-3468(05)80329-x. [DOI] [PubMed] [Google Scholar]
- Rizzo A, Davis PC, Hamm CR. Powell RW. Intraoperative vesical pressure measurements as a guide in the closure of abdominal wall defects. Am. Surg. 1996;62:192–196. [PubMed] [Google Scholar]
- Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–1732. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- Osifo OD. Efobi AC. Challenges of giant ventral hernia repair in children in an African tertiary care center with limited resources. Hernia. 2009;13:143–147. doi: 10.1007/s10029-008-0439-0. [DOI] [PubMed] [Google Scholar]
- Schlatter M, Norris K, Uitvlugt N, DeCou J. Connors R. Improved outcomes in the treatment of gastroschisis using a preformed silo and delayed repair approach. J. Pediatr. Surg. 2003;38:459–464. doi: 10.1053/jpsu.2003.50079. discussion 459–464. [DOI] [PubMed] [Google Scholar]
- Shah SK, Aroom KR, Walker PA, Xue H, Jimenez F, Gill BS, et al. Effects of nonocclusive mesenteric hypertension on intestinal function: implications for gastroschisis-related intestinal dysfunction. Pediatr. Res. 2012;71:668–674. doi: 10.1038/pr.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaussen T, Steinau G, Srinivasan PK, Otto J, Sasse M, Staudt F, et al. Recognition and management of abdominal compartment syndrome among German pediatric intensivists: results of a national survey. Ann. Intensive Care. 2012;2(Suppl.1):S8. doi: 10.1186/2110-5820-2-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]


